Joint-adjacent Adipose Tissue by MRI is Associated With Prevalence and Progression of Knee Degenerative Changes: Data from the Osteoarthritis Initiative
Abstract
Background
Adipose tissue has recently gained interest as an independent imaging biomarker for osteoarthritis.
Purpose
To explore 1) cross-sectional associations between local subcutaneous fat (SCF) thickness at the knee and the extent of degenerative changes in overweight and obese individuals and 2) associations between local fat distribution and progression of osteoarthritis over 4 years.
Study Type
Retrospective cohort study.
Population
338 obese and overweight participants from the Osteoarthritis Initiative cohort without radiographic evidence of osteoarthritis.
Field Strength
3T: 3D-FLASH-WE; 3D-DESS-WE; T1w-SE; MSME.
Assessment
Baseline SCF thickness was measured in standardized locations medial, lateral and anterior to the knee and the average joint-adjacent SCF (ajSCF) was calculated. Right thigh SCF cross-sectional area was assessed. Quantitative cartilage T2 relaxation times and semi-quantitative whole organ MRI scores (WORMS) were obtained at baseline and 4-year follow-up. WORMSsum was calculated as sum of cartilage, bone marrow edema, subchondral cyst, and meniscal scores.
Statistical Tests
Associations of SCF measures with baseline, and 4-year change in T2 and WORMS were analyzed using regression models. SCF measurements were standardized using the equation 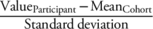 . Analyses were adjusted for age, sex, physical activity, and BMI.
. Analyses were adjusted for age, sex, physical activity, and BMI.
Results
Cross-sectionally, significant associations between lateral SCF, lateral compartment WORMS and T2 were found ( , [95% CI]: 0.53, [0.12–0.95], P < 0.05; ΔT2: 0.50, [0.02–0.98], P < 0.05). Moreover, greater lateral SCF was associated with faster progression of lateral WORMSsum gradings (OR = 1.50, [1.05–2.15], P < 0.05). No significant positive associations were found for thigh SCF and WORMSsum (P = 0.44) or T2 measurements (medial: P = 0.15, lateral: 0.39, patellar: P = 0.75).
, [95% CI]: 0.53, [0.12–0.95], P < 0.05; ΔT2: 0.50, [0.02–0.98], P < 0.05). Moreover, greater lateral SCF was associated with faster progression of lateral WORMSsum gradings (OR = 1.50, [1.05–2.15], P < 0.05). No significant positive associations were found for thigh SCF and WORMSsum (P = 0.44) or T2 measurements (medial: P = 0.15, lateral: 0.39, patellar: P = 0.75).
Data Conclusion
Joint-adjacent SCF thickness was associated with imaging parameters of knee osteoarthritis, both cross-sectionally and longitudinally, while thigh SCF was not, suggesting a spatial association of SCF and knee osteoarthritis. Based on these findings, joint-adjacent SCF may play a role in the development and progression of knee osteoarthritis.
Level of Evidence
4
Technical Efficacy
Stage 5
Known risk factors for knee osteoarthritis (OA) include previous knee injury, malalignment, and obesity.1, 2 Joint-adjacent adipose tissue has recently gained interest as a possible independent risk factor for the development and progression of knee OA, and has been investigated as a potential imaging biomarker.3-7 One mechanism by which adipose tissue may drive OA development is through adipocytes that produce inflammatory factors, such as adipokines.8 Adipokines play various roles in inflammatory processes involved in the development and progression of OA: adiponectin and visfatin were both found to increase cartilage degradation, while resistin showed the potential to increase synovial hypertrophy in addition to cartilage degradation.9-12 Another adipokine, leptin, has been found to increase IGF-1, TGF-beta, MMP-2, and MMP-9, which are associated with an accelerated breakdown of cartilage.13, 14 Thus, multiple possible pathways of OA acceleration are linked to inflammatory processes within SCF.
In addition to this background, magnetic resonance imaging (MRI) features of inflammatory processes within the intraarticular fat pads of the knee have been associated with knee OA.15 Although the highest joint-adjacent adipokine concentrations were found in the infrapatellar fat pad, subcutaneous fat (SCF) also contributes to the production of adipokines, thus, an association between SCF medial to the knee and OA of the femoro-patellar joint has been proposed.12, 16-18
Besides local adipose tissue, thigh SCF has been investigated in relation to knee OA. A greater increase of thigh SCF over 4 years was found in painful knees, compared to asymptomatic knees, and increases in thigh SCF have been linked to progression of OA of the knee in male participants.6, 19 However, the number of studies investigating the potential of joint-adjacent fat as an imaging biomarker for knee OA is too limited to draw firm conclusions.
Therefore, the aims of this exploratory study were 1) to investigate cross-sectional associations between joint-adjacent and thigh SCF and the degree of knee structural damage in overweight and obese individuals and 2) to analyze the impact of baseline joint-adjacent and thigh SCF on progression of knee OA over 4 years, using MRI-based semiquantitative imaging biomarkers and cartilage T2 relaxation time measurements as outcomes.
Materials and Methods
Subject Selection
This study analyzed data from the osteoarthritis initiative (OAI, https://oai.nih.gov), a longitudinal, observational multi-center study with a cohort size of n = 4796 subjects, assessing biomarkers in OA. Datasets include MRI of the knee and thigh as well as knee radiographs. Institutional review boards of each center approved informed consent documentation, study protocols and amendments. All investigations were carried out in compliance with the Helsinki Declaration.
A flow chart illustrating the inclusion and exclusion criteria is presented in Fig. 1. To reduce the length of the imaging protocol, the 3D FLASH WE sequence was only obtained at the right knees, and furthermore T2 measurements in the OAI cohort were only available at the right knee. Thus, we selected individuals with availability of a baseline and 4-year follow-up MRI study of the right knee. In order to focus on early structural changes of the cartilage, individuals without radiographic evidence of OA (Kellgren–Lawrence (KL) score of the right knee ≤1 at baseline) were included. Given the focus on obesity and joint-adjacent fat, overweight or obese (body mass index (BMI) ≥ 25 kg/body height in m2) individuals were selected. Moreover, individuals without a history of surgery or inflammatory arthropathy on either knee were included. We excluded individuals with incomplete demographic data (sex, age, and physical activity survey for the elderly (PASE)-score). Of the 338 subjects selected, 278 had an MRI exam of the right thigh at baseline.
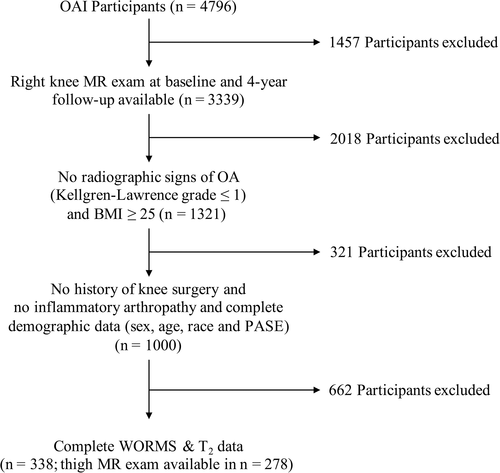
Image Acquisition
MR images were acquired at four centers (Columbus, Ohio; Baltimore, Maryland; Pittsburgh, Pennsylvania and Pawtucket, Rhode Island), using four identical 3.0 Tesla scanners (Siemens Magnetom Trio, Erlangen, Germany). Acquired sequences of the knee included: 1) coronal 2D intermediate-weighted (IW) turbo spin-echo (TSE) [repetition time (TR)/echo time (TE); spatial resolution; field of view (FOV); slice thickness; gap] [3700/29 ms; 0.365 mm × 0.456 mm; 140 mm; 3.0 mm; 0 mm), 2) sagittal, fat-saturated (FS) 2D IW TSE [3200 ms/30 ms; 0.357 mm × 0.511 mm; 160 mm; 3 mm; 0 mm), 3) coronal 3D fast low angle shot with water excitation (FLASH WE) [7.57 ms/20 ms; 0.313 mm × 0.313 mm; 160 mm; 1.5 mm; 0 mm] and 4) sagittal 3D dual-echo steady state sequence with water excitation (DESS WE) [4.7 ms/16.3 ms; 0.365 mm × 0.456 mm; 140 mm; 1.5 mm; 0 mm] with axial and coronal reformations. To allow quantitative assessment of cartilage T2 relaxation times, a sagittal 2D multi slice multi echo sequence (MSME) was also included [2700 ms/10–70 ms; 0.313 mm × 0.446 mm; 120 mm; 3.0 mm/0.5 mm]. Thigh imaging consisted of an axial T1 weighted spin echo sequence (T1W-SE) [500 ms/10 ms; 0.977 mm × 0.977 mm; 500 mm; 5 mm; 0 mm]. Detailed information on imaging protocols is available online (https://oai.epi-ucsf.org/datarelease/operationsManuals/MRI_ManualRev.pdf).
Quantification of Local and Thigh Subcutaneous Fat
Joint-adjacent SCF was quantified by measuring SCF thickness in five locations around the knee by two observers (A.H.O. and J.B., 1 and 3 years of experience, respectively), who were trained by an experienced musculoskeletal radiologist (T.M.L., 25 years of experience). As shown in Fig. 2, two measurements each were located on the medial and lateral side of the joint, respectively, while the fifth measurement was obtained anterior to the joint.
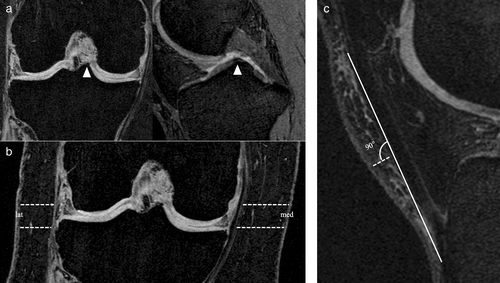
Lateral and medial measurements were acquired on the coronal 3D FLASH WE sequence. This sequence was chosen for its precise delineation of the SCF boundaries and the larger FOV compared to the other available coronal sequences. To improve the reproducibility of the measurements, a section was chosen, that was centered on the medial tibial spine, using sagittal and axial reformations of the DESS sequence (Fig. 2a). SCF thickness was measured on the coronal section at the level of the medial joint space and the superior boundary of the medial tibial spine, both medially and laterally (Fig. 2b).
Anterior SCF was quantified on the sagittal DESS sequence by choosing the section centered on the lateral tibial spine, which was localized analogous to the medial tibial spine. The length of the patellar tendon was measured and a perpendicular measurement of SCF thickness was acquired at the center of the tendon as shown in Fig. 2c.
Medial SCF was calculated by averaging both measurements on the medial side, and lateral SCF was calculated similarly. Moreover, the average of all joint-adjacent measurements of SCF within one subject (averaged joint-adjacent subcutaneous fat, ajSCF; 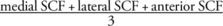 ) was calculated.
) was calculated.
Thigh SCF was semi-automatically segmented by two observers (A.H.O. and J.B.), using an in-house, spline-based segmentation software based on MatLab 2012b (The MathWorks, Inc. Natick, MA, USA) using the axial T1W-SE sequence (Fig. 3). Coverage of this sequence started 10 cm proximal to the distal epiphysis of the right femur, extending 7.5 cm proximally with 15 slices obtained. To adjust for differences in slice locations due to body height, we segmented a single slice, representing the location at approximately 33% of femoral length (distal to proximal), using a method developed by Dannhauer et al.20 To determine inter-observer reproducibility, SCF measurements were obtained in 10 individuals by two readers (A.H.O. and J.B.). Intra-observer reproducibility measurements were obtained in 10 individuals by a single reader (J.B.) with 2 months between readings.
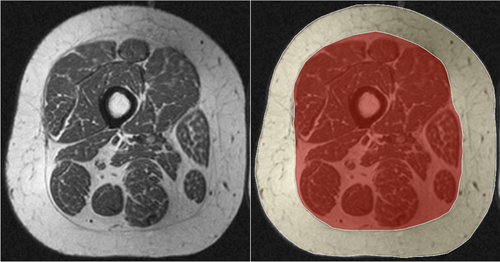
Image Analysis: Cartilage T2 Measurements and Semi-Quantitative WORMS
Cartilage segmentation was performed semi-automatically using the 2D MSME sequence. Regions of interest included the patellar cartilage (PAT) and cartilage of the medial and lateral femur (MF and LF, respectively) as well as the medial tibial (MT) and lateral tibial (LT) cartilage (Fig. 4). The trochlea was excluded from the segmentation due to flow artifacts of the popliteal artery, which limited delineation of the cartilage. Following cartilage segmentation, T2 maps were computed on a pixel-by-pixel basis using a previously validated and published method.21 Reproducibility measurements of ROI placement for T2 relaxation time measurements obtained in our workgroup has been provided before.22 Averages of T2 relaxation times were calculated for the medial and lateral compartments (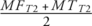 and
and 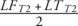 ).
).
All MR imaging studies were reviewed by members of our research group, who underwent training by an experienced musculoskeletal radiologist (T.M.L.) prior to the study, on picture archiving communication system (PACS)-workstations (Agfa, Ridgefield Park, NJ, USA), using a modified version of the Whole-Organ Magnetic Resonance Imaging Score (WORMS), which included grading cartilage, bone marrow edema-like lesions and subchondral cyst-like lesions in six regions (patella, trochlea, medial and lateral femur, medial and lateral tibia). Meniscal lesions were graded in six locations (anterior horn, body and posterior horn; for the medial and lateral meniscus, respectively). Inter- and intra-observer reproducibility of WORMS readings obtained by our research group has been demonstrated before.22
Each score was assigned to one of three compartments (patello-femoral, medial, lateral): The patello-femoral compartment included cartilage, bone marrow edema-like lesions and subchondral cyst-like lesion scores of the patella and trochlea, while medial and lateral cartilage, bone marrow edema-like lesions, subchondral cyst-like lesion and meniscal scores were assigned to the medial and lateral compartment, respectively. Total summation scores (WORMSsum) and feature specific summation scores (WORMScartilage, WORMSmeniscus) were calculated for each compartment.
Statistical Analysis
Statistical analysis was performed using STATA version 15 software (StataCorp LP, College Station, TX, USA), using a two-sided level of significance of α = 0.05. The inter- and intra-observer reproducibility of repeated SCF measurements was investigated using coefficients of variation (CV). Descriptive statistics for participant demographics (age, sex, BMI, PASE score, KL grade) and SCF measurements at baseline were analyzed using crosstabs for categorical data and means and standard deviation (SD) for continuous data. Changes in WORMS and T2 relaxation time measurements between baseline and 4 years were assessed using paired T tests.
Associations between BMI and baseline fat measurements were assessed using linear regression models, adjusted for age and sex. For the main analyses, outcome variables were designated as primary or secondary (exploratory), to limit potential multiple testing issues.23 Medial, lateral and patello-femoral WORMSsum were defined as primary outcomes. Secondary outcomes included WORMScartilage, WORMSmeniscus and T2 relaxation time measurements of each compartment (medial, lateral, patello-femoral). Baseline associations between SCF measures and outcomes were investigated using linear regression models, while associations between baseline SCF and change in outcomes over 4 years (binarized, as described below) were determined using logistic regression models.
Binary outcomes were defined as follows: first, absolute changes in WORMS/T2 between baseline and 4 years were calculated for each subject and in each compartment. Progression of WORMS was defined as positive if scores increased ≥ 1.0 over 4 years, and 0 otherwise. Progression of T2 relaxation time measurements was defined as positive if mean compartmental T2 at 4 years was greater than mean compartmental T2 at baseline, and 0 otherwise. Linear regression models investigating associations between SCF and osteoarthritis outcome measures were adjusted for age, sex, PASE and BMI, as these variables have been shown or are suspected of driving development and progression of KOA.24-28
All regression models were performed using standardized values for baseline SCF measurements as predictors that were calculated by subtracting the mean across all participants from each participant's value and dividing by the SD. The coefficients and odds ratios therefore are the change in outcome per standard deviation change in predictor ( ).
).
Results
Participant Demographics
Analyses of inter- and intra-observer reproducibility of SCF measurements demonstrated good reproducibility (CVinter-observer = 2.72%; CVintra-observer = 2.01%). A summary of the participant characteristics is given in Table 1. Subjects were on average 58.35 years old (± SD; ± 8.83), with a BMI of 29.20 kg/m2 (± 3.39 kg/m2) and a PASE score of 165.19 (± 81.14). The sex distribution showed a higher percentage of women than men (n (%); women: 208 (61.54); men: 130 (38.46)), reflecting the overall distribution in the OAI cohort. The majority of participants had a baseline KL score of 0 (n (%); 221 (65.38)), while a KL score of 1 was found in 117 (34.62) participants. Overall, the greatest thickness of SCF was found on the medial side (cm, mean ± SD; 1.68 ± 0.81), while anterior SCF was found to be thinnest on average (0.40 ± 0.19). The cohort showed significant progression of all investigated WORMS parameters over 4 years (P < 0.05), and the most substantial increases were found in the patello-femoral compartment (Table 1).
| Parameter | Baseline | 4-Year Follow-up | |
|---|---|---|---|
| Age (mean ± SD in years) | 58.35 ± 8.83 | ||
| Sex (n (%)) | Female | 208 (61.54) | |
| Male | 130 (38.46) | ||
| BMI (mean ± SD in kg/m2) | 29.20 ± 3.39 | ||
| PASE (mean ± SD) | 165.19 ± 81.14 | ||
| SCF measurements | Anterior (mean ± SD in cm) | 0.40 ± 0.19 | |
| Medial (mean ± SD in cm) | 1.68 ± 0.81 | ||
| Lateral (mean ± SD in cm) | 0.83 ± 0.58 | ||
| ajSCF (mean ± SD in cm) | 1.59 ± 0.76 | ||
| Thigh SCF (mean ± SD in cm2)a | 72.40 ± 36.26 | ||
| Kellgren–Lawrence gradeb (n(%)) | 0 | 221 (65.38) | 203 (61.14) |
| I | 117 (34.62) | 102 (30.72) | |
| II | 0 | 14 (4.22) | |
| III | 0 | 13 (3.92) | |
| WORMSsumc (mean ± SD) | Patello-femoral | 4.12 ± 3.99 | 5.63 ± 4.57 |
| Medial | 1.57 ± 2.43 | 2.67 ± 3.92 | |
| Lateral | 1.66 ± 2.50 | 2.57 ± 3.71 | |
| WORMScartilage (mean ± SD) | Patello-femoral | 2.76 ± 2.50 | 3.62 ± 2.71 |
| Medial | 0.57 ± 1.18 | 1.12 ± 1.91 | |
| Lateral | 0.83 ± 1.33 | 1.26 ± 1.76 | |
| WORMSmeniscus (mean ± SD) | Medial | 0.78 ± 1.43 | 1.17 ± 1.89 |
| Lateral | 0.53 ± 1.29 | 0.90 ± 1.84 | |
| T2 (mean ± SD in ms) | Patellar | 32.18 ± 3.25 | 33.91 ± 4.27 |
| Medial | 34.02 ± 2.34 | 35.05 ± 2.33 | |
| Lateral | 32.19 ± 2.77 | 33.31 ± 2.34 | |
- BMI = body mass index; PASE = Physical Activity Survey of the Elderly; SCF = subcutaneous fat; ajSCF = average joint-adjacent subcutaneous fat; WORMS = Whole-Organ Magnetic Resonance Imaging Score; SD = standard deviation.
- a Baseline thigh exams were available in n = 278;
- b Follow-up: n = 332;
- c WORMSsum: summation of cartilage-, bone marrow edema-like lesion-, subchondral cyst- and meniscal scores.
Compared to the patella-femoral compartment, progression of OA in the medial and lateral femoro-tibial joint compartments were more limited. Average T2 values also increased in all compartments over 4 years, with the greatest changes in the patellar cartilage. All SCF measurements were positively associated with BMI, with the lowest correlation coefficient found for the association of lateral SCF thickness and BMI (coefficient (change in BMI associated with 1 SD change in SCF) [95% CI], P value; medial SCF: 0.12 [0.10, 0.14], < 0.05; lateral SCF: 0.06 [0.04, 0.08], < 0.05; anterior SCF: 0.07 [0.04, 0.10]; ajSCF: 0.10 [0.08, 0.12], < 0.05; thigh SCF: 0.11 [0.08, 0.13], < 0.05).
Baseline Associations of SCF Measurements and WORMS/T2
Despite the statistical adjustment for BMI, the primary analysis showed positive associations between all SCF measurements (lateral, medial, anterior and thigh) and baseline lateral WORMSsum (Table 2). Associations between ajSCF and lateral WORMSsum (change in WORMSsum per 1 SD change in ajSCF, [95% CI], P value; 0.42, [0.02, 0.81], < 0.05), as well as lateral SCF and lateral WORMSsum (0.53, [0.12, 0.95], < 0.05) were statistically significant, while associations between medial, anterior and thigh SCF and lateral WORMSsum were not (P = 0.45, 0.18 and 0.44, respectively). Associations of any SCF measure and medial knee compartment WORMS were weak and not statistically significant (P ≥ 0.90). Notably, thigh SCF was negatively correlated with WORMSsum of the patello-femoral compartment (−0.79, [−1.55, −0.02], 0.05).
| Medial SCFa | Lateral SCFa | Anterior SCFa | Thigh SCFb | ajSCFa | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef | 95% CI | P | Coef | 95% CI | P | Coef | 95% CI | P | Coef | 95% CI | P | Coef | 95% CI | P | ||||||
| WORMSsumc | ||||||||||||||||||||
| Medial | 0.00 | −0.41 | 0.42 | 0.99 | −0.03 | −0.44 | 0.38 | 0.90 | 0.01 | −0.25 | 0.28 | 0.94 | −0.03 | −0.49 | 0.43 | 0.90 | 0.01 | −0.38 | 0.40 | 0.96 |
| Lateral | 0.17 | −0.26 | 0.59 | 0.45 | 0.53 | 0.12 | 0.95 | 0.01 | 0.19 | −0.09 | 0.47 | 0.18 | 0.19 | −0.30 | 0.69 | 0.44 | 0.42 | 0.02 | 0.81 | 0.04 |
| Patello-femoral | −0.05 | −0.72 | 0.61 | 0.88 | 0.05 | −0.60 | 0.71 | 0.87 | 0.16 | −0.28 | 0.59 | 0.47 | −0.79 | −1.55 | −0.02 | 0.05 | 0.15 | −0.47 | 0.78 | 0.63 |
| WORMScartilage | ||||||||||||||||||||
| Medial | 0.07 | −0.14 | 0.27 | 0.53 | −0.02 | −0.22 | 0.19 | 0.87 | −0.03 | −0.16 | 0.11 | 0.68 | −0.06 | −0.29 | 0.16 | 0.59 | 0.04 | −0.15 | 0.23 | 0.70 |
| Lateral | 0.09 | −0.14 | 0.32 | 0.46 | 0.26 | 0.03 | 0.48 | 0.03 | 0.01 | −0.14 | 0.16 | 0.85 | 0.06 | −0.21 | 0.34 | 0.65 | 0.17 | −0.05 | 0.38 | 0.12 |
| Patello-femoral | −0.06 | −0.48 | 0.35 | 0.76 | 0.08 | −0.33 | 0.49 | 0.72 | 0.10 | −0.17 | 0.37 | 0.47 | −0.41 | −0.89 | 0.06 | 0.09 | 0.04 | −0.35 | 0.43 | 0.83 |
| WORMSmeniscus | ||||||||||||||||||||
| Medial | −0.04 | −0.27 | 0.20 | 0.76 | −0.06 | −0.29 | 0.18 | 0.63 | 0.04 | −0.12 | 0.19 | 0.62 | 0.05 | −0.23 | 0.33 | 0.72 | −0.02 | −0.24 | 0.20 | 0.85 |
| Lateral | 0.03 | −0.19 | 0.25 | 0.78 | 0.17 | −0.04 | 0.39 | 0.18 | 0.13 | −0.01 | 0.28 | 0.06 | 0.04 | −0.20 | 0.28 | 0.77 | 0.76 | −0.13 | 0.28 | 0.46 |
| T2 | ||||||||||||||||||||
| Medial | 0.22 | −0.18 | 0.61 | 0.28 | 0.27 | −0.22 | 0.75 | 0.28 | 0.49 | 0.23 | 0.75 | <0.01 | 0.33 | −0.12 | 0.79 | 0.15 | 0.39 | 0.01 | 0.76 | 0.04 |
| Lateral | −0.16 | −0.63 | 0.31 | 0.50 | 0.50 | 0.02 | 0.98 | 0.04 | 0.48 | 0.16 | 0.79 | <0.01 | 0.25 | −0.33 | 0.84 | 0.39 | 0.38 | −0.07 | 0.82 | 0.10 |
| Patellar | −0.46 | −1.02 | 0.10 | 0.11 | 0.27 | −0.30 | 0.85 | 0.35 | 0.28 | −0.09 | 0.65 | 0.14 | −0.11 | −0.78 | 0.56 | 0.75 | −0.04 | −0.57 | 0.50 | 0.89 |
- All SCF measurements were standardized by converting to Z scores prior to the analyses. All analyses are adjusted for age, sex, body mass index and Physical Activity Survey for the Elderly (PASE)-scores. Secondary (exploratory) analyses are shaded in gray. Statistically significant results are printed bold.
- SCF = subcutaneous fat; ajSCF = average joint-adjacent subcutaneous fat; Coef = coefficient; 95% CI = 95% confidence interval.
- a n = 338.
- b n = 278.
- c WORMSsum: summation score of individual cartilage-, bone marrow edema-like lesion-, subchondral cyst- and meniscal scores.
The secondary analysis showed positive correlations between lateral and ajSCF and lateral WORMScartilage as well as lateral compartment T2. However, only the associations between lateral SCF and lateral WORMScartilage and T2 were statistically significant (WORMScartilage: 0.26 [0.03, 0.48], < 0.05; T2: 0.50 [0.02, 0.98], < 0.05). Although both, lateral SCF and ajSCF, were also positively associated with lateral WORMSmeniscus, associations were not statistically significant (P = 0.18 and 0.46). Notably, also associations between anterior SCF and medial/lateral compartmental T2 were positive and statistically significant, although correlations between anterior SCF and WORMS were limited (medial T2: 0.49 [0.23, 0.75], < 0.05; lateral T2: 0.48 [0.16, 0.79], < 0.05).
Progression of WORMS/T2 over 4 Years
In the primary analysis, knees with greater amounts of joint-adjacent SCF showed a higher probability of structural knee OA progression in the lateral compartment (Table 3): Logistic regression models revealed elevated odds ratios (OR) for lateral WORMSsum progression in knees with greater lateral SCF and anterior SCF (OR, [95% CI], P-value; 1.50, [1.05, 2.15], < 0.05; 1.29, [1.02, 1.63], < 0.05, respectively). Knees with greater ajSCF at baseline were also more likely to show WORMSsum progression in the lateral compartment, but this observation remained a statistical trend (1.37, [0.97, 1.92], 0.07). While greater medial SCF and thigh SCF were also positively associated with increased odds for structural knee OA progression (WORMSsum) of the lateral compartment, results were not statistically significant (P = 0.80 and 0.62, respectively). Associations of SCF measurements and medial compartment WORMSsum were not statistically significant (P ≥ 0.40), but greater lateral SCF measurement was associated with an elevated likelihood for medial WORMSsum progression. Associations of lateral, anterior, thigh and ajSCF and patello-femoral WORMSsum were positive, but not statistically significant (P ≥ 0.20).
| Medial SCFa | Lateral SCFa | Anterior SCFa | Thigh SCFb | ajSCFa | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||||||
| WORMSsumc | ||||||||||||||||||||
| Medial | 0.88 | 0.61 | 1.27 | 0.50 | 1.12 | 0.78 | 1.60 | 0.55 | 0.90 | 0.71 | 1.15 | 0.40 | 0.86 | 0.56 | 1.31 | 0.47 | 0.98 | 0.70 | 1.38 | 0.92 |
| Lateral | 1.05 | 0.73 | 1.50 | 0.81 | 1.50 | 1.05 | 2.15 | 0.03 | 1.29 | 1.02 | 1.63 | 0.04 | 1.11 | 0.74 | 1.67 | 0.62 | 1.37 | 0.97 | 1.92 | 0.07 |
| Patello-femoral | 0.99 | 0.70 | 1.40 | 0.94 | 1.19 | 0.84 | 1.69 | 0.33 | 1.16 | 0.92 | 1.47 | 0.20 | 1.10 | 0.73 | 1.65 | 0.66 | 1.22 | 0.87 | 1.70 | 0.25 |
| WORMScartilage | ||||||||||||||||||||
| Medial | 0.07 | −0.14 | 0.27 | 0.53 | 0.94 | 0.62 | 0.14 | 0.78 | 0.85 | 0.64 | 1.13 | 0.26 | −0.06 | −0.29 | 0.16 | 0.59 | 0.04 | −0.15 | 0.23 | 0.70 |
| Lateral | 0.09 | −0.14 | 0.32 | 0.46 | 1.19 | 0.80 | 1.77 | 0.40 | 1.16 | 0.89 | 1.50 | 0.27 | 0.06 | −0.21 | 0.34 | 0.65 | 0.17 | −0.05 | 0.38 | 0.12 |
| Patello-femoral | −0.06 | −0.48 | 0.35 | 0.76 | 1.19 | 0.85 | 1.68 | 0.32 | 1.18 | 0.94 | 1.48 | 0.16 | −0.41 | −0.89 | 0.06 | 0.09 | 0.04 | −0.35 | 0.43 | 0.83 |
| WORMSmeniscus | ||||||||||||||||||||
| Medial | −0.04 | −0.27 | 0.20 | 0.76 | 1.30 | 0.85 | 1.99 | 0.23 | 1.13 | 0.85 | 1.51 | 0.39 | 0.05 | −0.23 | 0.33 | 0.72 | −0.02 | −0.24 | 0.20 | 0.85 |
| Lateral | 0.03 | −0.19 | 0.25 | 0.78 | 1.65 | 1.07 | 2.52 | 0.02 | 1.49 | 1.12 | 1.99 | <0.01 | 0.04 | −0.20 | 0.28 | 0.77 | 0.08 | −0.13 | 0.28 | 0.46 |
| T2 | ||||||||||||||||||||
| Medial | 1.08 | 0.74 | 1.59 | 0.68 | 1.05 | 0.71 | 1.56 | 0.81 | 0.79 | 0.61 | 1.01 | 0.06 | 0.81 | 0.53 | 1.25 | 0.34 | 1.00 | 0.70 | 1.43 | 0.99 |
| Lateral | 1.37 | 0.93 | 2.02 | 0.11 | 1.21 | 0.81 | 1.80 | 0.35 | 0.88 | 0.69 | 1.13 | 0.32 | 0.83 | 0.54 | 1.27 | 0.38 | 1.11 | 0.77 | 1.60 | 0.59 |
| Patellar | 1.02 | 0.72 | 1.47 | 0.90 | 0.95 | 0.65 | 1.38 | 0.78 | 0.86 | 0.68 | 1.09 | 0.20 | 0.97 | 0.64 | 1.46 | 0.89 | 0.97 | 0.69 | 1.36 | 0.87 |
- All SCF measurements were standardized by converting to Z scores prior to the analyses. All analyses are adjusted for age, sex, body mass index and Physical Activity Survey for the Elderly (PASE)-scores. Secondary (exploratory) analyses are shaded in gray. Statistically significant results are printed bold.
- SCF = subcutaneous fat; ajSCF = average joint-adjacent subcutaneous fat; OR = odds ratio; 95% CI = 95% confidence interval.
- a n = 338.
- b n = 278.
- c WORMSsum: summation score of individual cartilage-, bone marrow edema-like lesion-, subchondral cyst- and meniscal scores.
In the secondary analysis, participants with greater amounts of joint-adjacent fat were found to show accelerated degradation of the lateral meniscus and lateral compartment cartilage. While associations with lateral WORMScartilage were not statistically significant, ORs for meniscal degradation were significantly increased (lateral SCF: 1.65, [1.07, 2.52], < 0.05; anterior SCF: 1.49, [1.12, 1.99], < 0.05). Associations between SCF and progression of T2 relaxation time measurements were not statistically significant (P ≥ 0.11). However, greater amounts of lateral SCF at baseline were associated with an increased likelihood of elevated T2 values in the lateral knee compartment after 4 years and, in contrast, participants with greater anterior SCF thickness demonstrated decreased odds for T2 progression (lateral SCF: 1.37 [0.93, 2.02], 0.11; anterior SCF: 0.88 [0.69, 1.13] 0.32).
Discussion
This study assessed the associations between thigh and joint-adjacent SCF measurements with the severity of knee osteoarthritis quantified using cartilage T2 and WORMS measurements (outcomes). Statistically significant associations between severity of joint structural damage and joint-adjacent SCF measurements were found, in particular for the lateral joint compartment, where higher WORMS scores (cross-sectional and longitudinal) and elevated T2 values (cross-sectional) were associated with greater SCF thickness. Moreover, individuals with greater amounts of joint-adjacent SCF had higher odds for cartilage and meniscal structural progression over 4 years measured by WORMS.
While obesity is considered as major risk factor for knee OA, BMI does not reflect local body composition and has limitations in assessing body fat.29 In a systematic review and meta-analysis, Long et al identified body composition measures (total body fat mass and body fat percentage) as possible risk factors for knee OA, but the impact of SCF in proximity to the knee remains unclear7: While the influence of the more distant thigh SCF on knee OA was found to be complex and with limited statistical significance, only one previous study investigated associations of medially sided joint-adjacent SCF and knee OA and found greater patello-femoral cartilage defects in patients with greater SCF thickness.5, 16, 19 In this exploratory study, we found significant associations of joint-adjacent SCF thickness and BMI. In order to limit the effects of a higher BMI and instead to focus on the impact local body composition has on progression of knee OA, all our analyses were adjusted for BMI. In light of the aforementioned limitations of BMI, our observations may thus provide an insight into associations of joint-adjacent SCF and OA, independently of BMI.
In the primary analysis, particularly for the lateral knee compartment, positive correlations between joint-adjacent SCF measurements and prevalent knee OA were found, while coefficients for the medial compartment were non-significant. The secondary analysis showed positive associations between lateral compartment cartilage and meniscus scores for both lateral and ajSCF, supporting the findings in our primary analysis. Also, we found positive associations between cartilage T2 relaxation times (a quantitative imaging biomarker for collagen integrity and water content indicating early cartilage deterioration), and greater lateral and ajSCF.30, 31 Thus, particularly OA of the lateral joint compartment showed remarkable associations with local SCF thickness.
Previous studies have shown associations of the supra- and infrapatellar fat pads to knee OA: Intra-capsular fat deposits have been investigated regarding their potential of modifying knee OA by inducing inflammatory processes within the knee joint.3, 17 Adipokines produced in the Hoffa fat pad, have moreover been identified as mediators contributing to cartilage and meniscal degradation.9-11, 13, 14, 32 With its intra-capsular location, adipokines produced by the Hoffa fat pad may reach the synovial fluid by permeating the synovium. However, SCF has also been shown to produce adipokines.18, 33 Thus, also the associations between joint-adjacent SCF and OA found in this study may be explained by local inflammatory processes induced by adipokines.
Findings for joint-adjacent SCF were contrasted by a negative association of thigh SCF and structural degeneration of the patello-femoral joint. This observation may be explained by differences in biomechanical joint loading patterns, as forces on the patello-femoral joint during slow walking activities are lower, compared to fast walking or running, which include a higher degree of knee-bending.34, 35 Since walking speed in overweight subjects is slower compared to individuals with normal weight, the decreased load in the patello-femoral joint may be responsible for the lower structural damage and T2 values found in this study.35-37 Moreover, findings for thigh SCF support the hypothesis of a spatial dependence of SCF-associated OA. Further, associations of thigh SCF and WORMS may be regarded as indicator for the independence of our observations from BMI, as a scenario in which adjustment for BMI reduces the impact of thigh SCF, but not joint-adjacent SCF, seems unlikely.
Longitudinal findings supported our cross-sectional findings, and demonstrated that baseline joint-adjacent SCF predicted knee OA: odds for lateral compartment WORMSsum progression were greater in participants with increased levels of joint-adjacent SCF, compared to those with less joint-adjacent SCF. Similar to the cross-sectional results, the secondary longitudinal analysis also showed positive associations between joint-adjacent SCF measures and both cartilage and meniscal degradation. However, findings in the primary analysis were most likely attributable to associations between lateral and anterior SCF and meniscal degradation, as these associations were statistically significant in the secondary analysis.
The positive associations between SCF and meniscal deterioration furthermore strengthen the hypothesis of adipokine-associated changes: Nishimuta et al reported, that adipokines may accelerate meniscal degradation by a resistin-induced release and depletion of sulfated glycosaminoglycans in menisci, impacting the meniscus' viscosity and its ability to withstand heavy loads.38 The menisci of individuals with greater amounts of joint-adjacent SCF may thus have lower glycosaminoglycan levels, leading to accelerated degradation.
Limitations
This study has limitations, including the employed statistical methods and the study design: First, the number of analyses may raise a multiple comparison issue. However, to reduce the number of comparisons, we employed primary and secondary outcomes. Also, this study is of an exploratory nature, since knowledge about the associations between SCF and knee OA is very limited.16 Most significant findings in this study were restricted to lateral SCF and the lateral knee compartment. Further studies investigating associations between joint-adjacent SCF and OA may thus focus on the lateral knee compartment. Second, since this study is based on data acquired through the OAI, we were unable to acquire data on subcutaneous adipokine levels and to correlate these with our findings. However, adipokine production in SCF of the lower limb has previously been shown.18, 33 While diffusion of the molecules may lead to a direct increase in intra-articular adipokine-levels, subcutaneous adipokines may also indirectly increase intra-articular levels by inducing inflammatory changes of the Hoffa fat pat or the synovium.39 Third, we excluded patients with a BMI < 25 kg/m2 from our study, possibly limiting the external validity of this study. However, the estimated ratio of overweight or obese people in the U.S. population consistently exceeds 65% and thus, our findings may still be applicable to the largest part of the U.S. population.40 Fourth, although the analyzed cohort of 338 OAI participants was large compared to previous studies, many of the discovered associations remained borderline significant. Studies investigating a larger cohort may resolve this limitation. A fifth limitation involves the exploratory nature of the study design: the study focuses on investigating statistically significant associations of SCF and structural measures of OA. This does not allow for conclusions on causal relationships or generate concrete clinical implications. Overall, further studies are needed to overcome these limitations and confirm our observations.
Conclusion
This study reported positive associations between joint-adjacent subcutaneous fat at the knee and osteoarthritic changes of the adjacent knee compartments, and findings were independent of BMI. Particularly, associations of lateral subcutaneous fat and lateral compartment structural changes were the strongest, cross-sectionally and longitudinally, and findings were supported by increased T2 relaxation times, consistent with cartilage compositional deterioration. Joint-adjacent subcutaneous fat may be of interest for further investigation regarding its role in the etiology of OA. Less significant results for thigh subcutaneous fat indicate the importance of changes in local tissue composition around the knee for progression of OA.
Acknowledgments
The analyses in this study were funded through NIH/NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01AR064771). The study was supported by the Osteoarthritis Initiative, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262), with research conducted by the Osteoarthritis Initiative Study Investigators. The study was also funded in part by the Intramural Research Program of the National Institute on Aging, NIH. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
John Lynch, PhD, contributed substantially to study conception and design and helped identifying variables from the OAI datasets, that were relevant to this study. Open Access funding enabled and organized by Projekt DEAL.