Displacement Encoding With Stimulated Echoes Enables the Identification of Infarct Transmurality Early Postmyocardial Infarction
This research was supported by the Chief Scientist Office (ETM/263). Dr Mangion was supported by a Fellowship from the British Heart Foundation (BHF FS/15/54/31639). Professor Berry was supported by a Senior Clinical Fellowship from the Scottish Funding Council and from funding from the British Heart Foundation Centre of Excellence Award (RE/13/5/3017).
Relationship with industry: The University of Glasgow holds a research agreement with Siemens Healthcare, who provided the DENSE work-in-progress CMR sequence and data analysis software. There are no other relevant disclosures.
Abstract
Background
Segmental extent of infarction assessed by late gadolinium enhancement (LGE) imaging early post-ST-segment elevation myocardial infarction (STEMI) has utility in predicting left ventricular functional recovery.
Hypothesis
We hypothesized that segmental circumferential strain with displacement encoding with stimulated echoes (DENSE) would be a stronger predictor of infarct transmurality than feature-tracking strain, and noninferior to extracellular volume fraction (ECV).
Study Type
Prospective.
Population
Fifty participants (mean ± SD, 59 ± 9 years, 40 [80%] male) underwent cardiac MRI on day 1 post-STEMI.
Field-Strength/Sequences
1.5T/cine, DENSE, T1 mapping, ECV, LGE.
Assessment
Two observers assessed segmental percentage LGE extent, presence of microvascular obstruction (MVO), circumferential and radial strain with DENSE and feature-tracking, T1 relaxation times, and ECV.
Statistical Tests
Normality was tested using the Shapiro–Wilk test. Skewed distributions were analyzed utilizing Mann–Whitney or Kruskal–Wallis tests and normal distributed data using independent t-tests. Diagnostic cutoff values were identified using the Youden index. The difference in area under the curve was compared using the z-statistic.
Results
Segmental circumferential strain with DENSE was associated with the extent of infarction ≥50% (AUC [95% CI], cutoff value = 0.9 [0.8, 0.9], −10%) similar to ECV (AUC = 0.8 [0.8, 0.9], 37%) (P = 0.117) and superior to feature-tracking circumferential strain (AUC = 0.7[0.7, 0.8], −19%) (P < 0.05). For the detection of segmental infarction ≥75%, circumferential strain with DENSE (AUC = 0.9 [0.8, 0.9], −10%) was noninferior to ECV (AUC = 0.8 [0.7, 0.9], 42%) (P = 0.132) and superior to feature-tracking (AUC = 0.7 [0.7, 0.8], −13%) (P < 0.05). For MVO detection, circumferential strain with DENSE (AUC = 0.8 [0.8, 0.9], −12%) was superior to ECV (AUC = 0.8 [0.7, 0.8] 34%) (P < 0.05) and feature-tracking (AUC = 0.7 [0.6, 0.7] −21%) (P < 0.05).
Data Conclusion
Circumferential strain with DENSE is a functional measure of infarct severity and may remove the need for gadolinium contrast agents in some circumstances.
Level of Evidence
2
Technical Efficacy Stage
5 J. MAGN. RESON. IMAGING 2020;52:1722–1731.
THE TRANSMURAL EXTENT of myocardial infarction per myocardial segment, acquired using late gadolinium enhancement (LGE) imaging early post-ST-segment elevation myocardial infarction (STEMI), has prognostic importance for the recovery of myocardial contractile function and prediction of major adverse cardiac events in the longer term.1, 2
In the acute phase post-STEMI, segmental LGE extent overestimates infarction due to coexistent myocardial edema, inflammation, and hemorrhage.3 In fact, a segmental cutoff of 75% has been proposed in patients imaged early post-STEMI to determine viability rather than the 50% threshold used in chronic coronary syndromes.4
T1 mapping and extracellular volume fraction (ECV) estimation enable an alternative and complementary assessment of infarct severity to LGE-derived segmental extent of infarction. Segmental T1 values have been reported to be strongly correlated with the extent of infarction on LGE imaging, and ECV has been reported to be a superior marker than LGE to identify segmental myocardial functional improvement.5, 6 However, these conventional techniques require the use of gadolinium contrast agents and are contraindicated in patients with chronic kidney disease.
Myocardial strain has the potential to provide new insights into the pathophysiology of STEMI.7-11 The advantages of quantitative methods beyond the left ventricular ejection fraction (LVEF) and wall-motion scoring include improved diagnostic sensitivity and specificity, less interobserver and intraobserver variability, and novel parameters of biomechanical function.7
Displacement encoding with stimulated echoes (DENSE) has advantages over tagging, which is historically regarded as the gold standard technique for cardiac magnetic resonance imaging (MRI)-derived strain.12-18 Specifically, the accuracy of DENSE strain is equivalent to that of tagging, and interobserver reproducibility of DENSE is better than that of tagging.19, 20 Furthermore, the time required to perform strain analysis of DENSE images is less than that for conventional tagging analysis.19 Studies previously demonstrated that DENSE-derived global circumferential strain had incremental benefit over acute infarct size to predict major adverse cardiac events, and segmental circumferential strain with DENSE provided incremental utility over the segmental extent of infarction to predict myocardial functional recovery by wall-motion scoring.10, 11 Further work needs to be carried out to assess if DENSE can obviate the use of gadolinium contrast agents in patients postmyocardial infarction.
The aims of the study were to investigate if segmental strain with DENSE would be: 1) a stronger predictor of infarct transmurality than feature-tracking strain, and 2) noninferior to ECV in acute STEMI patients.
Materials and Methods
Study Population
A prospective MR single-center cohort study was performed between January 26, 2015, and September 19, 2016, in a regional cardiac center. The study had ethics approval (reference 14/WS/0085) and was publicly registered (https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/cathepsins-in-stemi/). Clinical research staff screened STEMI admissions between January 24, 2015, and March 21, 2016. Acute STEMI management followed contemporary guidelines.21, 22 Inclusion criteria included ≥18 years of age, with symptom onset of ≤6 hours and an occluded culprit artery that is Thrombolysis in Myocardial Infarction (TIMI) flow grade 0/1. Exclusion criteria included an inability to give informed consent, cardiogenic shock, contraindication to MRI (eg, defibrillator, glomerular filtration rate <30 mL/min/1.73 m2).
MRI Acquisition
MRI was performed using a 1.5T scanner (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany), using an anterior phased-array body coil (12-element) and a posterior phased-array spine coil (24-element) within the first 3 days postmyocardial infarction (MI).23
The MRI protocol included cine (balanced steady-state free precession [b-SSFP]), 2D-spiral DENSE, pre- and postcontrast T1 mapping and LGE phase-sensitive inversion recovery (PSIR) pulse sequences.24, 25
LV dimensions were assessed using b-SSFP cinematographic breath-hold sequences. The heart was imaged in multiple parallel short-axis planes 8-mm thick separated by 2-mm gaps, as well as in the 2-chamber, 3-chamber, and 4-chamber long-axis views. Typical sequence parameters were: bandwidth 930 Hz/pixel, echo time (TE) 1.2 msec, flip angle 77°, spatial resolution 180 × 256 mm, and temporal resolution 46 msec.
DENSE imaging was acquired in three short-axis slices (basal, mid-ventricular, apical). Typical DENSE imaging parameters were as follows: TE 1.08 msec; repetition time (TR) 15 msec; flip angle 20°; slice thickness 8 mm; field of view 360 mm × 270 mm; matrix size 112 × 84; displacement encoding of 0.08 cycles/mm; spiral interleaves: 6.
A modified Look–Locker inversion recovery prototype sequence (work-in-progress sequence 488, Siemens Healthcare) was used for T1 mapping and performed in three short-axis slices at mid-diastole (basal, mid-LV, apical), before and 20 minutes postgadolinium contrast, in line with recommendations.26 Typical parameters were: bandwidth ~1090 Hz/pixel; flip angle 35°; TE 1.1 msec; inversion time (TI) of first experiment 100 msec; TI increment 80 msec; matrix 192 × 124 pixels; spatial resolution 2.2 × 1.8 × 8.0 mm; slice thickness 8 mm; scan time 17 heartbeats. Each study participant had a blood sample acquired prior to the MR scan in order to obtain a hematocrit.
LGE images covering the entire LV were acquired 10–15 minutes after intravenous injection of 0.15 mmol/kg of gadoterate meglumine (Gd2+-DOTA, Dotarem, Guebert, Villepinte, France) using segmented PSIR turbo fast low-angle shot. Typical imaging parameters were: matrix 256 × 156, flip angle 25°, TE 3.36 msec, bandwidth 130 Hz/pixel, echo spacing 8.7 msec, and trigger pulse 2. The voxel size was 1.8 × 1.3 × 8 mm3.
Image Analysis
Data analysis was carried out in a core lab by two researchers (cardiac MRI experience: K.M.: 5 years; M.M.L.: 3 years), utilizing dedicated software (Medis Suite V2.1, Medis Solutions, Leiden, the Netherlands) for LVEF (K.M.), T1 and ECV estimation (K.M.), infarct size (M.M.L.); freely available DENSEanalysis software27, 28 for DENSE-derived strain (K.M.); and Diogenes feature-tracking software (TomTec Imaging Systems, Germany) for cine strain analysis (K.M.). Imaging contours and data spreadsheets were reviewed by an observer (C.B.) with >10 years of cardiac MR experience.
Infarct Definition And Size
Infarction was defined by the occurrence of myocardial LGE revealed in orthogonal planes including phase swap acquisitions as appropriate by the scanning radiographers or fellow to rule out artifact. Microvascular obstruction was defined as a dark zone within an area of LGE.29, 30 The extent of late gadolinium enhancement (% LV mass) was assessed utilizing the full-width half-maximum thresholding method.31
Transmural extent of infarction and the presence/absence of microvascular obstruction (MVO) were also analyzed on a segmental basis at basal, mid-LV, and apical short-axis slices. The transmural extent of infarct was then binarized into LGE >50% (yes/no) and in view of recent data, LGE >75% (yes/no).4
Segmental T1 and Extracellular Volume Fraction Quantification
Color-coded T1 and extracellular volume (ECV) maps were generated based on inline-generated, motion-corrected raw images using QMap software V2.1 (Medis). Endocardial and epicardial contours were manually drawn and the LV was segmented according to the American Heart Association 16-segment model with respect to the anterior right ventricular insertion point. Segmental regions of interest comprising the middle 80% of the myocardial thickness with 10% myocardium omitted on the endo- and epicardial sides were constructed to avoid partial volume effects from neighboring tissue and blood pool.
Strain Analyses
The 2D DENSE images were analyzed off-line using DENSE analysis. Segmentation of the LV myocardium was performed semiautomatically after endo- and epicardial contours were drawn by K.M., using the anterior right ventricular insertion point as a reference. Spatiotemporal phase unwrapping was then carried out on the LV myocardium pixels, and displacement vectors were calculated.28, 32 Lagrangian strain was computed from these displacements and then projected into radial, circumferential (or longitudinal in long-axis acquisitions) directions relative to the LV center of mass.
Diogenes feature-tracking software (TomTec Imaging Systems) was used to quantify strain from short-axis cine b-SSFP images. The same operator derived strain following a standard protocol taught by the software manufacturer.33 The relevant Digital Imaging and Communications in Medicine (DICOM) folders were loaded into the program. The endocardial border was contoured on the end-diastolic image. Strain was generated based on border displacement.
Strain analysis was carried out by K.M. with C.M. (10 years MRI experience) carrying out interobserver variability assessment.
Statistical Analysis
Statistical analysis was performed using SPSS (IBM, Armonk, NY) or R v. 2.15 (Vienna, Austria) or higher. Normality was tested using the Shapiro–Wilk test. Continuous variables were expressed as mean ± standard deviation (SD) or median (Q1, Q3), depending on distribution. Skewed distributions were analyzed utilizing Mann–Whitney or Kruskal–Wallis tests; normal distributed data was analyzed using independent t-tests, as required. No correction was made for multiple comparisons. Correlation analyses were either Pearson or Spearman tests, depending on the distribution of variables. P < 0.05 was considered statistically significant. Diagnostic cutoff values were identified from the “OptimalCutpoints” package34 using the Youden index.35 The difference in area under the curve (AUC) was compared using the z-statistic using DENSE as the reference standard. Noninferiority was defined as a z-statistic P > 0.05 when compared with DENSE circumferential strain. AUCs were classified as 0.9–1 = excellent; 0.80–0.90 = good; 0.7–0.8 = fair; 0.6–0.7 = poor; 0.5–0.6 = fail.36
Ten randomly identified datasets were deidentified. They were analyzed by K.M. for intraobserver repeatability and by C.M. for interobserver repeatability.
Sample Size Calculation
2D strain information was calculated and described in terms of segmental strain and correlated with the transmural extent of MI. The AUC for 2D strain for identification of MI on a per-segment basis was assessed. For a Type 1 error (α) of 0.05, power of 80% and 90%, and AUC of 0.85 and 0.90 with 2D strain, respectively, then 351 and 478 data points would be needed, respectively.37
Results
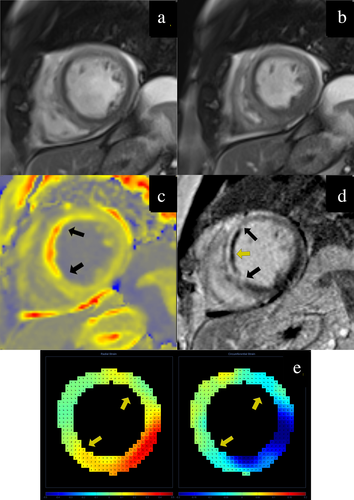
Out of 62 patients who underwent MR, DENSE was not acquired in three of them, was unsuitable for analysis in two of them, and seven patients did not have T1 maps acquired or the maps were unsuitable for analysis (Fig. 1). The analysis dataset included 50 patients, including 10 (20%) women and 40 (80%) men. The mean age was 59 ± 9 years. Demographics and a typical case are presented in Table 1 and Fig. 2, respectively. The MR scans were carried out, on average, 1 ± 0.5 days post-MI.
| Baseline characteristicsa (N = 50) | Values |
|---|---|
| Age, years | 59 ± 9 |
| Male sex, n (%) | 40 (80) |
| Hypertension, n (%) | 15 (30) |
| Current smoking, n (%) | 21 (42) |
| Hypercholesterolemia, n (%) | 8 (16) |
| Diabetes mellitusb, n (%) | 5 (10) |
| Previous myocardial infarction, n (%) | 5 (10) |
| Presenting characteristics | |
| Heart rate, bpm | 75 ± 3 |
| Systolic blood pressure, mmHg | 123 ± 23 |
| Diastolic blood pressure, mmHg | 73 ± 12 |
| Time from symptom onset to reperfusion, min | 114 ± 59 |
| Coronary angiography | |
| Culprit artery, n (%) | |
| Left anterior descending | 22 (44) |
| Left circumflex | 5 (10) |
| Right coronary | 23 (46) |
| Medication on discharge from hospital, n (%) | |
| Aspirin | 50 (100) |
| Ticagrelor | 50 (100) |
| Angiotensin converting enzyme inhibitor | 48 (96) |
| Beta-blocker | 48 (96) |
| Statin | 50 (100) |
- a Values are expressed as number (percentage) or mean ± standard deviation.
- b Diabetes mellitus was defined as a history of diet-controlled or treated diabetes.
Reproducibility Analyses
DENSE had statistically less intra- and interobserver variability as assessed by bias, limits of agreements, intraclass correlation coefficient, and correlation, when compared with feature-tracking (Supplementary Tables 2 and 3).
Per Patient Analysis
The mean LVEF was 52 ± 10%, and median infarct size was 13 (9, 21) % LV mass (Table 2). Global circumferential strain with both DENSE and feature-tracking had moderate negative correlations with LVEF; global radial strain (with feature-tracking) had a moderate correlation with LVEF. Global circumferential strain with both DENSE and feature-tracking had moderate correlations with acute infarct size (Table 3).
| Parametersa | Values |
|---|---|
| Left ventricular ejection fraction, % | 52 ± 10 |
| Left ventricular end-diastolic volume, indexed to body surface area, mL/m2 | 74 ± 12 |
| Left ventricular end-systolic volume, indexed to body surface area, mL/m2 | 32 (28, 42) |
| Infarct size, % left ventricular mass | 13 (9, 21) |
| Microvascular obstruction, % left ventricular mass | 0.0 (0.0, 1.0) |
| Global circumferential strain, % (DENSE) | −14 ± 3 |
| Global radial strain, % (DENSE) | 13 ± 5 |
| Global circumferential strain, % (feature-tracking) | −20 ± 5 |
| Global radial strain, % (feature-tracking) | 33 ± 10 |
| Infarct zone | |
| Infarct zone circumferential strain, % (DENSE) | −8 ± 4 |
| Infarct zone radial strain, % (DENSE) | 9 ± 8 |
| Infarct zone circumferential strain, % (Feature-tracking) | −13 ± 10 |
| Infarct zone radial strain, % (Feature-tracking) | 17 ± 15 |
| Infarct zone T1 time, msec | 1061 ± 122 |
| Infarct zone extracellular volume fraction, % | 43 ± 11 |
- DENSE = displacement encoding with stimulated echoes.
- a Values are expressed as mean ± standard deviation or median (interquartile range).
| Global values | Segmental values | |||
|---|---|---|---|---|
| Left ventricular ejection fraction | Acute infarct size | Segmental extent of infarction | ||
| Global circumferential strain, % (DENSE) | −0.6, P < 0.05 | 0.6, P < 0.05 | ||
| Global radial strain, % (DENSE) | 0.2, P = 0.097 | −0.3, P = 0.037 | ||
| Global circumferential strain, % (feature-tracking) | −0.7, P < 0.05 | 0.6, P < 0.05 | ||
| Global radial strain, % (feature-tracking) | 0.5, P < 0.05 | −0.5, P < 0.05 | ||
| Infarct zone | Microvascular obstruction | 0.6, P < 0.05 | ||
| Infarct zone circumferential strain, % (DENSE) | −0.4, P < 0.05 | 0.5, P < 0.05 | Circumferential strain, % (DENSE) | 0.6, P < 0.05 |
| Infarct zone radial strain, % (DENSE) | 0.4, P < 0.05 | −0.4, P < 0.05 | Radial strain, % (DENSE) | −0.2, P < 0.05 |
| Infarct zone circumferential strain, % (feature-tracking) | −0.3, P = 0.084 | 0.2, P = 0.196 | Circumferential strain, % (feature-tracking) | 0.3, P < 0.05 |
| Infarct zone radial strain, % (feature-tracking) | 0.4, P < 0.05 | −0.4, P < 0.05 | Radial strain, % (feature-tracking) | −0.4, P < 0.05 |
| T1 time, msec | −0.1, P = 0.425 | 0.2, P = 0.240 | T1 time, msec | 0.1, P < 0.05 |
| Extracellular volume fraction, % | 0.1, P = 0.510 | 0.1, P = 0.397 | Extracellular volume fraction, % | 0.5, P < 0.05 |
- DENSE = displacement encoding with stimulated echoes.
Per Segment Analysis
Out of 800 segments acquired with DENSE, 20/800 were excluded due to not having interpretable DENSE data acquired (ie, artifact). Out of 780 segments, 34 were excluded due to artifact on pre- or postcontrast T1 mapping. The final number of segmental datasets used was 746 (Table 4). Ninety-seven (13%) segments had ≥50% LGE, 46 (6%) had ≥75% LGE, and 65 (9%) had MVO.
| 746 segments | AUC (95% CI) | Cutoff value | Sensitivity | Specificity | Z statistic | P value |
|---|---|---|---|---|---|---|
| Late gadolinium myocardial enhancement ≥50% (N = 97) | ||||||
| DENSE ECC, % | 0.9 (0.8, 0.9) | −10 | 0.8 | 0.8 | Reference | |
| DENSE ERR, % | 0.4 (0.3, 0.4) | 64 | 0.0 | 1.0 | — | |
| FT ECC, % | 0.7 (0.7, 0.8) | −19 | 0.8 | 0.5 | 5 | <0.05 |
| FT ERR, % | 0.2 (0.2, 0.3) | 0.0 | 1.0 | 0.0 | — | |
| T1, msec | 0.6 (0.5, 0.7) | 1055 | 0.5 | 0.7 | 8 | <0.05 |
| ECV, % | 0.8 (0.8, 0.9) | 37 | 0.8 | 0.7 | 2 | 0.117 |
| Late gadolinium myocardial enhancement ≥75% (N = 46) | ||||||
| DENSE ECC, % | 0.9 (0.8, 0.9) |
−10 | 0.9 | 0.8 | Reference | |
| DENSE ERR, % | 0.3 (0.3, 0.4) | 64 | 0.0 | 1.0 | — | |
| FT ECC, % | 0.7 (0.7, 0.8) | −13 | 0.8 | 0.7 | 3 | <0.05 |
| FT ERR, % | 0.2 (0.1, 0.2) |
0.0 | 1.0 | 0.0 | — | |
| T1, msec | 0.6 (05, 0.7) | 1105 | 0.4 | 0.8 | 5 | <0.05 |
| ECV, % | 0.8 (0.7, 0.9) | 42 | 0.7 | 0.9 | 2 | 0.132 |
| Microvascular obstruction (N = 65) | ||||||
| DENSE ECC, % | 0.8 (0.8, 0.9) | −12 | 0.9 | 0.7 | Reference | |
| DENSE ERR, % | 0.4 (0.3, 0.4) | 5 | 0.8 | 0.2 | — | |
| FT ECC, % | 0.7 (0.6, 0.7) | −21 | 0.8 | 0.5 | 5 | <0.05 |
| FT ERR, % | 0.2 (0.2, 0.3) | 0.0 | 1.0 | 0.0 | — | |
| T1, msec | 0.6 (0.7, 0.5) | 1009 | 0.7 | 0.5 | 7 | <0.05 |
| ECV, % | 0.8 (0.7, 0.8) | 34 | 0.9 | 0.6 | 2 | <0.05 |
- AUC = area under the curve; CI = confidence interval; DENSE = displacement encoding with stimulated echoes; ECV = extracellular volume fraction; ECC = circumferential strain; ERR = radial strain; FT = feature-tracking.
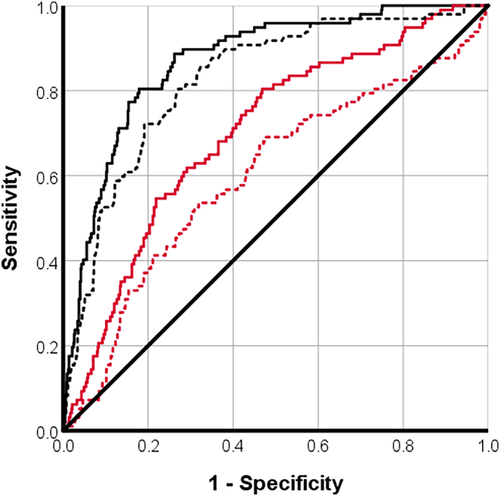
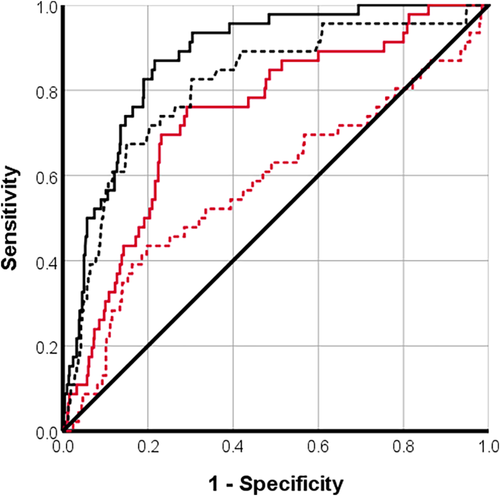
Microvascular obstruction (presence/absence) and segmental circumferential strain with DENSE had moderately strong correlations with segmental extent of infarction (Table 3). Segmental circumferential strain with DENSE was a good predictor of segmental extent of infarction ≥50% (AUC [95% confidence interval, CI], cutoff value = 0.87 [0.83, 0.91], −10.0%), as was segmental ECV (AUC = 0.8 [0.8, 0.9], 37%) (Fig. 3). For the detection of segmental infarction ≥75%, circumferential strain with DENSE (AUC = 0.9 [0.8, 0.9], −10%) and segmental ECV (AUC = 0.1 [0.7, 0.9], 42%) were classified as good predictors (Fig. 4). For the detection of MVO, only circumferential strain with DENSE was classified as a good predictor (AUC = 0.8 [0.80, 0.9], −12%) (Fig. 5). Segmental ECV was noninferior to DENSE to identify segmental extent of infarction ≥50% (P = 0.117) or ≥75% (P = 0.132).
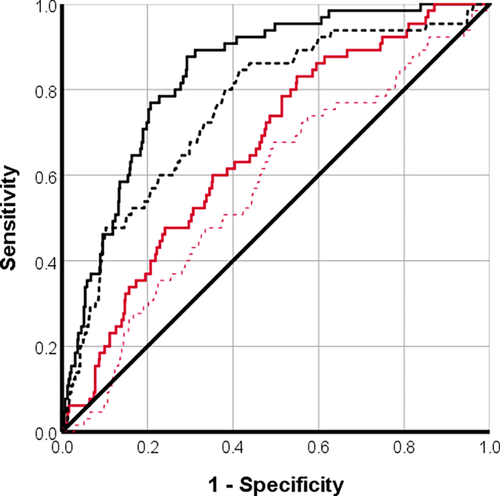
Discussion
The main findings of the study were: 1) For the detection of segmental infarction, circumferential strain with DENSE was noninferior to ECV and superior to feature-tracking; 2) For the detection of MVO, circumferential strain with DENSE was superior to ECV and to feature-tracking; 3) Segmental radial strain with both techniques was a poor predictor of infarct characteristics.
Observer Repeatability Analysis
DENSE had superior intraobserver and interobserver variabilities when compared with feature-tracking. This has important implications in studies investigating regional contractility post-STEMI,1, 2 as smaller sample sizes would be required to identify an improvement in myocardial functional recovery postintervention.
Segmental Strain and Infarct Characteristics
Segmental circumferential strain with DENSE was the strongest predictor of infarct transmurality >50%. We also investigated the utility of strain to identify segmental extent of infarction >75%, as recently identified by Bulluck et al,4 as a possible threshold for identifying infarcted segments post-MI with less potential for functional recovery.
In this study, segmental circumferential strain with DENSE (AUC = 0.9) and feature-tracking (AUC = 0.7) were strong predictors for identifying segments with >75% infarct transmurality. This compared well with other studies utilizing strain-encoded MRI (n = 38 patients, AUC = 0.96) or with feature-tracking (74 patients, AUC = 0.91).8, 9 A possible explanation for the lower AUC in our study is that one of these previous studies included non-ST-segment elevation MI besides STEMI participants, and both studies excluded patients with previous MI or adverse characteristics.
In this study, circumferential strain with DENSE >-10% indicates severe infarct pathology but does not distinguish between subtypes of infarct severity. As the cardiac MRI was carried out 1 ± 0.5 days post-MI, this could be due to myocardial stunning postreperfusion.,
Given recent safety concerns of using gadolinium-based contrast agents (GBCAs) in patients with impaired renal function, DENSE has potential to remove the use of gadolinium-based imaging in post-MI patients. Up to 1 in 3 patients presented with STEMI have renal dysfunction38; thus, contrast-free techniques enable a comprehensive MRI assessment in all patients. Furthermore, techniques such as DENSE can be used for serial cardiac imaging in view of reports of gadolinium accumulation in the brain.39
Segmental radial strain with both DENSE and feature-tracking was a poor predictor of infarct characteristics. It also had poor interobserver reproducibility when compared with circumferential strain. This could explain why there is a lack of information on the clinical utility of radial strain in detecting infarcted segments in patients with acute STEMI.7
Extracellular Volume Fraction and Infarct Characteristics
Segmental T1 was a poor predictor of the segmental extent of infarction or the presence of MVO. Remote zone ECV has been reported to be associated with adverse LV remodeling and segmental ECV expansion was associated with a reduction in segmental LV wall thickening,40, 41 and can predict segmental myocardial contractile recovery independent of segmental infarct size.6 In our study, ECV performed as well as circumferential strain with DENSE to detect >50% and > 75% LGE in myocardial segments.
Limitations
The MRI scan was carried out hyperacutely, 1 ± 0.5 days post-MI due to clinical service pressures, with potential infarct size overestimation due to edema in the infarct zone territory.5 Only three short-axis slices were acquired with DENSE and T1 mapping, and tagging imaging was not acquired due to time constraints. Only STEMI patients with TIMI flow grade 0 or 1 at presentation were included in this study. This was a single-center study with small patient numbers (N = 50).
Conclusion
Segmental circumferential strain by DENSE has the potential to detect segmental acute severity of infarction without the need of GBCAs. This was a single-center observational study and further work is warranted.
Acknowlegments




