MRI sequence optimisation methods to identify cranial nerve course for radiotherapy planning
Abstract
Introduction
Magnetic resonance imaging (MRI) is being increasingly used to improve radiation therapy planning by allowing visualisation of organs at risk that cannot be well-defined on computed tomography (CT). Diagnostic sequences are increasingly being adapted for radiation therapy planning, such as the use of heavily T2-weighted 3D SPACE (Sampling Perfection with Application optimised Contrasts using different flip angle Evolution) sequence for cranial nerve identification in head and neck tumour treatment planning.
Methods
A 3D isotropic T2 SPACE sequence used for cranial nerve identification was adapted for radiation therapy purposes. Distortion was minimised using a spin-echo-based sequence, 3D distortion correction, isocentre scanning and an increased readout bandwidth. Radiation therapy positioning was accounted for by utilising two small flex, 4-channel coils. The protocol was validated for cranial nerve identification in clinical applications and distortion minimisation using an MRI QA phantom.
Results
Normal anatomy of the cranial nerves CI-CIX, were presented, along with a selection of clinical applications and abnormal anatomy. The usefulness of cranial nerve identification is discussed for several case studies, particularly in proximity to tumours extending into the base of skull region. In-house testing validated that higher bandwidths of 600 Hz resulted in minimal displacement well below 1 mm.
Conclusion
The use of MRI for radiation therapy planning allows for greater individualisation and prediction of patient outcomes. Dose reduction to cranial nerves can decrease late side effects such as cranial neuropathy. In addition to current applications, future directions include further applications of this technology for radiation therapy treatments.
Introduction
Radiation treatment requires accurate identification of targets and organs at risk to achieve a therapeutic ratio. Magnetic resonance imaging (MRI) is increasingly being used in radiation therapy planning due to its superior soft tissue contrast compared to conventional computed tomography (CT). With the advent of MRI simulators and MRI LINACS, it has become necessary to understand the concepts of MRI and adaption for radiation treatments. It is common practice to import diagnostic images to radiation therapy planning systems, however, diagnostic images can result in suboptimal image fusion due to differences in patient position and inherent distortions in diagnostic scans, particularly in the translation of small structures.1 Deformable image registration techniques are currently utilised clinically to overcome these image registration obstacles. However, if appropriate deformable software is unavailable to a clinic, or in the absence of reference CT, such as in the case of MRI-based treatment planning, it is important to investigate the adaptation of MRI protocols for distortion minimisation to improve registration accuracy.2
Integration of diagnostic procedures into radiation therapy-focused protocols can increase the usefulness of MRI in radiation therapy, particularly in cranial imaging. One area of interest in cranial imaging is the identification of cranial nerves for radiation therapy planning, with MRI affording the ability to visualise cranial structures and organs at risk which cannot be appreciated on conventional CT alone. Malignancies such as cutaneous squamous cell carcinoma and adenoid cystic carcinomas can present with perineural spread. These presentations along with nerve-related pain syndromes, that is, trigeminal neuralgia, need accurate identification of the nerves to improve the therapeutic response to treatment. While in other instances, identification of cranial nerves would be useful in sparing the nerve and/or reduce hotspots when treating benign conditions like acoustic neuromas and pituitary adenoma.
Diagnostic MRI sequences used for the identification of cranial nerves include True FISP/Dual excitation acquisitions such as Siemens' 3D CISS (three-dimensional constructive interference of steady state) and T2-weighted turbo spin echo techniques such as a 3D SPACE (Sampling Perfection with Application optimised Contrasts using different flip angle Evolution).3 Studies have identified that an increase in spatial resolution and decreased slice thickness in the sequence used resulted in an increased reliability in identifying the cranial nerves, particularly cranial nerves III-XII.3, 4 The comparisons between 3D CISS and 3D SPACE sequences for cranial nerve found that the 3D SPACE is superior for identifying cranial nerves V-XII, as well as having fewer image-degrading artefacts.4, 5 As the cranial nerves carry a sleeve of cerebral spinal fluid (CSF) until it exits the cranium, the use of a heavily T2-weighted sequence allows for the visualisation of the nerve, against the bright CSF, while the 3D isotropic acquisition allows for retrospective reformatting of the data in multiple view angles to allow greater assessment of the anatomical area being examined.
In this report, we describe our adaptation of a T2-weighted 3-dimensional SPACE diagnostic sequence in radiation therapy planning, for cranial nerve identification in head and neck tumour treatment planning. The adaptation to radiation therapy treatment positioning and distortion minimisation requirements are supported using a turbo-spin echo-based sequence, reducing B0, chemical shift and patient-specific distortions.6 This sequence offers additional advantages such as lower Specific Absorption Rate (SAR) and the ability to pair with advanced acceleration techniques for high-resolution 3D images in a shorter time frame.
Materials and Equipment
Technical challenges
Sequence adaptation
Image distortion in MRI is attributed to the non-linearity of the magnetic field gradient, in-homogeneity of the static magnetic field (B0) and magnetic susceptibility effect specific to the imaging subject.6 Mitigation of the effects can be achieved largely through the use of vendor distortion correction, as well as through appropriate image readout bandwidth selection, albeit at the expense of signal-to-noise ratio (SNR). As diagnostic imaging is primarily concerned with the identification and characterisation of disease processes, distortion minimisation is typically compromised in order to maximise SNR. While the purpose of MRI for radiation therapy planning is to aid in the spatial location of the tumour region and organs at risk, as such distortion minimisation in MRI is prioritised at the expense of SNR. The highly conformal treatment dose distributions in radiation therapy mean that image distortion leads to the incorrect spatial location of disease or organs at risk (OARs), causing under-dosing of treatment regions or overdosing and inadequate sparing of surrounding critical structures.
Therefore, due to the advantages of reduced distortion in a spin echo sequence compared to gradient echo sequence, lower energy deposition, greater identification of cranial nerves and fewer artefacts, a diagnostic 3D isotropic T2-weighted SPACE sequence was adapted for radiation therapy purposes.6 Image distortion was further minimised by scanning at the isocentre, applying 3D distortion correction, and increasing the readout bandwidth to 600 Hz/pixel, to reduce the fat water shift at 3 T, at the expense of SNR. To compensate, the image voxel size was increased (0.8 mm isotropic) and the field of view (FOV) was increased to 240 mm.
Patient setup
To adapt to the radiation therapy treatment position and s-frame immobilisation cast, a QFix (QFix, Avondale, PA 19311 USA) flat couch top was positioned atop the spine array coil. Two 4-channel flex coils were positioned behind the head portion of the couch top, slightly overlapping the superior portion of the spine array coil and wrapped anteriorly over the s-frame cast. Inferiorly to this, an 18-channel body coil is positioned to abut the flex coils anteriorly, while the spine array coil is utilised posteriorly (Fig. 1).
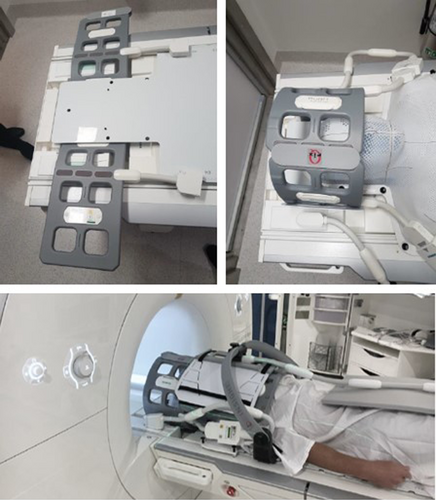
Validation of protocol adaptations
To assess system performance with respect to total magnetic field distortion and bandwidth dependency, a T1 weighted spoiled gradient echo sequence with a large field of view coverage was used with a commercial phantom (MODUS QUASAR MRI3D) to quantify distortion due to B0 inhomogeneity and susceptibility (dB0 + dBS) as well as gradient non-linearity (dBG). The clinical T2 SPACE was not used for this testing, due to limitations of the scan parameters and field of view requirements of the phantom.
Methods
Acquisition for radiation therapy planning
The need for cranial nerve identification was noted by the treating radiation oncologist prior to the planning scans. Ethics approval for the study was obtained through the Hunter New England Human Research Ethics Committee for low-risk MRI research (2020/ETH02542: Radiation Oncology Imaging Metaproject). The participants in the study provided written informed consent to participate in this study. The MRI coil arrangement was determined by the treatment region. For patients receiving intracranial treatment, the isotropic nature of the entire MRI protocol allowed for the use of a 20-channel head and neck coil, or a 32-channel head coil for image acquisition. For patients who were receiving treatment to the head region, extending outside of the cranium and into the neck, neck flexion caused difficulty in the rigid registration process between scans, therefore the radiation therapy treatment position had to be accounted for.1
The region of interest and cranial nerves of interest were requested by the treating radiation oncologist. The first 12 patients identified were used for the initial validation phase for radiation oncologist feedback and sequence optimisation, before routine clinical application. The isotropic T2 SPACE sequence was added to the MRI protocol for these patients, pre-contrast, and positioned centrally over the identified region to minimise distortion.
Validation of protocol adaptations
To simulate human brain scanning conditions, a 200 mm diameter of spherical volume (DSV) was assessed at isocentre. The imaging sequence was acquired twice with opposing phase encoding directions in the A-P direction at both 290 Hz/pixel and 600 Hz/pixel readout bandwidth, both with 3D distortion correction applied and with no distortion correction. The QUASAR MRID3D Geometric Distortion Analysis System software, which corrects for phantom susceptibility-induced distortion was used to quantify average absolute distortion, dB0 and dBG within the spherical volume of interest at both bandwidths.
Results
Acquisition for radiation therapy planning
Normal anatomy
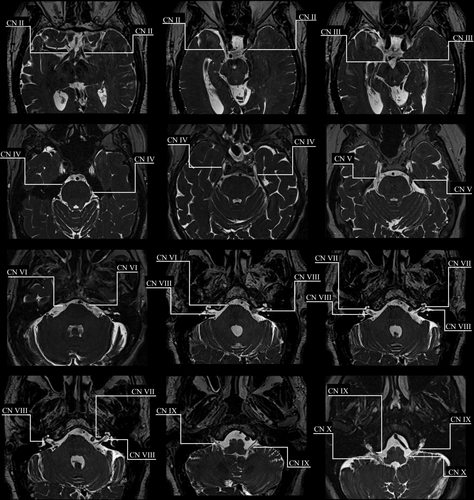
The identification of cranial nerves was successfully adopted in our clinic. An example of a T2 SPACE modified for radiation therapy planning purposes to visualisation normal anatomy for cranial nerves II–X is shown in Figure 2. Figure 3 depicts a comparison of the isotropic T2 SPACE and a typical T2 sequence acquired axially for radiation therapy planning purposes. The isotropic T2 SPACE is shown to be superior in cranial nerve identification to the conventional T2 sequence due to the heavier T2 weighting, higher image resolution and greater visibility in three imaging planes.
Case studies
Clinical applications of the modified protocol, in the form of case studies, are presented below.
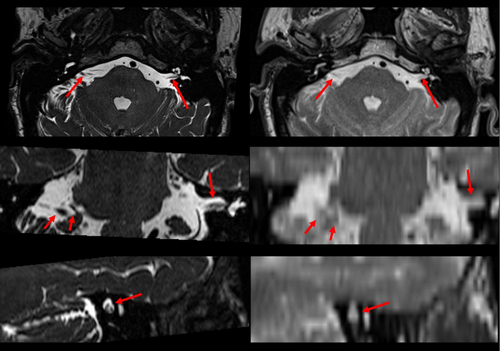
Case one (Fig. 4) depicts a meningioma, arising from the anteromedial inferior tentorium, extending to the left acoustic meatus and cavernous sinus. The lesion extends to the left optic foramen, and the left trigeminal nerve appears to be displaced medially. Figure 4 depicts the tumour volume and the clinically significant cranial nerves III–VI & VIII, which were visualised for treatment planning considerations. Hot spots were avoided in the adjacent II, IV and V cranial nerves during the treatment planning process.
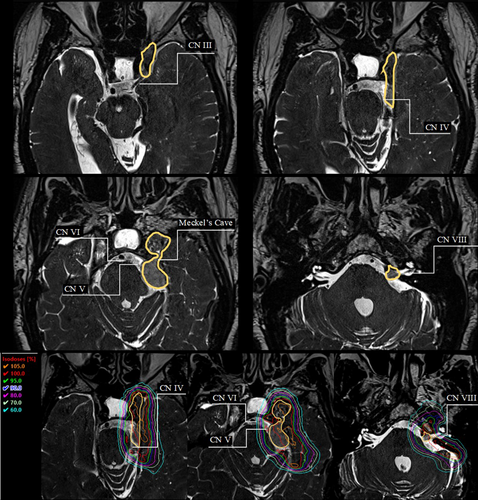
Case two (Fig. 5) depicts a case of a pituitary adenoma that surrounds and encases the left cavernous internal carotid artery. The mass extends into the foramen ovale. Figure 5 depicts the tumour volume and cranial nerves II–V which were visualised for treatment planning considerations. Hot spots were avoided in the adjacent cranial nerves, with cranial nerve III of particular concern during the treatment planning process as it passes through the middle of the gross tumour volume.
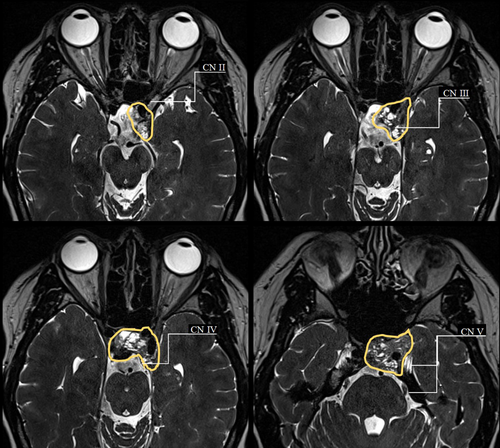
Case three (Fig. 6) depicts a meningioma of the right middle cranial fossa, extending from the left orbital apex and along the right tentorium, with a history of a left sphenoid wing meningioma. Figure 6 shows the tumour volume and proximity of cranial nerves III–VIII for treatment planning considerations. Hot spots were avoided over-involved nerves during the treatment planning process.
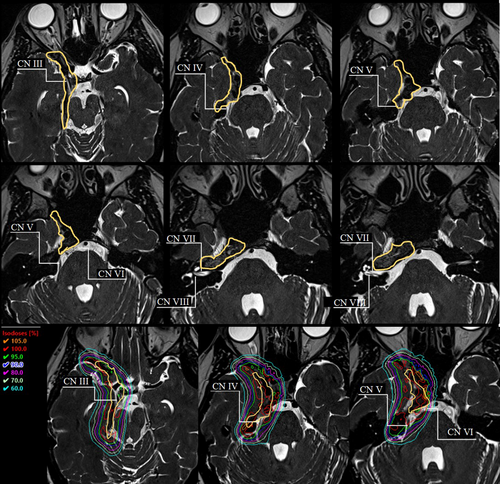
In the clinical cases depicted here, the visualisation of the cranial nerves was significant to the treatment planning and treatment volume definition for radiation therapy treatment. The increasing adoption and adaptation of diagnostic imaging tools allowed for a greater individualisation of treatment planning and information to predict patient outcomes. Figures 4 and 6 present how the identified cranial nerves were accounted for in the planning process, by reducing higher doses in the nerve regions. Greater dose control around cranial nerves can lead to a reduction in late side effects such as cranial neuropathy.
Validation of protocol adaptation
A maximum absolute distortion of 2.94 mm was measured at 290 Hz/pixel (no distortion correction applied), with a 95% quartile value of 1.98 mm within the 200 mm DSV, while a maximum absolute distortion of 0.92 mm was measured at 600 Hz/pixel (3D distortion correction applied), with a 95% quartile value of 0.60 mm within the 200 mm DSV (Table 1). The results showed also that dB0 is largely suppressed with an increased readout bandwidth, with dBG the main contributor to absolute distortion at increased bandwidths. However, at a 200 mm DSV, the greatest suppression of distortion results from the application of vendor-supplied 3D distortion correction software.
| Bandwidth | Mean (mm) | Max (mm) | P95 (mm) | % above 1 mm | |
|---|---|---|---|---|---|
| 290 Hz/px No distortion correction | Ave total | 0.74 | 2.94 | 1.98 | 24.72 |
| B0(X) | 0.10 | 0.21 | 0.13 | 0.00 | |
| BG(X) | 0.71 | 2.93 | 1.97 | 24.53 | |
| 290 Hz/px 3D distortion correction | Ave total | 0.32 | 0.95 | 0.64 | 0.04 |
| B0(X) | 0.28 | 0.48 | 0.37 | 0.00 | |
| BG(X) | 0.30 | 0.92 | 0.62 | 0.00 | |
| 600 Hz/px No distortion correction | Ave total | 0.71 | 2.89 | 1.95 | 24.14 |
| B0(X) | 0.05 | 0.08 | 0.06 | 0.00 | |
| BG(X) | 0.71 | 2.88 | 1.94 | 23.89 | |
| 600 Hz/px 3D distortion correction | Ave total | 0.30 | 0.92 | 0.60 | 0.00 |
| B0(X) | 0.07 | 0.16 | 0.11 | 0.00 | |
| BG(X) | 0.27 | 0.88 | 0.57 | 0.00 |
- Resulting average total image distortion, B0 inhomogeneity and gradient non-linearity (dBG) if reported for 290 and 600 Hz/px, both without distortion correction and with 3D distortion correction applied.
Discussion
This article described the adaptation of a diagnostic imaging technique for radiation therapy purposes. The challenges of adapting the sequence for specific radiation therapy uses were addressed. In-house testing showed that a distortion minimisation techniques resulted in a 1.4 mm reduction in the 95% quartile value of distortion and a 2 mm reduction in the maximum distortion within the image. At a DSV of 200 mm, the application of vendor-supplied 3D distortion correction had the greatest impact on distortion minimisation in this protocol. A 1.4 mm in the 95% quartile value of distortion within the image, is clinically significant when compared to the measured diameters of the cranial nerves. It should be noted that the vendor-supplied software for phantom-based assessment of distortion, performs automatic correction of susceptibility-induced distortion from the phantom itself. Patient-specific distortion, therefore, will have an additional component due to the magnetic susceptibility of tissue. This will be more prominent at lower receiver bandwidths but will be largely mitigated through the selection of a 600 Hz/pixel bandwidth. Furthermore, the application of 3D distortion correction will have the largest impact on spatial fidelity through the correction of gradient non-linearity. It should also be noted that the 3D gradient echo-based FLASH sequence used for phantom testing, does not contain a 180° re-focusing pulse, which can correct for signal de-phasing due to inhomogeneity of the main field (B0), unlike the 3D turbo spin echo-based SPACE sequence. This technique is particularly important for addressing cranial nerve dose in treatment planning, as the size of the cranial nerves is in the order of millimetres, requiring accurate spatial location, to ensure precise treatment planning and estimation of dose deposition to the nerves. The image technique has been routinely implemented in our centre for patients who have been pre-identified by the radiation oncologist as being at risk of cranial neuropathy from the radiation therapy treatment. We are now looking to expand to extracranial applications and dose limitation and response management.
Over the past few decades, with improved treatments, the number of cancer survivors is continuing to increase and the survivorship of this cohort needs tailored approaches.7 Cranial nerve palsies tend to present late and can be quite debilitating with intractable symptoms of pain, hearing loss, dysguesia, dysphagia, vision impairment, hearing loss, etc.8, 9 The contributing factors to nerve damage are multifactorial and dependent on volume, technique, total dose, fraction, presence of hotspots, extent of post-treatment fibrosis and inherent patient-related factors including response to the injury.10, 11 The rate and severity of the cranial neuropathies have been shown to correlate with the fractionation dose and the length of nerve involved in the high-dose treatment region.9, 12 The use of cranial nerve-specific sequences would allow for nerve preservation planning techniques and greater information on nerve involvement or displacement, with implications on fractionation dose or treatment volumes.9, 13 Routine use of cranial nerve sequences would also allow for a retrospective study of nerve dose and treatment length, correlated with late-term side effects, to update the limited literature on cranial nerve dose tolerance.
The easier access to advanced treatment planning and delivery techniques has led to an increase in re-irradiation for head and neck cancers prompting American Radium Society to put out a practice guideline.14 There is paucity of dose-volume data from the more contemporary era. Guidelines to identify cranial nerves for CT-based contouring have been published.15, 16 Older studies have identified the difficulty previously in identifying the cranial nerves from CT alone, theorising that greater visualisation of the cranial nerves would allow accurate measurements of the exact volume of cranial nerve irradiated from the length and diameter of the nerve within the irradiated volume.12 The addition of a dedicated planning MRI will allow clinicians and dosimetrists to confidently include these structures in the dose optimisation interfaces of the planning systems.
The use of an optimised and dedicated MRI simulation sequence would allow to prospectively collect accurate dose-volume data to inform cranial nerve dose tolerances. Radiation treatment delivery has increasingly become more accurate with the introduction of sub-millimetre accuracy in positioning, and continuous monitoring of the treatment isocentres with either kilo-voltage imaging or surface guidance. Advanced treatment planning software also allows for controlling dose around small volumes both for treatment as well as avoidance. The combination of these techniques will improve the identification of organs at risk like the cranial nerves thereby ensuring better therapeutic ratios and patient outcomes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and patient consent
The studies involving human participants were reviewed and approved by Hunter New England Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Open Research
Data availability statement
Ethics approval for this study does not allow for sharing of individual patient scans. Other study data, which does not include patient data sets, are available from the corresponding author upon reasonable request.




