Inborn disorders of the malate aspartate shuttle
Judith J. M. Jans and Nanda M. Verhoeven-Duif contributed equally to this study.
Communicating Editor: Jean-Marie Saudubray
Funding information: Metakids, Grant/Award Number: 2017-075
Abstract
Over the last few years, various inborn disorders have been reported in the malate aspartate shuttle (MAS). The MAS consists of four metabolic enzymes and two transporters, one of them having two isoforms that are expressed in different tissues. Together they form a biochemical pathway that shuttles electrons from the cytosol into mitochondria, as the inner mitochondrial membrane is impermeable to the electron carrier NADH. By shuttling NADH across the mitochondrial membrane in the form of a reduced metabolite (malate), the MAS plays an important role in mitochondrial respiration. In addition, the MAS maintains the cytosolic NAD+/NADH redox balance, by using redox reactions for the transfer of electrons. This explains why the MAS is also important in sustaining cytosolic redox-dependent metabolic pathways, such as glycolysis and serine biosynthesis. The current review provides insights into the clinical and biochemical characteristics of MAS deficiencies. To date, five out of seven potential MAS deficiencies have been reported. Most of them present with a clinical phenotype of infantile epileptic encephalopathy. Although not specific, biochemical characteristics include high lactate, high glycerol 3-phosphate, a disturbed redox balance, TCA abnormalities, high ammonia, and low serine, which may be helpful in reaching a diagnosis in patients with an infantile epileptic encephalopathy. Current implications for treatment include a ketogenic diet, as well as serine and vitamin B6 supplementation.
1 INTRODUCTION
In the last few years, several inherited metabolic disorders (IMDs) affecting the malate aspartate shuttle (MAS) have been discovered. The MAS is a redox shuttle which plays an essential and indispensable role in the redox balance both in mitochondria as well as the cytosol. Nutrients that enter the cytosol are oxidized in metabolic pathways by many different enzymes that use NAD+ as a cofactor. These metabolic pathways generate cytosolic reducing equivalents in the form of NADH which need to enter the mitochondrion to be used for energy production. As the inner mitochondrial membrane is impermeable to NADH molecules, the MAS transports the cytosolic reducing equivalents, or electrons, from the cytosol across the membrane.1 Eventually, electrons are supplied to the electron transport chain in the form of NADH for ATP production. Simultaneously, the MAS regenerates cytosolic NAD+. This cytosolic NADH re-oxidation system maintains the highly compartmentalized NAD+/NADH balance, which is essential for cellular energy metabolism and is also the driving force for NAD+-dependent reactions in the cytosol for instance in the biosynthesis of serine.2 The MAS is thus a redox shuttle that supports oxidative pathways as well as oxidative phosphorylation.3
The MAS is particularly important for the central nervous system,4 where it is also involved in the synthesis of aspartate and glutamate for neurotransmission.5 The fact that patients with a deficiency in one of the components of the MAS present with predominant neurological signs and symptoms emphasizes the importance of the MAS for neurological functioning.6-12 Early infantile epileptic encephalopathy is a common phenotype observed in patients with a defect in the MAS but is currently attributed to over 80 different genetic diseases in the OMIM database13 and over 30 in the IEMbase.14 Since these clinical phenotypes are aspecific, insights into the clinical and biochemical phenotypes of MAS deficiencies could help narrowing down the differential diagnosis.
In this literature review, the clinical phenotypes and biochemical characteristics of the different MAS deficiencies identified up to now will be discussed. These MAS deficiencies are, at least in part, amenable to targeted treatments, making early diagnosis important. In order to gain additional insight in the MAS and MAS disorders, biochemical clues and possible biochemical mechanisms linking the clinical phenotypes will be explored.
2 HISTORICAL PERSPECTIVE
In 1949, Friedkin and Lehninger proved that NADH links key metabolic pathways and processes such as the citric acid cycle and mitochondrial ATP synthesis,15, 16 setting the path to the discovery that ATP synthesis and NADH oxidation by oxygen are coupled. Later work showed that these processes are linked to proton translocation across the inner mitochondrial membrane, and led to the concept of a protonmotive force (about 200 mV) driving chemiosmotically ATP synthesis.17 A key requirement for mitochondrial NADH oxidation is the continuous entry of reducing equivalents such as NADH into the mitochondria. However, the pioneer experiments of Kennedy and Lehninger with isolated mitochondria18 led to the conclusion that cytosolic NADH is not directly oxidized by isolated rat-liver mitochondria, indicating that mitochondrial membranes are impermeable to NADH.19 Consequently, NADH had to be re-oxidized in the cytosol, reducing another acceptor that could permeate into mitochondria.1 In 1962, Piet Borst postulated that reducing equivalents could be shuttled across the mitochondrial membrane by using substrate cycles involving malate and aspartate, thus formulating the malate-aspartate cycle.20, 21 Today, the MAS is a well-established mitochondrial electron shuttle.22-24 An excellent historical and personal perspective of the MAS has been published recently by its discoverer, Piet Borst.25
3 ENZYMES AND TRANSPORTERS OF THE MAS
Four enzymes and two mitochondrial carriers cooperate to transport reducing equivalents across the inner mitochondrial membrane (Figure 1). Enzymes of the MAS include cytosolic and mitochondrial NAD(H)-dependent malate dehydrogenase (EC 1.1.1.37; respective encoding genes, MDH1 and MDH2), as well as cytosolic and mitochondrial aspartate aminotransferase (EC 2.6.1.1; respective encoding genes, GOT1 and GOT2). The two mitochondrial carriers are the oxoglutarate/malate carrier (OGC; gene SLC25A11) and the two isoforms of the aspartate-glutamate carrier, AGC1 (also known as Aralar; gene SLC25A12) or AGC2 (also known as Citrin; gene SLC25A13).
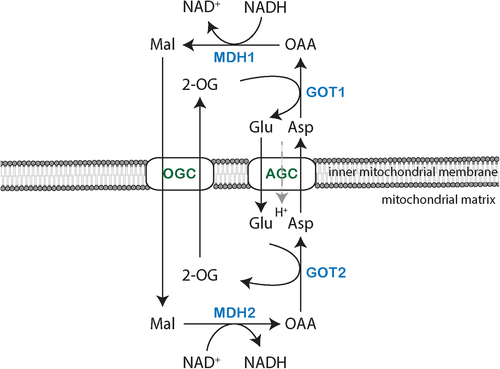
The MAS components are mainly expressed in high-energy demanding tissues, such as the brain, heart and liver, reflecting their critical roles in energy production. Malate dehydrogenases, aspartate aminotransferases, and OGC show relatively high expression in the brain, heart, and skeletal muscle.26 Isoforms of AGC are expressed in a tissue-specific manner.27 Whereas AGC1 is highly expressed in the brain, heart, central nervous system, and skeletal muscle, AGC2 is only abundant in the epithelial lining of the intestine and in the liver.27-29 Both isoforms are regulated by cytosolic calcium.27 MDH2 is more ubiquitously expressed than other MAS genes, probably reflecting its direct involvement in the TCA cycle. Not every MAS gene is expressed in all the tissues. In liver, AGC2, MDH2, GOT1, and GOT2 are strongly expressed, but MDH1 and OGC have low expression.26 Some tissues, such as the lung, have low expression of all MAS genes.
The MAS can be considered to start in the cytosol with the reduction of oxaloacetate to malate by MDH1, which is obligatory coupled to the oxidation of NADH to NAD+, thus regenerating NAD+ in the cytosol. Malate can enter the mitochondrial matrix by an electroneutral antiporter mechanism that exchanges malate for mitochondrial matrix 2-oxoglutarate, which is mediated by the OGC.24, 30 Next, malate is re-oxidized in the mitochondrial matrix by MDH2 and NAD+ to yield NADH and oxaloacetate. In this way, reducing equivalents are introduced into the mitochondria without passage of NADH across mitochondrial membranes. The oxaloacetate produced in the mitochondrial matrix can be converted to aspartate by transamination by the pyridoxal phosphate-dependent enzyme GOT2, using glutamate as the amino group donor and as the source of the 2-oxoglutarate that is exchanged for malate by OGC. Mitochondrial aspartate is then exported to the cytosol across the inner mitochondrial membrane by the Aralar or Citrin carriers (AGC isoforms), in exchange for cytosolic glutamate and a proton. The electrogenic nature of the glutamate/aspartate antiporting mechanism implies that AGC is essentially irreversible, at least under energized conditions.31, 32 Lastly, cytosolic oxaloacetate is regenerated by transamination from aspartate to cytosolic 2-oxoglutarate by GOT1 (Figure 1), regenerating the cytosolic glutamate needed for exchange with aspartate.
4 THE ROLE OF THE MAS IN METABOLISM
A major role of the MAS in metabolism is the net transfer of NADH over the inner mitochondrial membrane for oxidative phosphorylation. Simultaneously, the MAS maintains the cytosolic redox state by balancing the NAD+/NADH ratio via regeneration of NAD+. NAD+ is an important cofactor that serves as a carrier for the net removal of electrons from metabolites (oxidation).33 NAD+ impacts metabolism in several ways, both directly as well as via the NAD+/NADH ratio.34 Indeed, the absolute NAD+ pool plays a key role in cellular signaling, transcriptional regulation, and DNA damage repair via protein and nucleic acid modifications, since NAD+ is the driving force behind different enzymes including the various members of the sirtuins family as well as the different poly (ADP)-ribose polymerases (PARPs).35, 36 In these reactions, NAD+ is not reduced to NADH but instead converted into other metabolites. On the other hand, the NAD+/NADH ratio determines the preferred direction of reactions that are NAD(H)-dependent. In general, cells favor a high cytosolic NAD+/NADH ratio (~500-1000) which drives oxidative pathways, whereas the mitochondrial NAD+/NADH ratio is much lower (~5-10).34, 37, 38 Transfer of cytosolic reducing equivalents to the electron transport chain (ETC) against this 10- to 100-fold difference in NAD+/NADH ratio, as mediated by the MAS, is energetically unfavorable. It can only take place because the electrogenic AGC is driven by the electrical potential gradient across the inner mitochondrial membrane (~200 mV), which is generated by proton pumping coupled to the electron transport chain.24 Thus, MAS activity is intimately linked to electron transport through the respiratory chain. In addition, AGC1-mediated cytosolic calcium sensing has recently been shown to be essential for the control of oxidative phosphorylation by regulating mitochondrial pyruvate supply, as demonstrated in a knockout mouse model in which the mitochondrial calcium transporter was deleted.39
Metabolic flux through NAD+-dependent pathways affects cytosolic and mitochondrial NAD+/NADH ratios. Glycolysis is an important NAD+ dependent pathway that both affects and is affected by the cytosolic NAD+/NADH ratio. For glycolysis to continue, a high NAD+ /NADH ratio is required for the conversion of glyceraldehyde 3-phosphate into 1,3-bisphosphoglycerate by glyceraldehyde 3-phosphate dehydrogenase (GAPDH, EC 1.2.1.12), in which NAD+ is converted into NADH.40, 41 Re-oxidation of NADH can either be accomplished via the conversion of pyruvate to lactate by lactate dehydrogenase (LDH, EC 1.1.1.27) or by the conversion of oxaloacetate into malate by MDH1 (Figure 2). Converting glucose into lactate consumes and regenerates an equal amount of NAD+,33, 37 and additionally results in the production of 2 ATP molecules per glucose molecule. However, re-oxidation of NADH via MDH1, and the subsequent transfer of reducing equivalents to the ETC, coupled with the transfer of pyruvate across the mitochondrial membrane and its subsequent full oxidation to CO2 and H2O, results in the production of 36 ATP molecules per glucose molecule. Therefore, re-oxidation of NADH via the MAS provides more energy as it supports both glycolysis and mitochondrial respiration.42 In addition, sufficient NAD+ regeneration is also necessary for oxidative synthesis of nucleotides and amino acids, such as serine. The first step of serine biosynthesis is catalyzed by phosphoglycerate dehydrogenase (PHGDH, EC 1.1.1.95), another NAD+-dependent enzyme. The synthesis of serine from glucose includes two oxidative steps catalyzed by GAPDH and PHGDH, requiring two molecules of NAD+. Therefore, other metabolic pathways that need serine, including synthesis of cysteine, glycine, but also one-carbon metabolism, indirectly require NAD+.37 The MAS plays an essential role in redox balance and consequently in the continuation of the metabolic pathways mentioned earlier.
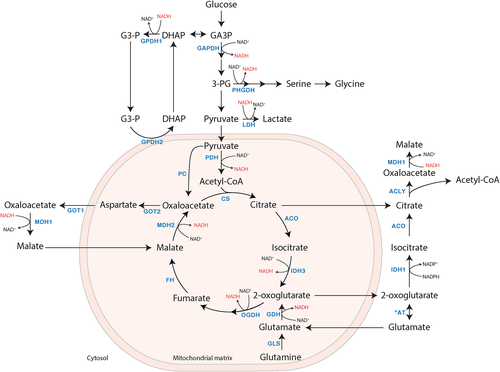
Next to their roles in cellular redox balance, some of the MAS enzymes play a role in anabolic and catabolic processes. Via transamination of oxaloacetate and glutamate by GOT2, the MAS is involved in the synthesis of aspartate. Whereas a cycle of the MAS as discussed above only results in a net transfer of electrons, de novo synthesis of aspartate can take place if oxaloacetate is supplied via pyruvate carboxylase. However, the major source of aspartate is oxidative glutamine metabolism. Here, glutamine carbons enter the TCA cycle as 2-oxoglutarate, which is further oxidized into oxaloacetate via MDH2.43 Oxaloacetate is then further converted to aspartate via GOT2. Inhibition of the ETC impairs aspartate biosynthesis via a drop in the mitochondrial NAD+/NADH ratio, which implies that mitochondrial respiration sustains aspartate biosynthesis.43, 44 Aspartate is essential for cell proliferation, as it is a precursor for purine and pyrimidine synthesis. In liver, aspartate is also involved in the urea cycle. Thus, the MAS is important for aspartate synthesis by providing its precursor oxaloacetate via MDH2 followed by transport of aspartate to the cytosol by AGC, thereby impacting several other metabolic pathways.
5 THE GLYCEROL 3-PHOSPHATE SHUTTLE
An alternative shuttle linked to the MAS via transfer of redox equivalents may be of importance if MAS activity is deficient.1, 45 In addition to the MAS, the glycerol 3-phosphate shuttle also transfers cytosolic reducing equivalents into the ETC. In 1958, two independent groups proposed the glycerol 3-phosphate shuttle, also known as the glycerophosphate (GP) shuttle.46, 47 In this shuttle, cytosolic glycerol 3-phosphate dehydrogenase (GPD1, EC 1.1.1.8) transfers reducing equivalents of NADH to dihydroxyacetone phosphate with concomitant formation of glycerol 3-phosphate and NAD+ (Figure 2). Via the enzyme GPD2 (EC 1.1.5.3), which is bound to the cytosolic face of the mitochondrial inner membrane, glycerol 3-phosphate is re-oxidized into dihydroxyacetone phosphate, while flavin adenine dinucleotide (FAD) is reduced to FADH2. FADH2 then transfers two electrons to ubiquinone (coenzyme Q), reducing it to ubiquinol, which subsequently enters the ETC at complex III. Two GPD enzymes form the GP shuttle, of which GPD2 is rate-limiting. Mammalian GPD2 is mainly expressed in brown adipose tissue, muscle, and brain, with a lower expression in liver and heart.48 Various glycolytic cells have expression of the GP-shuttle, including beta-pancreatic cells.49-51 Whereas the MAS connects glycolysis, the TCA cycle, and the ETC, the GP-shuttle acts as a connection between glycolysis, glycerol metabolism, and ETC.48
6 INBORN DISORDERS OF THE MAS
Like the majority of IEM, MAS deficiencies form a group of rare diseases, with only a few reported patients. The most common disorder is AGC2 or citrin deficiency, which occurs at a relatively high frequency in Japan and Southeast Asia. The molecular basis of this deficiency was identified in 1999.52 A few years later, the role of the AGC2 isoform AGC1, or aralar, was reported in a mouse model and the first patient with AGC1 deficiency was reported in 2009.9, 53 MDH2 deficiency was first described in 2017 and in 2019 deficiencies in GOT2 and MDH1 were published.6-8 No deficiencies in GOT1 and OGC have been reported to date.
As all MAS genes are highly expressed in brain, it is not surprising that the majority of MAS deficiencies affect the central nervous system. Deficiencies in GOT2, MDH1, MDH2, and AGC1 all present with early infantile epileptic encephalopathy, with abnormalities in myelination, hypoplasia and atrophy in the central nervous system. Since AGC2 is only expressed in liver, AGC2 deficiency leads to a more liver-specific disease. Table 1 comprises published data composed of biochemical and phenotypical findings. Because of the distinct phenotype of AGC2 deficiency compared to the other four MAS deficiencies, AGC2 was excluded from a more comprehensive overview of the neurological phenotypes, which is provided in Table 2.
| Disorder (OMIM) protein; gene | High tissue expression | Clinical phenotype | Biochemical | N | Reference |
|---|---|---|---|---|---|
MDH1 deficiency (618959) Malate dehydrogenase 1; MDH1 |
Brain, skeletal muscle, heart, kidney | Global developmental delay,infantile epileptic encephalopathy, microcephaly | Plasma: Lactate ≈ Dried blood spots: Glutamate↑, Glycerol 3 Phosphate ↑ |
2 | Broeks et al6 |
MDH2 deficiency (617339) Malate dehydrogenase 2; MDH2 |
Brain, skeletal muscle, heart, liver | Global developmental delay, infantile epileptic encephalopathy, hypotonia | Plasma: Lactate ↑, Lactate/Pyruvate ratio ↑ Urine: Malate ↑, Fumarate↑, Succinate (N) CSF: Lactate↑ MRS in brain: Lactate↑ Fibroblasts: Malate/Citrate ratio↑, Fumarate/Citrate ratio↑, RC-CI activity↓ Muscle biopsy: RC-CV activity↓-(N)Liver biopsy: RC-CV activity ↓ |
3 | Ait-El-Mkadem et al7 |
GOT2 deficiency (618721) Aspartate aminotransferase 2; GOT2 |
Brain, skeletal muscle, heart, kidney, liver | Infantile epileptic encephalopathy, progressive microcephaly | Plasma: Lactate ↑, Ammonia ↑, Citrulline ↑, Serine↓ | 4 | Van Karnebeek et al8 |
AGC1 deficiency (612949) Aralar; SLC25A12 |
Brain, skeletal muscle, heart | Global developmental delay, infantile epileptic encephalopathy, hypotonia | Plasma: Lactate ≈↑ CSF: Lactate ≈↑ MRS in brain: N-acetyl aspartate ↓, Myo-inositol ↑, Lactate≈↑, Choline ≈↑ Muscle biopsy: RC activity (N), ATP production (substrates Glu + Mal/Suc) ↓ |
5 | Wibom et al9; Falk et al10; Kavanaugh et al12; Pfeiffer et al11 |
Citrin deficiency (603 859/605814) Citrin; SLC25A13 |
Liver, kidney, small intestine | CTLN2: Hepatic encephalopathy, associated with neuropsychiatric symptoms NICCD: Neonatal intrahepatic cholestasis with or without failure to thrive and dyslipidemia |
Plasma: Ammonia ↑, Citrulline ↑, Arginine ↑, Threonine/Serine ratio ↑, Pancreatic secretory trypsin inhibitor ↑ Liver biopsy: ASS activity↓ |
>100 | Kobayashi et al52; Yasuda et al54; Ohura et al55, 56; etc. |
- Notes: ≈ around normal levels; ↑ increased levels; ↓ decreased levels; (N) normal; CSF cerebrospinal fluid; MRS magnetic resonance spectroscopy; RC respiratory chain; CI complex I; CV complex V; ASS argininosuccinate synthetase; GOT glutamic oxaloacetic transaminase; AGC aspartate glutamate carrier; CTLN2 adult-onset type II citrullinemia; NICCD neonatal intrahepatic cholestasis caused by citrin deficiency.
| Clinical features | HPO ID | Deficiency | |||
|---|---|---|---|---|---|
| MDH1 | MDH2 | GOT2 | AGC1 | ||
| n = 2 | n = 3 | n = 4 | n = 5 | ||
| Failure to thrive | HP:0001508 | 2/3 | 4/4 | ||
| Microcephaly | HP:0000252 | 2/2 | 4/4 | 3/5 | |
| Ocular | |||||
| Strabismus | HP:0000486 | 2/2 | 2/3 | ||
| Retinitis pigmentosa | HP:0008035 | 1/3 | |||
| Gastro-intestinal | |||||
| Constipation | HP:0002019 | 2/3 | 1/5 | ||
| Feeding difficulties | HP:0011968 | 4/4 | |||
| Neurological | |||||
| Developmental | |||||
| Global developmental delay | HP:0001263 | 2/2 | 3/3 | 4/4 | 4/5 |
| Intellectual disability, severe | HP:0010864 | 2/4 | 1/5 | ||
| Intellectual disability, profound | HP:0002187 | 2/4 | |||
| Absent speech | HP:0001344 | 1/2 | 2/3 | 4/4 | 4/5 |
| Epilepsy | |||||
| Epileptic encephalopathy | HP:0200134 | 2/2 | 3/3 | 4/4 | 5/5 |
| Seizures | HP:0001250 | 2/2 | 3/3 | 4/4 | 5/5 |
| Tonus abnormalities | |||||
| Dystonia | HP:0001332 | 2/3 | |||
| Hyporeflexia | HP:0001265 | 1/3 | |||
| Infantile axial hypotonia | HP:0009062 | 1/2 | 3/3 | ||
| Severe muscular hypotonia | HP:0006829 | 4/4 | 5/5 | ||
| Hypertonia | HP:0001276 | 1/2 | 1/5 | ||
| Spastic quadriplegia | HP:0002510 | 2/4 | 1/5 | ||
| Spastic paraparesis | HP:0002313 | 2/4 | |||
| Hyperreflexia | HP:0001347 | 1/2 | 2/4 | 1/5 | |
| Hyporeflexia | HP:0001265 | 1/3 | |||
| Pyramidal signs | HP:0007256 | 2/3 | |||
| Non-ambulatory | HP:0002540 | 2/3 | 3/4 | 3/5 | |
| Movement disorder | |||||
| Dyskinesia | HP:0100660 | 1/3 | |||
| Neuro-imaging (MRI/MRS) | |||||
| Cerebral atrophy | HP:0002059 | 2/3 | 3/4 | 4/4 | |
| Cerebellar atrophy | HP:0001272 | 1/3 | 1/4 | ||
| Delayed myelination | HP:0012448 | 1/3 | 3/4 | ||
| Cerebral hypomyelination | HP:0006808 | 3/4 | |||
| Hypoplasia of the pons | HP:0012110 | 1/2 | |||
| Hypoplasia of corpus callosum | HP:0002079 | 2/2 | 1/3 | 3/4 | 1/4 |
| Inferior vermis hypoplasia | HP:0007068 | 1/2 | 3/4 | ||
| Multicystic encephalomalacia | HP:0040197 | 1/4 | |||
| High myoinositol in brain by MRS | HP:0025460 | 3/3 | |||
| Elevated brain lactate level by MRS | HP:0012707 | 2/3 | 2/4 | ||
| Reduced brain N-acetyl aspartate level by MRS | HP:0012708 | 3/3 | |||
6.1 MDH1 deficiency
Two cousins from a highly consanguineous family were identified with a pathogenic homozygous variant in the NAD+-binding domain of MDH1.6 Both patients presented with an early neurological phenotype of global developmental delay, epilepsy and progressive microcephaly (Tables 1 and 2). In addition, dysmorphic features, such as plagiocephaly, a bulbous nose, deep eyes, micrognathia, and strabismus, were present in both patients. Epilepsy in one of the patients was controlled using antiepileptic medication. Magnetic resonance imaging (MRI) of the brain in one patient revealed partial agenesis of predominantly the splenium of the corpus callosum, prominent ventricles, and mild hypoplasia of the inferior vermis and pons. The other patient's brain MRI showed mild shortening of the corpus callosum and a normal pons and ventricles. In both individuals, amino acids, acylcarnitines, and lactate levels in plasma were normal, as were organic acids in urine. A definite diagnosis was made by exome sequencing. Further biochemical investigations in dried blood spots of the patients revealed increased levels of glutamate and glycerol 3-phosphate, suggesting that glycerol 3-phosphate may be a biomarker for MDH1 deficiency. Additional investigations at the cellular level revealed decreased levels of fumarate and increased levels of aspartate in MDH1 KO HEK293 cells compared to control.
The increased levels of glycerol 3-phosphate in dried blood spots may provide mechanistic insights in MDH1 deficiency. As described above, glycerol 3-phosphate dehydrogenase is a cytosolic enzyme that, similar to MDH1, is part of a NADH redox shuttle to recycle cytosolic NAD+. Although the GP shuttle is less efficient in ATP production than the MAS, it is potentially suitable as a compensatory mechanism for generating cytosolic NAD+ in MDH1 deficiency. As mitochondrial GPD2 is the rate-limiting step of the GP shuttle, increased activity of the GP shuttle may lead to accumulation of G3P in cytosol. In addition, untargeted metabolomics analysis in dried blood spots of the patients revealed increased levels of unsaturated fatty acids, which may also serve as an additional cytosolic NAD+ recycling mechanism.59
Findings from other studies provide supporting or additional biochemical insights in case of a defect in MDH1. These findings include accumulation of aspartate which was found in three different experimental models: after knockdown of MDH1 in PANC-1 cells, in MDH1 KO HEK293 cells and in a MDH1 KO model of Jurkat cells.6, 43, 60 In addition, accumulation of oxaloacetate and decreased levels of malate were reported in MDH1 knockdown PANC-1 cells. Furthermore, a knockdown model of MDH1 in fibroblasts showed a decreased NAD+/NADH ratio.61 These fibroblasts had a senescent phenotype with a low-proliferative state, suggesting a role for decreased MDH1 activity in cellular senescence.61 Secondary consequences of MDH1 deficiency include a decreased ratio between reduced and oxidized glutathione (GSH/GSSG) as was observed in MDH1 KD PANC-1 cells. MDH1 was suggested to play a role in antioxidant defense by supporting NADPH production via malic enzyme.62 All together these findings suggest that MDH1 deficiency could lead to increased oxidative stress by disruption of both NAD+/NADH and NADPH/NADP+ balances.
6.2 MDH2 deficiency
To date, MDH2 deficiency has been reported in three unrelated subjects.7 Patients presented with bi-allelic MDH2 variants as identified by exome sequencing. The phenotypes of these patients were characterized by early-onset generalized hypotonia, psychomotor delay, and refractory epilepsy, accompanied by elevated lactate in the blood and cerebrospinal fluid (Tables 1 and 2). Additional clinical findings shared by two of three patients included failure to thrive, obstinate constipation, dystonia, and strabismus. Epileptic seizure frequency decreased in response to a ketogenic diet in two of three patients. Brain MRI showed nonspecific findings including atrophy of the anterior part of the corpus callosum, delayed myelination of the frontal white matter, and cortical, frontal, and parietal atrophy. Repeat brain MRI in a second patient showed delayed myelination of the genu of the corpus callosum and cortical and subcortical atrophy of the frontal lobes. For the last patient, marked cerebral and cerebellar atrophy were observed. Urinary organic acid analysis revealed increased levels of malate and fumarate in two of three patients, whereas succinate levels were normal in all patients. One of the patients had also increased concentrations of urinary ketone bodies, lactate, pyruvate, and 3-methylglutaconic acid. An important note for diagnostics is that MDH2-individuals can show nearly normal concentrations of urinary organic acids in a non-metabolic decompensation state.
No clear biomarkers were identified in patients with MDH2 deficiency, but additional insights from MDH2 KO cells have revealed increased intracellular levels of malate and fumarate.63 High concentrations of malate and fumarate can trigger an hypoxia response.64 Eventually, this can lead to inactivation of the PDH complex, pyruvate accumulation in the cytosol, and consequently increased lactate formation.65 Another consequence of the hypoxia response is an increase in glutamine catabolism that feeds in the 2-oxoglutarate pool.66 When mitochondrial pyruvate import is impaired, TCA cycle activity can be maintained via oxidative glutamine metabolism, as glutamine-derived malate can be converted into pyruvate via malic enzyme 2.67 Furthermore, glutamine may also undergo reductive carboxylation to support fatty acid biosynthesis. To this end, glutamine-derived 2-oxoglutarate is then carboxylated by the mitochondrial NADP-linked enzyme isocitrate dehydrogenase (IDH2) to produce isocitrate, which is, after conversion into citrate, exported from the mitochondrial matrix into the cytosol where citrate can be cleaved into both oxaloacetate and acetyl-CoA via the cytosolic enzyme citrate lyase.67 Similar to MDH1, activation of MDH2 also indirectly supports fatty acid biosynthesis in adipocytes by increasing intracellular NADPH levels via malic enzyme.68 Thus, these findings suggest that MDH2 deficiency might affect MAS and TCA cycle substrates, but also glutathione dependent antioxidant defense via decreased NADPH and glutamine levels.
6.3 GOT2 deficiency
Four patients with GOT2 deficiency from three independent families have been reported.8 Homozygous and compound heterozygous GOT2 variants were identified using exome sequencing. All patients presented with similar clinical features including progressive microcephaly, epileptic encephalopathy, and failure to thrive (Tables 1 and 2). Additional phenotypic features included feeding difficulties, hypotonia, frequent infections, intellectual and motor disabilities, such as spastic paraparesis and severe spastic quadriplegia. MRI brain scan showed multicystic encephalomalacia and wide cerebral atrophy for one of the patients. In addition, two of four patients revealed mild cerebral atrophy with a hypoplastic vermis and a thin corpus callosum. Brain MRI in the last patient showed (mainly frontoparietal) cerebral atrophy, asymmetric dilated lateral ventricles, hypoplastic vermis, and hypoplasia of the corpus callosum. Biochemically, patients had high plasma lactate and hyperammonemia. All patients had normal urinary organic acids and plasma acylcarnitines. In addition, amino acids in plasma were normal in three of the four patients. Low serine levels and high citrulline levels were found in one of the patients. This finding was followed up by additional functional analysis of serine biosynthesis in patients' fibroblasts and GOT2 KO HEK293 cells. These studies revealed that de novo serine biosynthesis was impaired which prompted institution of a new therapeutic option involving serine supplementation in two patients. In addition, these patients were given pyridoxine supplementation to boost residual enzyme activity. The epilepsy and overall neurodevelopmental status in these patients were serine and pyridoxine responsive.
Impaired de novo serine biosynthesis may provide mechanistic insights in GOT2 deficiency. Since the first step of serine synthesis is catalyzed by the NAD+-dependent enzyme 3-phosphoglycerate dehydrogenase, a secondary serine synthesis defect may occur as a consequence of cytosolic NAD+/NADH imbalance. The notion that GOT2 deficiency can cause an overall deficiency in the MAS and thereby decrease the cytosolic NAD+/NADH ratio is supported by a GOT2 knockdown model in pancreatic cancer cells that had impaired net transfer of cytosolic NADH into mitochondria.69 The addition of glycerol in cultured GOT2 KO HEK293 cells, as an attempt to restore the NAD+/NADH balance through the glycerol 3-phosphate shuttle, was not sufficient to correct the impaired serine biosynthesis.8 This suggests that the MAS is the predominant NADH shuttle in HEK293 cells. This is in line with the finding that glycerol 3 phosphate was not increased in MDH1 HEK293 cells.6 Interestingly, pyruvate supplementation restored serine biosynthesis in vitro,8 presumably by correcting the NAD+/NADH balance. However, since increasing pyruvate may result in increased lactate formation this may not be suitable for treatment. To circumvent glycolytic NADH production, and consequently lactate accumulation in the cytosol, the authors suggested a diet low in carbohydrates, high in fat, and supplementation with ketone bodies.
Hyperammonemia and hypercitrullinemia in patients with a GOT2 deficiency point to a secondary defect of the urea cycle. In GOT2 deficiency, mitochondrial aspartate production from oxaloacetate is decreased, as supported by findings in GOT2 knockdown cells.70 GOT2 is closely linked to the urea cycle via the production of aspartate, which reacts with citrulline to form argininosuccinate. A lack of aspartate will lead to accumulation of citrulline and dysfunction of the urea cycle. Thus, the hyperammonemia and hypercitrullinemia as observed in GOT2 deficient patients can be explained by a deficient aspartate provision to the cytosol. Hyperammonemia may also have consequences for the TCA cycle, since glutamate plays an important role in ammonia detoxification by forming glutamine.71 The high levels of ammonia can thereby deplete TCA cycle intermediates by withdrawing 2-oxoglutarate for the formation of glutamate and consequently glutamine. This can contribute to dysregulation of the TCA cycle. Thus, a deficiency in GOT2 may affect redox homeostasis, MAS substrates, the urea cycle as well as the TCA cycle.
6.4 AGC1 (Aralar/SLC25A12) deficiency
AGC1 deficiency is also known as Aralar deficiency. Five patients from four unrelated families with AGC1 deficiency have been reported. The phenotype of the first three reported patients, including two siblings, is characterized by severe infantile-onset encephalopathy with epilepsy, global developmental delay, generalized hypotonia, and abnormal myelination, accompanied by reduced cerebral N-acetyl aspartate (NAA) content (Tables 1 and 2).9, 10 In addition, two of three patients had frequent infections. A fourth patient presented with a similar phenotype, with the exception that this patient had normal myelination and absence of microcephaly or dysmorphic features.11 MRI findings included cerebral volume loss, delayed myelination, bilateral symmetric abnormal signal in the putamina,9 global lack of myelination in the cerebral hemispheres, and a slightly smaller than normal putamen and globus pallidus.10 The cerebellum, brainstem, and thalami were essentially normal. Lactate was reported normal to mildly elevated in these four patients. A fifth patient was identified with compound heterozygous variants at the age of 12.12 The clinical phenotype of this patient was characterized by small stature, microcephaly, dysmorphic features, early-onset global developmental delay, severe intellectual disability (non-verbal), hypotonia, epilepsy, nonambulatory spastic quadriplegia albeit a happy disposition. Neuroimaging findings indicated cerebral atrophy with early hypomyelination, but were more consistent with a leukodystrophy of the leuko-axonopathy category. A lactate peak was found upon MRS, which may have reflected the ongoing seizures at that time. Seizures of all patients were successfully controlled with medication. The oldest patient was free of seizures between the age of 3 and 12, without medical seizure control.12
For the first reported patient treatment with a ketogenic diet was published, reporting improved psychomotor development and eye contact as well as increased alertness.9, 72 After 20 months on the diet, the patient remained seizure free and neuro-imaging showed increased brain volume, as well as improved myelination. Subsequently, another patient was published in whom ketogenic diet was successful in terms of seizure control.11 These reports suggest the ketogenic diet as a viable therapeutic option for AGC1 deficiency.55
Recently, an alternative to a ketogenic diet was explored in a mouse model, by administrating β-hydroxybutyrate (B-OHB), the main metabolic product in a ketogenic diet.73 B-OHB was able to boost aspartate and NAA synthesis in neurons and myelin proteins in brain from the Aralar-KO mice. NAA is produced from aspartate and acetyl-CoA by the enzyme aspartate-N-acetyltransferase.74 As the B-OHB dependent recovery of aspartate and NAA did not require lowering of carbohydrates, this treatment may provide an alternative therapy and deserves further investigation.
At first, reduced levels of neuronal-generated NAA have been proposed to contribute to the observed hypomyelination in AGC1 deficiency,9, 10, 72 in which NAA would be a precursor for myelin lipid synthesis. However, one of the patients had normal myelination.11 In addition, in one of the reports serial MRI findings were less characteristic of a primary or permanent hypomyelinating disorder, pointing to AGC1 deficiency as a leukodystrophy of the leuko-axonapathy category.12 Therefore, the observed hypomyelination was later suggested to be secondary to neuronal dysfunction, which argues against AGC1 deficiency as a primary hypomyelinating disease.75 This conclusion was supported by the results of studies in mice which showed that the degeneration of neuronal processes in AGC1 deficiency occurred independent of hypomyelination.76 Moreover, an increase in aspartate and NAA after B-OHB administration in Aralar-KO mice was also not associated with myelin recovery.73 In addition, studies in mice in which both the NAA synthesizing enzyme (NAT8L) as well as the NAA hydrolyzing enzyme (aspartoacylase, ASPA) were inactivated, revealed no abnormalities in myelination, despite undetectable NAA levels.77
Another interesting observation is the variable presence of lactic acidemia in the reported cases. A proposed mechanism for lactic acidemia in patients with AGC1 deficiency is a secondary form of mitochondrial dysfunction, due to the inability of the MAS to transfer reducing equivalents across the mitochondrial membrane. A drastic decrease in respiration was found in AGC1 deficient mouse brain tissue when using malate plus glutamate as substrate.53 Still, 12% of residual activity persisted, which may be explained by the presence of other glutamate carriers (GC1, GC2), which also transport glutamate into mitochondria for respiration. In line with this, analysis of brain extracts from AGC1-deficient mice revealed a striking 86% decrease in the levels of aspartate, but only a modest 25% decrease in glutamate.78 Interestingly, the most prominent drop in amino acid concentrations was observed for glutamine and serine with decreases of 77% and 75% compared to control, respectively. This may suggest that AGC1 deficiency, similar to GOT2 deficiency, may be associated with a secondary serine synthesis deficiency as a consequence of a dysfunctional MAS and a cytosolic NAD+/NADH imbalance.
6.5 AGC2 (Citrin/SLC25A13) deficiency
AGC2 or citrin deficiency is the most extensively described and studied MAS deficiency (Table 1). Over hundred patients have been reported, often in East Asia.79, 80 AGC2 deficiency can manifest in several stages of life, dependent on age. In newborns or infants, it can present as neonatal intrahepatic cholestasis (citrin deficiency, NICCD), in older children as failure to thrive and dyslipidemia (citrin deficiency, FTTDCD), and in adults as recurrent hyperammonemia with neuropsychiatric symptoms in citrullinemia type II (CTLN2).
NICCD was discovered in neonates suffering from cholestasis and multiple amino acidemia including citrulline, threonine, methionine, lysine, arginine, tyrosine, serine, and phenylalanine.55, 81 Some of these may be detected in newborn screening, including citrulline, tyrosine, methionine, and phenylalanine, and a decreased ratio of alanine/citrulline.82 Other biochemical abnormalities were hypoproteinemia, galactosemia, and hypoglycemia.55, 81, 83, 84 Further symptoms include diffuse fatty liver with hepatomegaly and parenchymal cellular infiltration with hepatic fibrosis (cirrhosis), decreased coagulation factors (echinocytosis), elevated bilirubin (bilirubinemia), hemolytic anemia, and variable liver dysfunction. Most patients showed clinical improvement between 6 and 12 months upon treatment with a diet containing fat-soluble vitamin supplementation, lactose-free therapeutic formulas, and/or medium-chain triglyceride enriched therapeutic formulas.56, 85 However, some may develop cirrhosis or severe infections, or may later develop symptoms of adult-onset citrin deficiency.79, 83
FTTDCD has been proposed as a post-NICCD phenotype before the onset of CTLN2.86 Some children were found to have laboratory and/or clinical abnormalities after NICCD. Clinical symptoms included growth restriction, hypoglycemia, pancreatitis, but also severe fatigue and impaired quality of life.87 Laboratory abnormalities included dyslipidemia manifesting as higher levels of triglyceride and total- and LDL-cholesterols, but lower levels of HDL-cholesterol.86 Other findings were an increased lactate-to pyruvate ratio, higher levels of urinary oxidative stress markers, and marked abnormalities in tricarboxylic acid cycle metabolites.88, 89 Often FTTDCD is characterized by the patient's preference for protein-rich and/or lipid-rich foods and aversion to carbohydrate-rich foods.
CTLN2 patients also have a particular preference for protein-rich and/or lipid-rich foods, such as beans and peanuts.79 Clinical symptoms of CTLN2 patients are characterized by recurring episodes of hyperammonemia and neurologic and psychotic symptoms that closely resemble those of hepatic encephalopathy or genetic urea cycle disorders, including nocturnal delirium, aberrant behaviors (aggression, irritability, and hyperactivity), delusions, disorientation, restlessness, drowsiness, loss of memory, flapping tremor, convulsive seizures, and coma.79 In general, the onset is sudden, usually between the ages of 20 and 40. The age of patients diagnosed varies from 11 to 70 years with a mean age of 34.4 years.54 Although prognosis is bad, liver transplantation is the most effective therapy, preventing episodic hyperammonemia and eliminating the preference for protein-rich foods.90-92 Pancreatitis, hyperlipidemia, and hepatoma are major complications of CTLN2.91 Biochemical abnormalities of CTLN2 patients include citrullinemia, abnormal liver enzymes, low albumin, increased serum triglycerides, and decreased activity of argininosuccinate synthetase (ASS) in the liver (however, normal levels of ASS1 in other tissues).
Since the major function of the AGC is to supply aspartate to the cytosol, AGC2 deficiency causes a deficiency of cytosolic aspartate in the liver. A lack of aspartate limits synthesis of proteins and nucleotides, resulting in hypoproteinemia in NICCD. In addition, lack of aspartate for the ASS reaction causes accumulation of citrulline and eventually hyperammonemia.79 Beans and peanuts are the most prominent dietary sources of aspartate and asparagine, which might be the reason why CTLN2 patients show extraordinary liking for beans and peanuts. Administration of sodium pyruvate with l-arginine proved effective in treatment of a 13- and 73-year-old citrin-deficient patient.93, 94 Arginine was shown to be effective in lowering blood ammonia in hyperammonaemic patients, potentially via urea cycle enzymes.95 Administration of sodium pyruvate may increase the cytosolic NAD+/NADH ratio, which activates ureagenesis, as demonstrated in mice.96
Komatsu et al. hypothesized that the hepatic steatosis observed in patients with CTLN2 may result from a compensatory upregulation of the citrate-malate shuttle. This shuttle catalyzes the net transfer of acetyl-CoA from mitochondria to the cytosol, to promote fatty acid synthesis.23 In addition to the transfer of acetyl-CoA, the pathway regenerates cytosolic NAD+ via MDH1.62 An increase in cytosolic citrate and acetyl-CoA may result in an overproduction of fatty acids in hepatocytes and contribute to hyperlipidemia.97 In addition, since the glycerol 3-phosphate shuttle exerts low expression in liver, glycerol and glycerol 3-phosphate accumulation can also contribute to hyperlipidemia. Increased levels of urine glycerol and glycerol 3-phosphate have been observed in patients with FTTDCD.98
7 DISCUSSION
The current review presents an overview of clinical and biochemical aspects of the known genetic MAS deficiencies. Due to the expression pattern of the different MAS components, most of the MAS deficiencies affect the central nervous system and present with a neurological phenotype. An exception is AGC2 deficiency, which presents with a primary hepatic phenotype due to its liver-specific expression. MAS deficiencies with a neurological phenotype include MDH1, MDH2, GOT2, and AGC1. Common clinical features in these disorders are epilepsy, hypotonia, and global developmental delay (Table 2). In addition, MDH1, GOT2, and AGC1 deficiencies present with microcephaly. Common MRI findings include an affected corpus callosum in MDH1, MDH2, and GOT2 deficiencies. The most striking finding in AGC1 deficiency is delayed or hypomyelination.
As one of the major roles of the MAS is to maintain redox homeostasis, a dysfunctional MAS will result in a disturbed NAD+/NADH-redox balance. Indeed, in all reported MAS deficiencies a disturbed redox balance is reflected by different flux through other redox dependent pathways. These pathways include the glycerol 3-phosphate shuttle and the conversion of pyruvate to lactate, which are both directly connected to the cytosolic NAD+/NADH balance.37, 46 Increased activities of glycerol phosphate dehydrogenase and lactate dehydrogenase result in regeneration of NAD+ (Figure 2). Interestingly, both in MDH1 and AGC1 deficiencies, lactate was normal to slightly increased in plasma. In MDH1 deficiency, glycerol 3-phosphate shuttle activity appears increased, as glycerol 3-phosphate levels were high in blood (Table 1).6 In AGC1 deficiency, increased activity of the glycerol 3 phosphate shuttle has also been suggested,99 especially since a clear increase in lactate production is lacking. Since astrocytes in the brain lack AGC1,78, 100 it is postulated that the glycerol phosphate shuttle is the main NADH redox shuttle in astrocytes. Moreover, the lack of or only slight increase in lactate may be explained by the fact that mainly the unaffected astrocytes are responsible for lactate production.99
In contrast to the presumed absence of increased lactate levels in MDH1 and AGC1 deficiencies, patients with MDH2 and GOT2 deficiency present with a clear lactic acidosis (Table 1). Both MDH2 and GOT2 enzymes are located in the mitochondria and are functionally coupled to the TCA cycle and ATP production, thus, defects in these enzymes can result in overall mitochondrial dysfunction. Mitochondrial dysfunction is often partly compensated by increased glycolytic ATP production, leading to increased conversion of glucose to pyruvate as glycolytic end-point, with secondary conversion of pyruvate to lactate. Supporting this notion, high levels of urinary pyruvate were observed in MDH2 deficiency. However, GOT2 deficiency appears to result in low levels of pyruvate, as observed in KO HEK293 cells. This may indicate disturbed glycolysis in GOT2 deficiency as an additional consequence of redox imbalance, the enzyme GAPDH being dependent on NAD+/NADH ratio. Although overall glycolytic flux may be decreased in GOT2 deficiency, high levels of lactate are likely the result of increased pyruvate to lactate conversion due to increased NADH levels in the cytosol.
Disturbed redox homeostasis also affects other NAD(H)-dependent metabolic pathways like serine biosynthesis (Figure 2). This has been experimentally demonstrated to occur in GOT2 deficiency,8 and low serine levels were found in brains of AGC1 deficient mice.78 Serine is a nonessential amino acid and a precursor of essential compounds including phosphatidylserine, sphingomyelin, glycine, and d-serine. Genetic defects in serine biosynthesis illustrate that this pathway is the main source of serine in the brain, as dietary sources are typically not sufficient to compensate for these defects.101 Interestingly, defects in any of the three enzymes involved in the de novo serine biosynthetic pathway mainly present with a neurological phenotype. Clinical characteristics include microcephaly, developmental delay, irritability, feeding difficulties, poor psychomotor development, spastic tetraplegia, epilepsy, nystagmus, brain atrophy, and hypomyelination. The diseases are biochemically characterized by low serine and glycine in CSF and blood in fasting state.102 Many of the clinical characteristics overlap with the clinical phenotype of MAS deficiencies, including microcephaly, epilepsy, developmental delay, poor psychomotor development, and hypomyelination (Table 2). This may support the notion that part of the clinical phenotype is due to a secondary serine biosynthesis defect, especially as oral l-serine supplementation ameliorated the neurologic phenotype in GOT2 deficiency.
Next to the expected disturbed redox homeostasis, a dysfunctional MAS also affects aspartate and glutamate. As demonstrated in AGC1 deficiency, lack of cytosolic aspartate leads to a reduction of N-acetylaspartate and subsequently cerebral hypomyelination may occur. As GOT2 is responsible for aspartate production, N-acetylaspartate may also be low in this defect, but was not reported in GOT2 deficient patients. In AGC2 deficiency, similar to GOT2 deficiency, a lack of cytosolic aspartate leads to substrate reduction for the urea cycle. In both deficiencies, aspartate cannot react with citrulline in the urea cycle, causing accumulation of citrulline (Table 1). In addition, impaired activity of the urea cycle to dispose toxic ammonia leads to hyperammonemia. As MAS activity is required for aspartate synthesis by GOT2, MAS activity is also necessary for glutamate synthesis by GOT1 in the brain.4, 5 A deficiency in MAS can therefore affect the availability of glutamate for neurotransmission, potentially contributing to the epileptic phenotype seen in these deficiencies.103
A viable option for treatment of MAS deficiencies is provided by a ketogenic diet, which was shown to be effective in MDH2 and AGC1 deficiency.7, 11, 72 MAS deficiencies lead to inefficient use of glucose by neurons, since the decreased NAD+/NADH ratio prevents pyruvate from entering the TCA cycle by reducing it to lactate. A ketogenic diet contains ketone bodies that serve as an alternative to glucose as fuel for the brain. Therefore, a ketogenic diet can bypass the NAD+ dependent glycolytic metabolic pathways, and may be a beneficial therapeutic option in MAS deficiencies. Interestingly, serine supplementation might also ameliorate some of the neurological phenotypes in MAS deficiencies, as proven to be effective in GOT2 deficiency. In addition, vitamin B6 supplementation is advocated in GOT2 deficiency, as the residual enzyme activity might deplete other vitamin B6-dependent enzymes from their cofactor.
Although a ketogenic diet can bypass NAD+-dependent glycolytic metabolic pathways, a recent report demonstrated a novel approach to alleviate the intracellular redox balance.104 A well-known method in vitro to correct the intracellular NAD+/NADH redox imbalance is the conversion of pyruvate into lactate; however, this would not work well in patients as this would lead to an even further increase in lactate levels.8 Hence, a fusion protein of bacterial lactate oxidase and catalase was constructed, LOXCAT, which was able to convert lactate and oxygen into pyruvate and water.104 As proof-of-principle they demonstrated that the lactate/pyruvate ratio in medium could be lowered, hence intra-cellularly. In addition, LOXCAT had alleviated intracellular NAD+/NADH redox imbalance in heart and brain of mice with metformin induced complex I inhibition. In the context of MAS deficiencies, which have a low cytosolic NAD+/NADH ratio, this invention holds great promise.105
Important to note is that, since the MAS is essential for the brain, most reported deficiencies are hypomorphic in nature with residual enzyme activity. It is expected that complete inactivity of the MAS components are lethal in humans, as demonstrated by homozygous knockouts of MDH1, MDH2, GOT1, GOT2 in mice.8, 106, 107 For GOT1 and OGC, no deficiencies in humans have been reported yet. However, in the older order Amish population, a rare in-frame 3 bp deletion in GOT1 that was strongly associated with low serum aspartate aminotransferase activity was observed in heterozygous state. Functional studies showed that the mutant recombinant protein had no enzyme activity. No individuals with homozygosity for this deletion were found, suggesting that complete loss of function of GOT1 is indeed not compatible with life.108 Expected biochemical effects of GOT1 deficiency could be extrapolated from knockout and knockdown studies in cells, in which increased levels of aspartate were reported.43, 70 Allelic variants of OGC have been identified in paragangliomas, in which tumor tissue showed absence of the protein.109 Interestingly, loss of MDH2 was also associated with pheochromocytoma and paraganglioma.63 Knockdown of SLC25A11 in mouse chromaffin cells showed low 2-oxoglutarate and increased aspartate and glutamate. The cells acquired metastatic properties, which suggested that SLC25A11 can act as a tumor-suppressor gene.109 Other expected biochemical effects of OGC deficiency could be extrapolated from SLC25A11 knockdown in A549 cells, in which levels of fumarate, malate, citrate, NADH, and ATP were decreased.110
8 CONCLUSION
The current review provides insights into the biochemical and clinical characteristics of MAS deficiencies. Many of these deficiencies have no specific markers to support clinical diagnosis. The disturbed redox homeostasis in MAS deficiencies affects multiple redox dependent pathways, with complex consequences due to compartment- and tissue-specific expression of the MAS components. In general, biochemical clues for MAS deficiencies include high lactate, high glycerol 3-phosphate, a disturbed redox balance, TCA abnormalities, high ammonia, and low serine. Although not specific, these profiles may narrow down the long differential diagnosis of possible IMDs causing infantile epileptic encephalopathies.111 Interestingly, implications for treatment of MAS deficiencies are offered by the study of GOT2 deficiency, in which it was demonstrated that a cytosolic redox imbalance leads to a secondary serine biosynthesis defect and serine supplementation exerted a significant clinical benefit. Other treatment possibilities may include vitamin B6 supplementation (cofactor for GOT2) and a ketogenic diet. Pyruvate supplementation or other redox-correcting supplements may be interesting potential treatments for future research. All together, these IMD of MAS expands the list of treatable intellectual disabilities.112 Future studies are needed to reveal more specific biomarkers and to obtain more insights in the pathophysiological mechanisms of MAS deficiencies.
ACKNOWLEDGMENT
This work was supported by Metakids (2017-075 to Judith J. M. Jans). This article does not contain any studies with human or animal subjects performed by any of the authors.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




