Successful treatment of severe MSUD in Bckdhb−/− mice with neonatal AAV gene therapy
Abstract
Maple syrup urine disease (MSUD) is rare autosomal recessive metabolic disorder caused by the dysfunction of the mitochondrial branched-chain 2-ketoacid dehydrogenase (BCKD) enzyme complex leading to massive accumulation of branched-chain amino acids and 2-keto acids. MSUD management, based on a life-long strict protein restriction with nontoxic amino acids oral supplementation represents an unmet need as it is associated with a poor quality of life, and does not fully protect from acute life-threatening decompensations or long-term neuropsychiatric complications. Orthotopic liver transplantation is a beneficial therapeutic option, which shows that restoration of only a fraction of whole-body BCKD enzyme activity is therapeutic. MSUD is thus an ideal target for gene therapy. We and others have tested AAV gene therapy in mice for two of the three genes involved in MSUD, BCKDHA and DBT. In this study, we developed a similar approach for the third MSUD gene, BCKDHB. We performed the first characterization of a Bckdhb−/− mouse model, which recapitulates the severe human phenotype of MSUD with early-neonatal symptoms leading to death during the first week of life with massive accumulation of MSUD biomarkers. Based on our previous experience in Bckdha−/− mice, we designed a transgene carrying the human BCKDHB gene under the control of a ubiquitous EF1α promoter, encapsidated in an AAV8 capsid. Injection in neonatal Bckdhb−/− mice at 1014 vg/kg achieved long-term rescue of the severe MSUD phenotype of Bckdhb−/− mice. These data further validate the efficacy of gene therapy for MSUD opening perspectives towards clinical translation.
1 INTRODUCTION
Maple syrup urine disease (MSUD; MIM: 248600) is a rare, autosomal recessive metabolic disorder caused by the dysfunction of the mitochondrial enzyme complex, branched-chain 2-ketoacid dehydrogenase (BCKD), involved in the second step of branched-chain amino acids (BCAA) catabolism, which leads to massive increase of BCAA and associated branched-chain 2-ketoacids (BCKA) in tissues and biofluids.1, 2 Accumulation of leucine and 2-ketoisocaproic acid are responsible for the neurotoxicity observed in MSUD.3 MSUD is caused by mutations in any of 3 genes, BCKDHA, BCKDHB, and DBT (45%, 35%, and 20% of MSUD patients, respectively) which code for different subunits of the BCKD multimeric enzyme complex (branched-chain keto acid decarboxylase alpha and beta subunits [E1α and E1β], dihydrolipoyl transacylase [E2] subunit, respectively).2 85%–95% of MSUD patients exhibit the severe form of the disease with less than 3% residual BCKD activity, presenting shortly after birth with coma, cerebral edema and early death in the absence of treatment.4
Long-term management of MSUD, relying on severe and life-long BCAA dietary restriction associated with oral BCAA-free amino acid medical food, represents an unmet need as it is associated with a poor quality of life and does not prevent the risk of long-term neuropsychiatric complications.4-6 Orthotopic liver transplantation (OLT), despite specific risks,7 is an effective treatment for MSUD, allowing removal of dietary restrictions, providing metabolic and clinical stability, protection against acute decompensation and stabilization of neurocognitive impairments.7-9 As many autosomal recessive monogenic disorders of metabolism, MSUD represents a relevant target for gene therapy, with well-characterized pathophysiology and highly informative biochemical readouts and the demonstration provided by OLT that even restoration of only liver BCKD activity (representing 9%–13% of body BCKD activity)10 is therapeutic, even if the enzyme is ubiquitously expressed.
Adeno-associated virus (AAV) vectors are suitable tools to perform gene transfer to the liver or other organs11 and have been used in the field of inborn errors of metabolism to treat animal models of urea cycle disorders,12-16 organic acidemia,17, 18 phenylketonuria19 and others, and are used in humans in first clinical trials (ornithine transcarbamylase deficiency (OTC) (NCT02991144) for instance). For MSUD, two proofs of concept studies of AAV gene therapy efficacy in mice have been recently published.20, 21 In a Bckdha−/− mouse model, which recapitulates the severe human MSUD phenotype, we tested two vectors, carrying the human version of BCKDHA gene under the control of either an ubiquitous EF1α or liver-specific hAAT promoter, encapsidated in AAV capsids of serotype 8 which mainly targets the liver but also other organs (such as heart), and injected at birth. We demonstrated that intravenous neonatal gene therapy with the AAV8-EF1α-hBCKDHA vector achieved sustained disease rescue while the AAV8-hAAT-hBCKDHA vector showed a more limited efficacy.20 In a hypomorphic mouse model for the Dbt gene with a milder phenotype, it was shown that DBT gene therapy (under the control of the ubiquitous CB7 promoter encapsidated in muscle-tropic AAV9 capsids) provided substantial disease rescue after intramuscular or intravenous injection in young adult mice (P21-28), while liver-specific and muscle-specific strategies achieved more limited efficacy.21 To our knowledge, no mouse model for Bckdhb has been reported.
In this study, we provided characterization of a Bckdhb−/− mouse model, which recapitulates the severe neonatal human phenotype of MSUD. Based on our experience in Bckdha−/− mice, we demonstrated that AAV gene therapy expressing the human BCKDHB gene, performed at birth in Bckdhb−/− mice, achieved long-term disease rescue (>6 months).
2 MATERIALS AND METHODS
2.1 Plasmid and vector production
The hBCKDHB gene (NCBI gene ID: 594) comprises 10 coding exons, spanning around 240 Kb on chromosome 6, resulting in a cDNA of 1.18 Kb. The BCKDHB transgene was a wild-type version of the human BCKDHB coding sequence, encoding the full-length hBCKDHB protein. Similar to our previous work on the BCKDHA gene,20 the transgene expression cassette, termed EF1α-hBCKDHB, was assembled by cloning the hBCKDHB cDNA into a modified AAV expression cassette including the wild-type AAV2 inverted terminal repeats (ITRs) and a ubiquitous human elongation factor 1-alpha promoter (hEF1α) with an EF1α intronic sequence to increase the expression of the transgene. To improve mRNA stability, we also included the Woodchuck Hepatitis Virus (WHP) Posttranscriptional Regulatory Element (WPRE), and the bovine growth hormone gene polyadenylation signal (bGH poly[A]). All DNA sequences used in this study were synthesized by GeneCust. The AAV vectors were produced as already described.20 The AAV serotype used was AAV8.
2.2 Animal study procedures
Mouse studies were performed according to the French and European legislation regarding animal care and experimentation (2010/63/EU) and approved by the local institutional ethical committee (Paris Descartes ethics committee CEEA34, APAFIS#22754-2018092017287553 v3). Bckdhb+/− mice were purchased from The Jackson laboratory (C57BL/6NJ-Bckdhbem1[IMPC]J/Mmjax). These mice carried a 528-bp deletion of Chr9 from 83.988.526 to 83.989.053 at the heterozygous state. Balb/c mice were purchased from Charles River laboratories France (BALB/cByJ). In order to avoid mother-associated cannibalism, we obtained Bckdhb+/− mice on the mixed C57Bl/6NJ X Balb/c background (Bckdhb KO mice generated on the C57BL/6NJ background and subsequently backcrossed for 5 generations to Balb/c). We subsequently crossed males and females to generate Bckdhb−/− mice. We performed single intravenous injections in the temporal vein of AAV8-EF1α-hBCKDHB transgene vectors in neonate mice at P0 at 1014 vg/kg. The volume of injection was 10 μL. We injected the whole litters and genotyped the pups at P7. Mice were housed in a temperature (20–22°C) and humidity (40%–50%)-controlled environment with 12 h/12 h light/dark cycle and fed ad libitum with a standard diet. Blood samples were collected every 2 weeks and at sacrifice. Plasma was separated by centrifugation at 3000g for 15 min and stored at −80°C until analysis. After sacrifice, organs were snap-frozen in liquid nitrogen and stored at −80°C for further analysis.
2.3 Genotyping
DNA extraction was performed as previously described.20 The first couple of primers, flanking the deleted region in Bckdhb (forward: 5′-gcactgtgtagttttctctttaccc-3′; reverse: 5′-atggtgaggctcccacagtt-3′), were used, giving either a 715 bp amplicon for the wild-type allele (without the deletion) or a 178 bp amplicon for the mutated allele (with the deletion). The second set of primers was used with the antisense primer spanning the deleted region of Bckdhb (forward: 5′-gcactgtgtagttttctctttaccc-3′; reverse: 5′-tcacacaacggggtgttaaa-3′), giving a 316 bp amplicon for the wild-type allele (without deletion), or no amplicon for the mutated allele (with the deletion). Amplification conditions were 95°C for 10 min then 40 cycles of 95°C for 30 s, 62°C for 30 s, 72°C for 1 min.
2.4 Vector genome copy number analysis and RT-PCR
Vector genome copy number analysis was performed as previously described.20 RNA extraction and retro-transcription to cDNA were performed as previously described.20 For hBCKDHB mRNA and mice Bckdhb expression analyses, the qPCR on cDNA was performed using Sybergreen and primers annealing specifically on the WT hBCKDHB transgene sequence (hBCKDHB forward, 5′-ctagaggctcctatatcaaga-3′; hBCKDHB reverse, 5′-ggcatcataacacttccat-3′) and the mice Bckdhb sequence respectively (Bckdhb forward, 5′-tgcagtatgggcaaactcaga-3′, Bckdhb reverse, 5′-ccaaatattactgcagtggggt-3′). Mouse Gapdh gene transcripts were used to normalize Bckdhb or hBCKDHB expression (Gapdh forward, 5′-ggcattgtggaagggctcat-3′; Gapdh reverse, 5′-gtcttctgggtggcagtgat-3′). Data were recorded with QuantStudio (version 5.2).
2.5 Western blot analysis
Protein extraction was performed as previously described.20 SDS-PAGE electrophoresis was performed in a 4%–12% polyacrylamide gel. After transfer, the membrane was blocked with a solution of 5% BSA and incubated with an anti-BCKDHB antibody (Rabbit polyclonal; Abcam; cat. no. ab201225; lot GR3244895; WB 1:200) and anti-GAPDH (Mouse monoclonal; Clone 6C5; Abcam; cat. no. ab8245; lot GR3317834; WB 1:1.000). The membrane was washed and incubated with the appropriate secondary antibody (donkey anti-IgG Rabbit; Donkey polyclonal; LiCor; cat. no. 926 32213; lot C70918; WB 1:10 000 or donkey anti-IgG Mouse; Donkey polyclonal; LiCor; cat. no. 926 68072; lot D00226; WB 1:10 000) and visualized with the Odyssey imaging system (LI-COR Biosciences) and Image Studio version 5.2. The polyclonal anti-BCKDHB antibody recognized both the mouse BCKDHB and human BCKDHB proteins. For quantification, the hBCKDHB and mouse BCKDHB protein expression was normalized to GAPDH.
2.6 Amino acid analysis and liver BCKD enzyme activity measurement
Plasma concentrations of amino acids were measured with liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) as previously described.20 Actual BCKD liver enzyme activity was measured with a spectrophotometric assay, monitoring NADH production and using 2-ketoisovaleric acid as substrate as previously described.20
2.7 Statistical analysis
All the data shown are reported as means ± SD. The statistical analyses were performed using GraphPad Prism (version 9.3.1). Nonparametric tests were performed when two groups were compared (two-sided Mann–Whitney test). Comparisons of survival curves were performed with the log-rank Mantel-Cox test.
3 RESULTS
3.1 Bckdhb−/− mice show typical features of the severe human phenotype of MSUD
The Bckdhb−/− knockout mice showed a severe early-onset phenotype. We observed a very early lethality with the death of all Bckdhb−/− mice before the end of the first week of life (Figure 1A). The Bckdhb−/− mice that survived until P5 exhibited severe growth delay (data not shown). At the biochemical level, in Bckdhb−/− mice we observed an increase of plasma branched-chain amino acids and alloisoleucine, the specific marker of MSUD, along with a decrease of alanine leading to high leucine/alanine ratio as described in MSUD patients (Figures 1B and S1).4 Relative to Bckdhb+/+ mice, mRNA Bckdhb transcript levels in the liver, heart and brain were decreased in Bckdhb+/− mice and barely detectable in Bckdhb−/− mice (Figure 1C). Western blot analysis confirmed the absence of the BCKDHB protein in Bckdhb−/− mice in these tissues (Figure 1D). BCKD enzyme activity was not detectable in the liver of Bckdhb−/− mice (Figure S2). Taken together, these results confirmed that the Bckdhb−/− mouse was a null model and demonstrated that this mouse exhibited clinical and biochemical features mimicking the severe human neonatal MSUD phenotype.
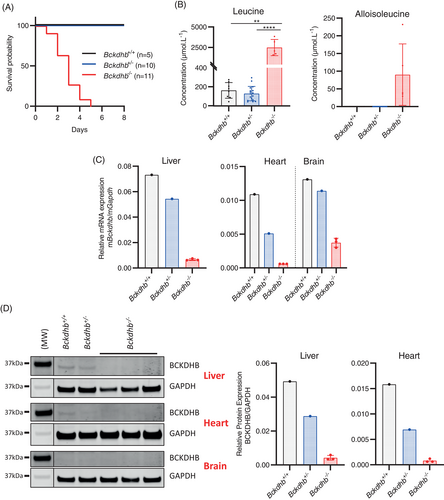
3.2 Neonatal AAV8-EF1α-hBCKDHB gene therapy provides long-term rescue of severe MSUD phenotype in Bckdhb−/− mice
Based on our previous results of successful gene therapy in the Bckdha−/− mouse model,20 we designed a cassette containing the EF1α (ubiquitous) promoter and the hBCKDHB transgene corresponding to the full-length human BCKDHB cDNA sequence and encapsidated in AAV8 capsids (AAV8-EF1α-hBCKDHB vector). To cope with early lethality of the Bckdhb−/− mice, we performed systemic temporal vein injection of the AAV8-EF1α-hBCKDHB vector at 1014 vg/kg at P0 (similar dose as previously used to rescue the Bckdha−/− knockout mouse model).20 We injected all the pups of the litters without prior knowledge of genotypes and performed genotyping at P7. Four pups died, eaten by the dam before genotyping. Of the 8 injected Bckdhb−/− mice, one died at P26, the 7 remaining individuals were alive at month 6, with a normal growth and without overt phenotypic abnormalities (Figure 2A,B). Hindlimb clasping in response to tail suspension was normal for all treated mice (not shown). At the biochemical level, BCAA accumulation in the plasma was dramatically reduced (less than two-fold above normal) until 6 months and alloisoleucine was not detectable (Figures 2C and S3). Three Bckdhb−/− mice were sacrificed at 6 months for tissue analyses. We detected the AA8 vectors in the liver and the heart and to a lesser extent in the brain (Figure 2D). Consistently, mRNA hBCKDHB transcripts were detectable in the liver and the heart and at lower levels in brain (Figure 2E). The BCKDHB protein was only detected in the liver and heart (Figure 2F). BCKD enzyme activity in the liver of treated Bckdhb−/− mice was about 16 ± 8% the value of age-matched untreated Bckdhb+/+ mice (Figure S2) at age 6 months.
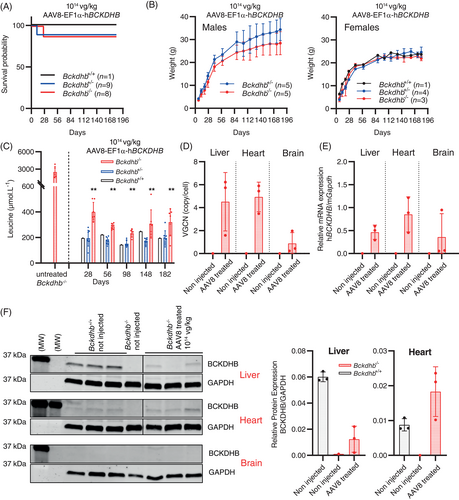
The four remaining Bckdhb−/− mice were followed beyond age 6 months. One died at day 254 despite a normal phenotype and good control of leucine plasma concentration. This death remained unexplained but probably unrelated to MSUD. A second mouse died accidentally at day 306 while showing also a good control of leucine plasma concentration and a normal phenotype. The 2 remaining Bckdhb−/− mice were still alive without overt phenotypical anomalies (Figure 3A) and normal hindlimb test at age 12 months (not shown). Plasma leucine concentrations and other amino acids disturbances were still well-controlled until this time point (Figures S3 and 3B). At age 12 months, we detected AAV8 genome copies, Bckdhb transcripts and hBCKDHB protein in the liver and heart as well as BCKD enzyme activity in the liver (Figures S2 and 3C–E).
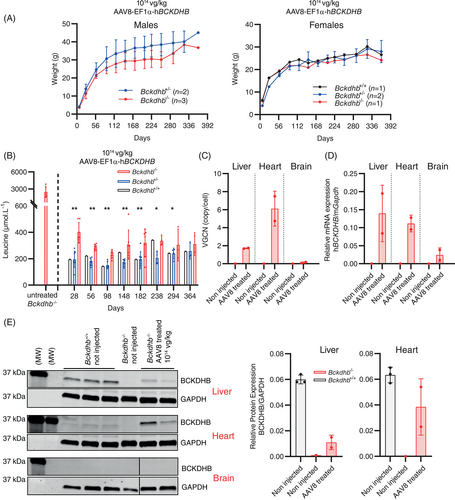
These results demonstrated that neonatal AAV8-EF1α-hBCKDHB gene therapy injected at 1014 vg/kg provides long-term phenotypic rescue of severe MSUD phenotype in Bckdhb−/− mice with a survival >6 months.
4 DISCUSSION
MSUD is an ideal target for gene therapy, because of its unmet medical needs, well-characterized pathophysiology, informative biomarkers and therapeutic efficacy of orthotopic liver transplantation in spite of incompletely restored BCKD enzyme activity. Proofs of concepts of efficacy of AAV gene therapy were recently published by us and others in a severe Bckdha−/− and in a mild Dbt−/− MSUD mouse models.20, 21 In this study, we extended this approach to the third MSUD gene, BCKDHB, mutated in 35% of patients,2 with extensive characterization of a Bckdhb−/− mouse model and demonstration that AAV gene therapy expressing the human BCKDHB gene can achieve long-term rescue of the severe MSUD phenotype. This work now completes the supply of gene therapy products for the 3 MSUD genes. We showed that homozygous Bckdhb−/− mice faithfully recapitulate the severe human MSUD phenotype with early-onset neurological symptoms leading to death during the first week of life, accompanied by massive accumulation of MSUD markers. A similar phenotype was observed in knockout mice with complete deficiency for Bckdha or Dbt genes.20, 22
Based on the most efficient settings from our previous experience in Bckdha−/− mice, we selected a cassette with the human BCKDHB transgene controlled by an ubiquitous promoter (EF1α), encapsidated in AAV8 capsids. We performed neonatal injections at a dose of 1014 vg/kg and obtained comparable results,20 that is, a long-term rescue of the clinical and biochemical MSUD phenotype. These observations further validate that a neonatal gene therapy providing hepatic and extra-hepatic BCKD activity is effective in treating severe MSUD in newborn mice.
One of the difficulties of AAV gene therapy in a neonatal setting is that transgene expression in proliferating tissues such as the liver declines with age.23-25 Furthermore, the vector dose used in our study is not readily translatable to human due to the potential risk of liver toxicity. Moreover, the effect of a given vector dose would likely be lower in human patients, in comparison with mouse, because AAV8 is less efficient at transducing human than mouse tissues (at least with regard to liver).26 In order to increase gene therapy efficacy in this particular setting (and also more generally), different strategies could be considered such as optimizing the transgene expression cassette,24 testing hybrid promoters,27 self-complementary AAV vectors28 and vectors with broader tropism than AAV8,29 in particular for heart and muscle. Injections later in life, after the main phases of liver tissue proliferation, could be envisioned in humans as patients can be managed with conventional dietary treatment. This may also allow decreasing vector doses relative to neonatal injections. This strategy was tested in a mild MSUD mouse model with injections at P21-P28.21 To perform comparable experiments in severe MSUD models, bridging therapies are required to maintain the mice alive long enough. Alternatively, a severe conditional MSUD model inducible in adulthood could be of interest.
In conclusion, we provide additional evidence for long-term efficacy of neonatal AAV gene therapy in a second severe MSUD mouse model.
ACKNOWLEDGMENTS
This work was supported by “DIM” (Domaine d'Intérêt Majeur) of the Paris Ile-de-France Region grant to CP, MSS, CG, AR, CO, MC and MS. The Authors are indebted to the AAV platform and the animal facility of Imagine Institute and the Centre d'Investigation Clinique of the Biotherapy Department, Necker Hospital.
FUNDING INFORMATION
This work was supported by “DIM” (Domaine d'Intérêt Majeur) of the Ile de France Region. We declare that CP, MSS, PDL, CO, MC, MS are designed as inventors of the European Patent Application EP20305079.4 filed on Jan 29, 2020 in the names of Inserm, Fondation Imagine, Université Paris Cité and APHP for “Gene therapy for MSUD.”
CONFLICT OF INTEREST STATEMENT
FM is employee and equity holder of Spark Therapeutics, a Roche company.
ETHICS STATEMENT
Mouse studies were performed according to the French and European legislation regarding animal care and experimentation (2010/63/EU) and approved by the local institutional ethical committee (Paris Descartes ethics committee CEEA34, APAFIS#22754-2018092017287553 v3).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




