Genotype and residual enzyme activity in medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: Are predictions possible?
Abstract
Medium-chain acyl-CoA dehydrogenase deficiency (MCADD) is the most common defect of mitochondrial β-oxidation. Confirmation diagnostics after newborn screening (NBS) can be performed either by enzyme testing and/or by sequencing of the ACADM gene. Here, we report the results from enzyme testing in lymphocytes with gene variants from molecular analysis of the ACADM gene and with the initial acylcarnitine concentrations in the NBS sample. From April 2013 to August 2019, in 388 individuals with characteristic acylcarnitine profiles suggestive of MCADD the octanoyl-CoA-oxidation was measured in lymphocytes. In those individuals with residual activities <50%, molecular genetic analysis of the ACADM gene was performed. In 50% of the samples (195/388), MCADD with a residual activity ranging from 0% to 30% was confirmed. Forty-five percent of the samples (172/388) showed a residual activity >35% excluding MCADD. In the remaining 21 individuals, MCAD residual activity ranged from 30% to 35%. The latter group comprised both heterozygous carriers and individuals carrying two gene variants on different alleles. Twenty new variants could be identified and functionally classified based on their effect on enzyme function. C6 and C8 acylcarnitine species in NBS correlated with MCAD activity and disease severity. MCADD was only confirmed in half of the cases referred suggesting a higher false positive rate than expected. Measurement of the enzyme function in lymphocytes allowed fast confirmation diagnostics and clear determination of the pathogenicity of new gene variants. There is a clear correlation between genotype and enzyme function underlining the reproducibility of the functional measurement in vitro.
Abbreviations
-
- MCAD
-
- medium-chain acyl-CoA dehydrogenase
-
- MCADD
-
- medium-chain acyl-CoA dehydrogenase deficiency
-
- NBS
-
- newborn screening
-
- VLCAD
-
- very long-chain acyl-CoA dehydrogenase
1 INTRODUCTION
Medium-chain acyl-CoA dehydrogenase (MCAD; EC1.3.8.7) catalyzes the first oxidation step of medium-chain fatty acids in the mitochondrial β-oxidation pathway. MCAD deficiency (MCADD, OMIM #201450) is the most common autosomal recessive disorder of fatty acid oxidation with an incidence between 1:4900 and 1:24900 newborns in Europe and North America.1 Patients with MCADD are prone to metabolic decompensation usually triggered by catabolic stress such as prolonged fasting or febrile illnesses due to unmet energy supply by β-oxidation. Symptoms of metabolic decompensations comprise hypoketotic hypoglycemia, muscular hypotonia, impaired vigilance, Reye-like encephalopathy, coma, and death.2-5 The inclusion of this disease in neonatal screening programs in many countries worldwide has dramatically improved the clinical outcome and reduced the mortality since patients can usually be identified before the onset of symptoms.1, 6, 7 Elevation of the following acylcarnitine concentrations and ratios is considered suggestive of MCADD: C6, C8, C10, C10:1 as well as the ratios C8/C2, C8/C6, C8/C10, C8/C12, C8/C12, and C8/C16.8, 9 Urinary excretion of hexanoylglycine, isohexanoylglycine, suberylglycine, and phenylpropionylglycine are indicative of MCADD as well, although confirmed patients lacking these metabolites in urine have also been reported.10-13 Confirmatory diagnosis of MCADD can be performed by molecular testing; however, the identification of one single or novel variant of unknown significance often leads to inconclusive results.4 Enzyme testing in lymphocytes, by measuring the oxidation rate of octanoyl-CoA or phenylpropionyl-CoA, is a faster and more efficient method for the confirmation of patients.14-16 There is evidence that residual enzyme function correlates with the expected phenotype. Different biallelic and monoallelic variants in the ACADM gene are associated with variable reductions of cellular enzyme function and subsequently differences in the risk of metabolic decompensation.14-16 However, it remains often difficult to evaluate at which level of residual enzyme function there is a risk of a metabolic derangement as after the “diagnosis” has been made it is impossible not to treat. Despite the diagnosis, the clinical relevance of mild MCADD has remained uncertain.
In this study, we present data from confirmation diagnostics in MCAD patients performed from April 2013 to August 2019 in the Laboratory of Clinical Biochemistry and Metabolism at the Center for Pediatrics and Adolescent Medicine of Freiburg. In addition to the genotype and residual enzyme function we included initial disease-specific acylcarnitine concentrations from newborn screening (NBS).
2 METHODS
Blood from 460 newborns with a positive NBS result suggestive of MCADD from Germany, Canada, and Italy was referred to our laboratory for confirmation diagnostics during the period from April 2013 to August 2019. In 171 out of 460 samples, we performed enzyme testing and molecular genetic analysis. Two hundred seventeen out of 460 samples received MCAD enzyme testing only, and in 72/460 samples we performed exclusively mutation analysis of the ACADM gene. Enzymatic data are available of 388 individuals. Results of enzyme testing were available between two and seven working days from arrival of the blood sample, with a median value of 2.5 days. The values described to exclude or confirm diagnosis were developed based on previous data.15 The defined reference values for discrimination of patients, carriers, and healthy individuals are described in the results section. Individuals with enzyme activities <30% were only defined as “patients” if two sequence variants on ACADM gene were found; otherwise they were classified as healthy heterozygous carriers.
We performed enzyme testing in lymphocytes and mutation analysis, with informed consent of the parents. The study was performed according to the rules of the Declaration of Helsinki and approved under study number 219/19 by the ethical review board of Albert-Ludwig University of Freiburg. Whenever the initial acylcarnitine profiles were available, data were included in the study (Supplementary Table 1).
2.1 Isolation of lymphocytes and octanoyl-CoA oxidation
The measurement of MCAD residual activity was achieved in lymphocytes by assaying the oxidation rate of octanoyl-CoA. Lymphocytes were isolated from 1 to 2 mL EDTA blood using Ficoll-PaqueTM Plus (Amersham Bioscience, Freiburg, Germany) and Leucosep H (Greiner Bio One, Solingen, Germany) tubes as described previously17 and stored at −80°C. Octanoyl-CoA oxidation rate was measured as described previously15 with minor modifications. The turnover rate of octanoyl-CoA was calculated using the sum of the peak area of the products C8:1-CoA and C8:OH-CoA and compared with the peak area of the substrate C8:0-CoA set at an initial concentration of 20 nmol. All tests were run in triplicate in the presence of two negative controls chosen randomly from our pool. In order to confirm lymphocyte quality the measurement of very long-chain acyl-CoA dehydrogenase (VLCAD) function was measured by the oxidation rate of palmitoyl-CoA as described previously.18 The reaction products C8:1-CoA and C8:OH-CoA and C8:0-CoA were identified by high-performance liquid chromatography (HPLC) followed by UV-detection. A Phenomenex C18 Kinetex column (5 μm, 100A, 150 × 4.6 mm) was used (Phenomenex, Aschaffenburg, Germany). For the separation of the metabolites, two mobile phases were applied, 25 mM KH2PO4 pH 6,9 and acetonitrile (ACN) with the following elution gradient maintained at 30°C: 0 to 4 minutes (10%-30% ACN), 4 to 8 minutes (30% ACN), 8 to 10 minutes (30%-80% ACN), 10 to 11 minutes (80% ACN), and 11 to 13 minutes (80%-10% ACN). The injection volume was 5 μL at a flow rate of 2 mL/min. The CoA-esters were detected at 254 nm. The interday variation was 4.3%. For practical reasons, it was not possible to measure the intraday value. As no external quality assessment programs exist so far for the MCAD enzyme testing, known samples from healthy controls and patients are retested regularly to ensure the quality of our results.
2.2 Mutation analysis
When requested, we performed also mutation analysis. Genomic DNA was extracted from EDTA blood and dried blood spots with the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany) and with the Dried Blood spot DNA Isolation Kit (Norgen Biotek Corp, Ontario, Canada). All exons and part of the flanking intronic regions of the ACADM gene were amplified by PCR and analyzed by Sanger fluorescent sequencing. The amplified sequences were analyzed using the SeqPilot software analysis (JSI Medical Sytems GmbH, Ettenheim, Germany). Single base pair variants of ACADM sequence (NM_000016.4) were assessed for pathogenicity by performing different in silico analyses using the Alamut Visual 2 software (http://www.interactive-biosoftware.com/alamut-visual/) that summarizes the results from four different tools covering different functional aspects (SIFT, https://sift.bii.a-star.edu.sg/; Mutation Taster, http://www.mutationtaster.org/; PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/; and Align GVGD, http://agvgd.hci.utah.edu/). All discovered variants were compared to data of The Human Gene Mutation Database professional version (http://www.hgmd.cf.ac.uk/ac/index.php) and the corresponding literature links. In the absence of variants or in case of heterozygosity in our samples, we performed additionally a multiplex ligation-dependent probe amplification (MLPA) analysis to exclude the presence of large deletions/duplications.
2.3 Statistical analysis
Graphs, statistical evaluation of the enzyme activities, and Spearman r correlation of genotype-enzyme activities as well as of aclycarnitine species and ratios were performed using GraphPad Prism 8 (La Jolla, California). Enzymatic data are presented as means ± SD.
3 RESULTS
Despite a positive NBS suggestive for MCADD, only in 50% of the individuals (196 out of 388) MCADD was confirmed with a residual activity ranging from 0% to 30% (Figure 1A). In 21 out of 388 individuals, we measured an MCAD residual activity of 30% to 35%. Approximatively one-fourth of the individuals displayed a residual activity of 35% to 50% (88/388). In 84 out of 388, residual activity was >50% (Figure 1A). These data are suggestive of a high false positive rate in NBS also for MCADD.
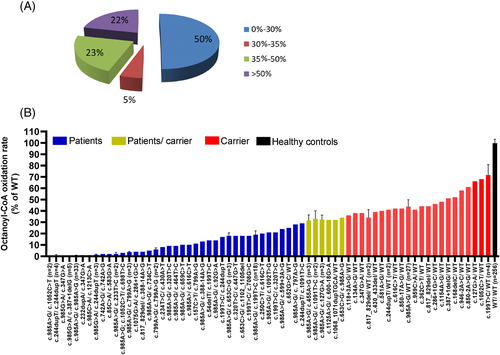
3.1 Correlation of gene variants with residual enzyme activity
The genotype of all sequenced patients identified by NBS is shown in Supplementary Table 1. Patients are listed in the order of the measured residual activity expressed as octanoyl-CoA oxidation rate. A total of 170 patients have been sequenced. In this cohort, 110 patients with a residual activity up to about 30% carried variants either in homozygous or compound heterozygous form confirming MCADD. We could observe a good correlation between genotype and enzyme function in that identical genotypes led to similar residual activities (Figure 1B; P < .0001). In particular, the most common mutation c.985G>A, found in 33 patients (patients #1-17, 28-42, and 48) in homozygosity was associated with a residual activity of 0.52% ± 0.44%, in line with previous reports of enzymatic measurements either with octanoyl-CoA or phenylpropionyl-CoA as substrate.15, 16 A further correlation could be observed also for patients #54 and #58 who carried the sequence variant c.985G>A in compound heterozygosity with the gene variant c.799A>G. In these cases, the residual activity was 3.67% ± 0.47% (Figure 1B). Samples from patients carrying the homozygous mutation c.799A>G (patients #92 and #100) resulted in a residual activity of 6% ± 1%. When c.985G>A was associated in compound heterozygosity with the known mild gene variant c.199T>C,8 the residual activity increased up to 18.89% ± 2.66% (Figure 1B). Several patients with residual activities in a range between 25% and 36% were either homozygous or compound heterozygous for variants in the ACADM gene. This group included five patients carrying five newly identified variants, namely c.652G>C, c.1068_1071dupTGCA, c.455A>G, c.653C>A, and c.1091T>C (Table 1). In case of the identification of variants in compound heterozygosity with c.985A>G, the measured residual activities allowed the classification of this newly identified mutation as being mild. All other sequenced individuals were heterozygotes and the variants were classified as severe (Table 1). Twenty-eight patients were heterozygous for the common mutation c.985G>A, and showed a residual activity of 44.25% ± 3.9%.
| Individual Nra | Allel 1 | Predicted aminoacid change | Exon | Allel 2 | Predicted aminoacid change 2 | Exon | Residual activity | Severityb |
|---|---|---|---|---|---|---|---|---|
| 1 | c.232dupA | p.(Ile78Asnfs*2) | 5 | c.347G>A | p.(Cys116Thr) | 5 | 1 | Severe |
| 2 | c.985G>A | p.(Lys329Glu) | 11 | c.1213C>A | p.(Gln405Lys) | 12 | 1 | Severe |
| 3 | c.85C>A | p.(Arg29Arg) | 2 | c.583G>A | p.(Gly195Arg) | 7 | 2 | Severe |
| 4 | c.1075G>A | p.(Ala359Thr) | 11 | c.286+1G>C | 4 | 4 | Severe | |
| 5 | c.234T>G | p.(Ile78Met) | 5 | c.430A>T | p.(Lys144*) | 6 | 8 | Both severe |
| 6 | c.659C>T | p.(Thr220Ile) | 8 | c.985G>A | p.(Lys329Glu) | 11 | 10 | Severe |
| 7 | c.54delT | p.(His19Ilefs*17) | 2 | c.199T>C | p.(Tys67His) | 3 | 14 | Severe |
| 8 | c.199T>C | p.(Tys67His) | 3 | c.708G>C | p.(Lys239Asn) | 8 | 18 | Severe |
| 9 | c.985G>A | p.(Lys329Glu) | 11 | c.1092T>G | p.(Ile364Met) | 11 | 21 | Mild |
| 10 | c.652G>C | p.(Ala218Pro) | 8 | WTa | 25 | Severea | ||
| 11 | c.455A>G | p.(Lys152Gly) | 6 | c.985G>A | p.(Lys329Glu) | 11 | 28 | Mild |
| 12 | c.1068_1071dupTGCA | p.(Lys358Cysfs*15) | 11 | WT | 32 | Severe | ||
| 13 | c.653C>A | p.(Ala218Asp) | 8 | c.985G>A | p.(Lys329Glu) | 11 | 34 | Mild |
| 14 | c.118+3A>G | WT | 36 | Severe | ||||
| 15 | c.581A>G | p.(Asn194Ser) | 7 | WT | 40 | Severe | ||
| 16 | c.850-17A>G | WT | 42 | Severe | ||||
| 17 | c.1154A>G | p.(Tyr385Cys) | 11 | WT | 48 | Severe | ||
| 18 | c.168delC | p.(Arg57Lysfs*21) | 3 | WT | 52 | Severe |
- a A possible variant on WT allele of individual Nr. 10 may escaped detection by Sanger sequencing and MLPA; in this case the variation p.(Ala218Pro) may be also considered as mild.
- b The severity of the sequence variation is referred to the newly identified sequence variants.
The allele frequency for the common variants c.985G>A and c.199T>C8 was 52.6% and 7.7% of the total alleles, respectively. As shown in Table 1, we found a total of 20 novel ACADM variants, including 13 missense variants, 4 small deletions/duplications, and 3 splice site variants (Table 1). In 12 patients, the novel variants were found in compound heterozygosity with a second either severe mutation, mostly c.985G>A, or a mild mutation, mostly c.199T>C. Patients #57 and #66 were carrying two novel variants each, c.1075G>A/c.286+1G>C and c.234T>G/c.430A>T, respectively. These four newly identified variants could be classified as severe since they resulted in a residual activity of 4% and 8%, respectively (Table 1). All further newly identified variants found in heterozygous form could also be classified as severe as affected patients showed a residual activity in the range of 36% to 52%.
Overall, measurement of the enzyme function in lymphocytes allowed determination of the pathogenicity of new gene variants. In addition, there is a clear correlation between genotype and enzyme function demonstrating also the reproducibility of the functional measurement in vitro.
3.2 Acylcarnitine species on NBS and enzyme activity
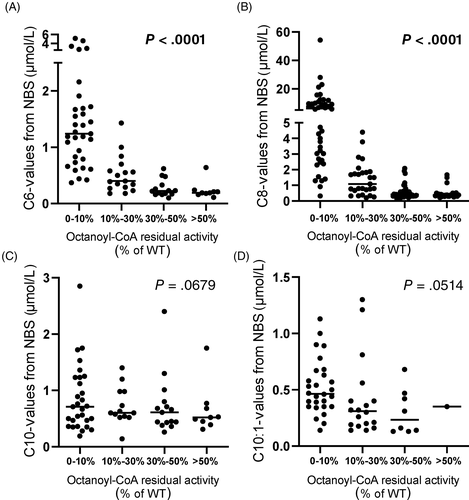
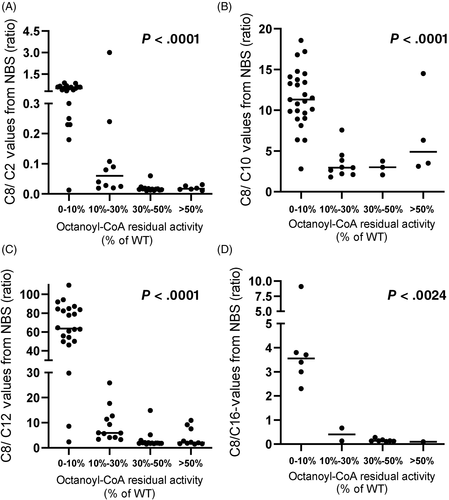
In order to investigate the correlation between acylcarnitine levels in NBS samples with MCAD activity, NBS results were analyzed. Individuals were clustered in different groups according to their residual activity. As the samples were collected from 19 different screening laboratories the method of analysis and the cutoffs for the reported acylcarnitines differed remarkably. As shown in Figure 2, a good correlation between residual activity and initial acylcarnitine values could be observed only for the species C6 and C8, in line with previous reports.8, 19 Patients with severe MCADD (range of residual activity 0%-10%) showed the highest C6 and C8 concentrations with reported values up to 54.3 μmol/L. A higher residual activity correlated with lower concentrations of C6 and C8 measured in NBS (Figure 2A,B). No correlation with enzyme residual activity was observed for the concentrations of C10 and C10:1 acylcarnitines. Indeed, similar concentrations of these acylcarnitine species were measured in false positive samples with a residual activity >30% and MCADD samples (Figure 2C,D). All ratios usually used in NBS for the diagnosis of MCADD appeared to be correlated with the measured residual enzyme activity (Figure 3). However, the exclusion of the group 0% to 10% showed clearly that for the ratios C8/C10 and C8/C16 this correlation was lost (Supplementary Figure 3B,D).
3.3 Reduction of the octanoyl-CoA oxidation rate is not accompanied by an upregulation of the palmitoyl-CoA oxidation rate
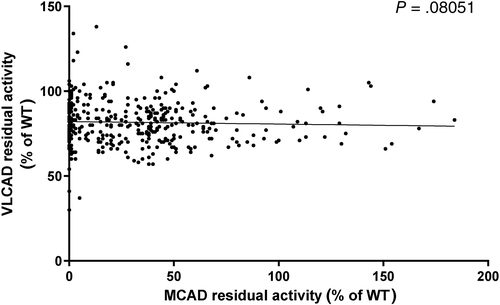
In order to evaluate the quality of the isolated lymphocytes and investigate whether VLCAD may in part compensate for a reduced octanoyl-CoA oxidation rate in MCADD we correlated the octanoyl-oxidation oxidation rate (corresponding to MCAD enzyme activity) with the palmitoyl-oxidation rate (corresponding to the VLCAD enzyme activity) (Figure 4; P = .08051). Surprisingly, we observed a palmitoyl-CoA oxidation rate >100% in few samples, especially in MCAD patients with a residual activity in the range 0% to 30%. However, since almost all the samples displayed a palmitoyl-CoA oxidation rate <100%, we assume that no compensatory upregulation of VLCAD enzyme activity occurred in case of MCADD. In analogy to cases reported previously,18 three patients #25 (0% MCAD; Supplementary Table 1), #26 (0% MCAD; Supplementary Table 1), and #106 (5% MCAD) showed a remarkably reduced VLCAD residual enzyme activity with values of 30%, 41%, and 37%, respectively. Sequencing of ACADVL confirmed heterozygosity for an ACADVL variant in these patients. The clinical significance of these findings such as synergistic heterozygosity is yet unclear.
4 DISCUSSION
MCADD is the most common defect of fatty acid oxidation. The introduction of MCADD as a target disease in NBS programs in many countries worldwide has remarkably reduced the risk of mortality and psychodevelopmental delay.20 Due to the high prevalence of the common classical mutation c.985A>G on exon 11, sequencing of the ACADM gene is often the first approach for confirmation diagnostics in case of positive NBS. However, diagnostic algorithms at least in Germany also include the performance of a functional test to quantify residual enzyme activity (https://www.awmf.org/leitlinien/detail/ll/027-021.html). In this study, we could show a clear correlation between enzyme activity and genotype in MCADD that allowed the unambiguous discrimination of patients and carriers as well as the classification of newly identified variants with respect to their impact on enzyme function.
Elevation of specific medium-chain acylcarnitines and related ratios is strongly indicative of MCADD.21 Despite the assumed high specificity of these metabolites only 50% of cases with a positive NBS suggestive of MCADD referred to our laboratory could be confirmed as true MCADD. The remaining samples showed either only a mild functional impairment typical for heterozygous carriers or no alteration of enzyme function. In the past, the correlation between biochemical phenotype and genotype has been extensively discussed especially with the known environmental factors influencing disease phenotype. Some studies described the lack of correlation between genotype and initial octanoylcarnitine levels.8, 15 However, the presence of the common homozygous mutation c.985A>G or other known highly deleterious variants is often associated with very high values of initial octanoylcarnitine.19, 22-24 In line with these studies, our results very clear showed that especially the initial levels of C6 and C8 acylcarnitines at NBS correlated with residual enzyme function. In fact, the highest C6 and C8 values have been reported in the group of patients with the lowest enzyme residual activity in the range of 0% to 10%. These data are strongly consistent with previous reports, although we plotted values of initial NBS from different screening laboratories that use different cutoffs for the identification of MCADD patients. With increasing residual activity we observed an overlapping of similar acylcarnitine values among mild patients (range, 10%-30%) but also in heterozygous carriers and healthy individuals. In this regard, Lehotay et al25 mentioned already in 2004 the difficulty of interpreting borderline elevation of acylcarnitines and the consequences for the decision of biochemical and clinical follow-up in these cases, especially with heterozygotes for the common c.985A>G mutation also presenting with elevated C8 levels.25 Also several other variables such as infectious diseases and metabolic distress due to prematurity and low birth weight may indeed trigger β-oxidation and induce accumulation of acylcarnitines.25, 26 In contrast, C10 and C10:1 species also currently used in NBS, appeared not to correlate with MCAD residual activity. It is conceivable that during catabolism when the degradation of fatty acids is upregulated, the accumulation of certain medium-chain acylcarnitines such as C10 and C10:1 indeed may reflect a physiological response of the organism as result of an overload of fatty acids in the mitochondrial β-oxidation. In a very similar way, the long-chain acylcarnitines C14:1 and C14:2 are typically increased also in healthy infants and individuals during fasting or metabolic situations.27
The use of ratios can remarkably increase the sensitivity of NBS tests and thereby the discrimination of patients with different severities of MCADD.12, 19, 22, 25 In this regard, C8/C2 and C8/C10 are considered most reliable with values about 10-fold higher in MCAD patients than in controls.28 This is only in part in line with our data. Although all reported ratios clearly correlate with enzyme activities in the range of classical MCADD, in patients and individual with a residual activity >10% values of ratios are very variable. Especially the ratios C8/C10 and C8/C16 showed overlapping values among patients, carriers, and healthy individuals that complicates the interpretation of the NBS results. However, the low number of reported values obtained by different NBS laboratories as well as the different methods of acylcarnitine methods in the different NBS laboratories and different used cutoff makes an accurate evaluation of the data difficult.
Overall, functional enzyme testing is necessary to discriminate severe from mild MCADD in cases of new genetic ACADM variants, and to discriminate healthy carriers and healthy individuals when initial acylcarnitine profiles are borderline and of unclear interpretation. With the optimized method based on Sturm et al,15 diagnostic quality was improved remarkably. However, the prediction of metabolic derangement may still remain difficult as this depends genetically on how the variance affects enzyme residual activity but also on several other variables such as lifestyle and environmental factors. It remains, therefore, a matter of debate whether MCADD patients with relatively high residual activity are at risk of metabolic decompensation and it still remains difficult to define the limit below which the development of symptoms is expected. The consistent enzymatic results obtained with the high number of samples with homozygosity for c.985A>G (0.52% ± 0.44%), compound heterozygosity for c.199T>C/c.985A>G (18.89% ± 2.66%) or heterozygosity for c.985A>G (44.25% ± 3.9%) allow a clear correlation between residual activity and genotype. With this method, compound heterozygous patients carrying the common mutation c.985A>G with a new variant or patients with two new variants can be easily diagnosed and classified based on the effect of the variation on the protein function. In this regard, we identified the variants c.653C>A/p.A218D involving the same codon and nucleotide of a known described pathogenic mutation (c.653C>G).29 Both variants were classified as pathogenic by the prediction software Alamut Visual 2; however, their impact on protein function was different. While the variant c.653C>G in compound heterozygosity with c.985A>G resulted in a residual activity of about 18% in our patient cohort, the newly identified variation showed a residual activity of 34%. Since this activity has been described also for the combination c.127G>A/c.985A>G, it is conceivable that despite the compound heterozygous presence of this variant the patients may very likely remain asymptomatic. Indeed, heterozygosity for an MCAD deficiency variant is not associated with a risk of metabolic decompensation. We believe that MCADD due to compound heterozygosity with at least one “high residual function” variant resulting in a residual activity >30% will also remain asymptomatic.
Initially meant as internal quality control for the viability of the lymphocytes, we also measured palmitoyl-CoA oxidation in the same patient samples. As acyl-CoA dehydrogenases share in part overlapping substrate specificity,30 we also investigated the effects of reduced MCAD enzyme activity on the palmitoyl-CoA oxidation rate. Of interest was the finding that palmitoyl-CoA oxidation rate was mildly reduced in many samples. Biochemical and computational data have demonstrated that higher concentration of palmitoyl-CoA as substrate for the β-oxidation induced a decline in flux and led to the accumulation of acylcarnitines.31 Taking advantage of the parallel measurement of VLCAD enzyme activity, we could also identify three MCAD patients with a VLCAD enzyme activity in the range of healthy carriers. These results were confirmed by molecular sequencing of ACADVL and revealed the presence of two common missense variants and one splice site variants. All three variants were previously described as disease-causing.18 The clinical consequences of variants in two different genes affecting the same metabolic pathway are still unknown.
5 CONCLUSION
The suspicion of MCADD in NBS is based on accumulating medium-chain acylcarnitines and respective ratios. Hexanoylcarnitine and octanoylcarnitine seem to be good predictors in the diagnosis of MCADD and high values associate with disease severity. However, in half of the referred suspected cases from NBS to our laboratory the disease could not be confirmed when functional assays were applied. These results are surprising since MCAD screening was reported to be relatively specific with few false positives.29, 32 In high patient numbers, we can demonstrate that the measurement of the enzyme function in lymphocytes allows clear determination of the pathogenicity of new gene variants. In addition, there is a clear correlation between genotype and enzyme function demonstrating also the reproducibility of the functional measurement in vitro. MCAD enzyme testing allows fast confirmation diagnostics for necessary prophylactic or therapeutic interventions.
ACKNOWLEDGMENT
Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Sara Tucci designed the research and drafted the manuscript, guarantor; Christine Wagner summarized the data and was responsible for the first draft of the manuscript; Sarah C. Grünert, Natalie Weinhold, Jeannette Klein, Francesco Porta, Marco Spada, Andrea Bordugo, Francesca Furlan, Francesca Menni, were primarily responsible for patient data collection; Uta Matysiak, Giulia Rodella and Anna Sajeva supported analysis and interpretation of the molecular genetics data; Ute Spiekerkötter co-drafted the article and revised it critically. Sara Tucci had primary responsibility for final content. All authors read and critically revised the article and approved the final version of the manuscript.
ETHICAL STATEMENT
The study was performed according to the rules of the Declaration of Helsinki and approved under study number 219/19 by the ethical review board of Albert-Ludwig University of Freiburg.




