Impact of newborn screening for very-long-chain acyl-CoA dehydrogenase deficiency on genetic, enzymatic, and clinical outcomes
Funding information ZonMw; Metakids; ZonMW
Abstract
Most infants with very-long-chain acyl-CoA dehydrogenase deficiency (VLCADD) identified by newborn screening (NBS) are asymptomatic at the time of diagnosis and remain asymptomatic. If this outcome is due to prompt diagnosis and initiation of therapy, or because of identification of individuals with biochemical abnormalities who will never develop symptoms, is unclear. Therefore, a 10-year longitudinal national cohort study of genetically confirmed VLCADD patients born before and after introduction of NBS was conducted. Main outcome measures were clinical outcome parameters, acyl-CoA dehydrogenase very long chain gene analysis, VLCAD activity, and overall capacity of long-chain fatty acid oxidation (LC-FAO flux) in lymphocytes and cultured skin fibroblasts. Median VLCAD activity in lymphocytes of 54 patients, 21 diagnosed pre-NBS and 33 by NBS was, respectively, 5.4% (95% confidence interval [CI]: 4.0-8.3) and 12.6% (95% CI: 10.7-17.7; P < 0.001) of the reference mean. The median LC-FAO flux was 33.2% (95% CI: 22.8-48.3) and 41% (95% CI: 40.8-68; P < 0.05) of the control mean, respectively. Clinical characteristics in 23 pre-NBS and 37 NBS patients revealed hypoglycemic events in 12 vs 2 patients, cardiomyopathy in 5 vs 4 patients and myopathy in 14 vs 3 patients. All patients with LC-FAO flux <10% developed symptoms. Of the patients with LC-FAO flux >10% 7 out of 12 diagnosed pre-NBS vs none by NBS experienced hypoglycemic events. NBS has a clear beneficial effect on the prevention of hypoglycemic events in patients with some residual enzyme activity, but does not prevent hypoglycemia nor cardiac complications in patients with very low residual enzyme activity. The effect of NBS on prevalence and prevention of myopathy-related complications remains unclear.
1 INTRODUCTION
Very-long-chain acyl-CoA dehydrogenase deficiency (VLCADD) (OMIM 201475) is an autosomal recessive inherited disorder of mitochondrial long-chain fatty acid oxidation (LC-FAO), in which energy homeostasis is compromised and long-chain (14-20 carbon atoms long) fatty acids accumulate.1 Clinical symptoms arise, or are provoked, during catabolic situations such as exercise, illness, or fasting. In infancy, major symptoms include hypoketotic hypoglycemia, rhabdomyolysis, cardiomyopathy, and arrhythmia, all of which can be fatal. Exercise intolerance, myopathy, and exercise-induced rhabdomyolysis often present at a later age.2-6 There is a marked variability in clinical severity among patients.
In the Netherlands, VLCADD has been included in the newborn screening (NBS) panel since 2007. Screening is performed by measuring the concentration of tetradecenoyl carnitine (C14:1) and acetylcarnitine (C2) in dried blood spots between 72 and 168 hours after birth. Since 2013, the cutoff value (COV) of the C14:1/C2 ratio for referral of newborns has been set at ≥0.023. Between 2007 and 2013, screening was solely based on C14:1 levels (COV ≥ 0.8 μmol/L), which failed to identify five patients who were only diagnosed retrospectively upon reevaluation of the blood spot results in 2010.7
NBS is widely regarded as a successful public health initiative that leads to improved treatment and disease outcomes. With the addition of VLCADD to NBS, the number of diagnosed patients rapidly increased. Most infants are asymptomatic at time of diagnosis and remain asymptomatic.5 The question is whether this is due to prompt diagnosis and start of therapy or because individuals with biochemical abnormalities who will never develop symptoms are identified. Phenotype prediction based on molecular analysis is challenging since various missense mutations or variants of uncertain significance with unknown effects on protein level and catalytic activity of the enzyme are observed in both severe and milder phenotypes.8 Functional studies of lymphocytes and fibroblasts measuring residual LC-FAO rates aid individualized therapeutic strategies but are only available in a few metabolic centers.9, 10
Treatment of VLCADD is aimed at preventing catabolism by avoidance of fasting.6, 11 In addition, a long-chain triglyceride (LCT)-restricted diet supplemented with medium-chain triglycerides (MCT) is traditionally advised to bypass long-chain fat oxidation for energy production.6, 11 Recent studies support a more relaxed dietary regimen without restrictions under healthy circumstances in patients with a milder phenotype.8, 9
The Dutch National Institute for Public Health and the Environment has facilitated a nationwide program to monitor clinical and biochemical features of all known VLCADD patients in the Netherlands. This program includes all VLCADD patients diagnosed by NBS (henceforth referred to as NBS patients) as well as patients born before the introduction of NBS (referred to as pre-NBS patients). The data collected in this program offer a unique opportunity to evaluate the effect of a decade of NBS in the Netherlands on the genetic and biochemical characteristics as well as clinical outcomes of VLCADD patients.
2 METHODS
2.1 Study design and setting
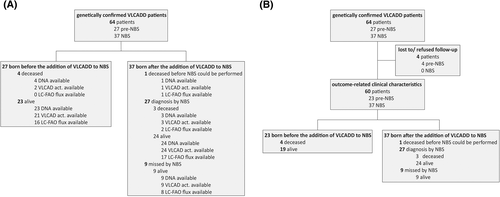
- Comparison of genetic and biochemical studies in genetically confirmed VLCADD patients born before the addition of VLCADD to NBS (pre-NBS) and patients born in the 11 years after implementation of VLCADD in NBS (January 2007-January 2018) (Figure 1A).
- Longitudinal evaluation of outcome-related clinical characteristics of the same cohort (March 2010-March 2018) (Figure 1B).
The study was approved by the medical ethics committee of the University Medical Center Utrecht (METC10-430/C; METC15-582/C). Informed consent was obtained for all patients participating in this study.
2.2 Patients
Patients were identified by using the Dutch Diagnosis Registration Metabolic Diseases (DDRMD, www.ddrmd.nl) and by contacting all Dutch metabolic centers. Date of birth was available in all patients. Age at diagnosis was available in 19 out of 27 pre-NBS patients and all NBS patients.
2.3 VLCAD activity
VLCAD activity measured in lymphocytes is currently standard practice once a patient is referred after a positive NBS test for VLCADD. VLCAD activity in lymphocytes and fibroblasts was measured using palmitoyl-CoA as substrate as described previously12; the results are shown as a percentage of the reference mean and were valid at the time of analysis. Older patients were usually diagnosed based on enzymatic testing of cultured skin fibroblasts, hence the inclusion of both cell types in this study.
2.4 Overall measurement of the LC-FAO flux
The LC-FAO flux in fibroblasts was defined as the production of radiolabeled H2O from [9,10-3H(N)]-oleic acid in nanomoles of fatty acid oxidized per hour per milligram of cellular protein as described previously.10, 13, 14 The LC-FAO flux is expressed as a percentage of the mean activity of skin fibroblasts of two healthy controls measured in the same experiment. As previously described by Diekman et al10 LC-FAO flux measured in fibroblasts correlates better to clinical severity compared to enzyme activity, which is why this assay was included in our study.
2.5 Genotype
Mutation analysis of the acyl-CoA dehydrogenase very long-chain gene (OMIM 609575) was performed by sequence analysis of all exons and flanking intronic sequences amplified by polymerase chain reaction from genomic DNA isolated from either fibroblasts or blood from the patients.
2.6 Clinical evaluation
In the Dutch FAO Expertise Center, patients were seen by a multidisciplinary team comprising metabolic specialists, research dieticians, neurologists, physical therapists, and cardiologists. During visits, patients received standardized interviews about medical history, current complaints, daily (sports) activities, and dietary habits.
Details on historical events were verified by collecting patient records from their local hospital.
Hypoglycemia was defined as documented blood glucose concentrations <45 mg/dL (<2.5 mmol/L).
Cardiomyopathy and/or arrhythmia was defined as abnormal results on echocardiography (with left or right ventricular wall thickness of at least one segment >2 SD, corrected for age) and/or electrocardiographic abnormalities.
The definition of myopathy was based on the previously reported clinical severity score for VLCADD10 (documented plasma creatine kinase [CK] concentration ≥ 250 U/L [reference values 70-170 U/L] as well as at least two of the following symptoms: myoglobinuria, myalgia, exercise intolerance, muscle weakness (Medical Research Council [MRC] grade 4 or less), and/or frequent fatigue). Rhabdomyolysis was defined as a plasma CK concentration ≥1000 U/L.
Physical examination was performed in all patients as well as validated motor development tests (Bayley Scales of Infant and Toddler Development [third edition], Movement Assessment Battery for Children [second edition], and Bruininks-Oseretsky Test of Motor Proficiency [second edition] in children and adolescents;15-17). Scores ≤ − 2 SD were considered abnormal.
2.7 Statistical analysis
Statistical analyses were performed using SPSS Statistics software version 23 (IBM Corp., Armonk, New York). Average levels of enzyme activity and LC-FAO flux and age of onset of clinical symptoms were compared between groups using the Mann-Whitney U test. A Fisher's exact test was used to compare categorical clinical data. All statistical tests were two-sided with a P-value of ≤0.05 considered significant.
2.8 Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had access to all data in the study and had responsibility for the decision to submit for publication. The study was approved by the medical ethics committee of the University Medical Center Utrecht (METC10-430/C; METC15-582/C). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study. Trial registration: Nederlands Trial Register, ID: NTR6582, http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6582
3 RESULTS
3.1 Comparison of genetic and biochemical studies in VLCADD patients diagnosed before and after the introduction of NBS
3.1.1 Patients
In the Netherlands, participation in the population NBS program is ~99.5%.18 On 1 January 2018, a total of 64 genetically confirmed VLCADD patients were registered in the DDRMD, 37 of whom were diagnosed by NBS (Figure 1). Table S1 shows the characteristics of all patients. On 1 January 2018, four deaths in the pre-NBS (before 1 January 2007) as well as four deaths in the NBS group (since January 2007) had been reported. In the NBS group 1, VLCADD deficient newborn passed away before NBS was performed and was diagnosed by post-mortem testing. The overall death rate was 15% in the pre-NBS group and 11% in the NBS group (including this patient). These number can, however, not be compared as it is highly likely that in the pre-NBS group deceased patients were not diagnosed, and therefore under-represented in the calculated death rate in the pre-NBS group. Before the introduction of NBS on VLCADD in 2007, an average of 0.6 VLCADD patients per year were born, a prevalence of 1:350.000. Since 2007, an average of 3.4 VLCADD patients per year are born, a prevalence of 1:55.000.
The median ages of living pre-NBS patients and living NBS patients were 23 (range 11-47) and 4 (0-10) years, respectively, on 1 January 2018. In the pre-NBS group (n = 27), the diagnosis was made in 23 patients after the development of symptoms. The remaining four were diagnosed by family testing. The median age at diagnosis was 6 (0-38) years. Of the NBS patients (n = 37), one died before screening could be performed and nine were not detected by initial NBS. Six out of these nine patients were later identified by retrospective analysis of bloodspot cards demonstrating an increased C14:1/C2 ratio, which led to the adaptation of the Dutch screening for VLCADD.7 This retrospective analysis was initiated because of identification of a patient missed by NBS. The six patients identified by this retrospective analysis were all asymptomatic at the time of their diagnosis, as well as the three affected siblings who were identified by subsequent family testing. Enzymatic data were available in 61 patients (24 pre-NBS vs 37 NBS). Three out of four deceased pre-NBS patients were diagnosed postmortem.
3.1.2 VLCAD activity
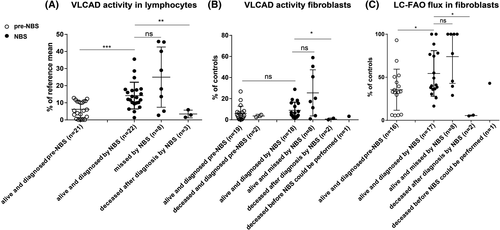
VLCAD activity was measured in lymphocytes (21 pre-NBS vs 33 NBS), leukocytes (3 NBS), and/or fibroblasts (21 pre-NBS vs 29 NBS) (Figure 2A and B). The median enzyme activity measured in lymphocytes of pre-NBS patients was 5.4% (95% CI: 4.0-8.3) compared to 12.6% (95% CI: 10.7-17.7; P < 0.001) of the reference mean in living NBS patients. Median VLCAD activity in lymphocytes of deceased NBS patients was significantly lower (2.9%; 95% CI: −2.1 to 9.0; P < 0.01) than that of living NBS patients. VLCAD activity measured in fibroblasts did not differ significantly between pre-NBS patients and NBS patients but was significantly lower in deceased NBS patients than that in the other patients (P < 0.05) (Figure 2B). There was no significant difference between the patients diagnosed and missed by NBS and other patients in any of the cell types.
3.1.3 LC-FAO flux
LC-FAO flux was measured in fibroblasts of 16 pre-NBS patients and 28 patients born after VLCADD was included in NBS. The median LC-FAO flux in pre-NBS patients was 33.2% (95% CI: 22.8-48.3) of mean activity in healthy control fibroblasts vs 41% (95% CI: 40.8-68.0; P < 0.05) in living NBS patients (Figure 2C). Median LC-FAO flux in deceased NBS patients was significantly lower (5.5%; 95% CI: −0.9 to 11.9; P < 0.02) than that in living NBS patients. No significant difference was found between patients missed and diagnosed by NBS. In the pre-NBS group, four patients had LC-FAO flux <10%, which is considered a severe impairment in LC-FAO flux.10 In the NBS group, only two patients had LC-FAO flux <10%. Forty-one patients had both LC-FAO flux in fibroblasrs as well as VLCAD activity in lymphocytes done. The relationship between LC-FAO flux and VLCAD activity is presented in Figure S1.
3.1.4 Genotype
Genotype information was available for all patients. Ten pre-NBS patients were homozygous (five carried missense mutations, three carried frameshift mutations, and two carried splice-site mutations). Eleven NBS patients were homozygous (nine carried missense mutations, one carried frameshift mutations, and one carried splice-site mutations). In both pre-NBS as well as NBS patients, the most often detected mutation was the c.848T>C (p.Val283Ala) mutation (9 out of 54 alleles of pre-NBS patients vs 31 out of 74 alleles of NBS patients). This mutation was only seen in combination with other mutations in the pre-NBS patients, whereas seven NBS patients carried this mutation homozygously. Seven out of nine patients missed by NBS were compound heterozygous (six for two missense mutations, one for a missense mutation, and a frameshift mutation) and two were homozygous for the c.848T>C (p.Val283Ala) mutation. The patient who died before NBS was compound heterozygous for a missense mutation and a frameshift mutation. Two out of three deceased patients diagnosed by NBS were homozygous for a missense mutation and a frameshift mutation, and the third was compound heterozygous for a missense mutation and a splice-site mutation. An overview of all observed mutations is presented in Table S2.
3.2 Outcome-related clinical characteristics of the VLCADD cohort (March 2010-March 2018)
Sixty patients (23 pre-NBS and 37 NBS) (Figure 1B).
3.2.1 Hypoglycemia
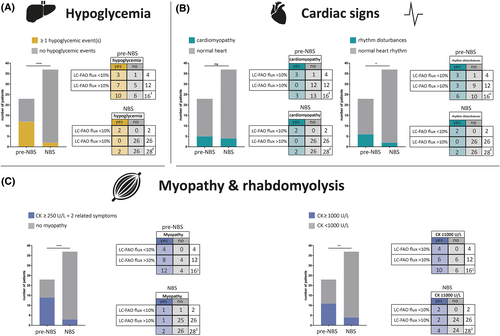
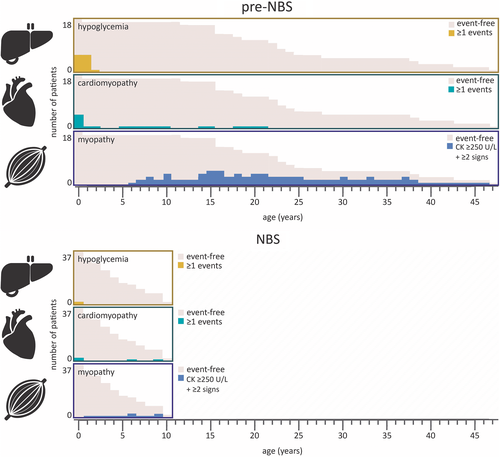
Hypoglycemic events were reported in 12 of 23 (52%) pre-NBS patients vs 2 out of 37 (5%) NBS patients (P < 0.0001) (Figure 3A). This includes one out of four deceased pre-NBS patients and two out of three deceased NBS patients. Median age of first hypoglycemic event was 9 (0-18) and 3.5 (0-7) months, respectively (P < 0.32) (Figure 4). Five out of 12 (42%) pre-NBS patients who had at least one hypoglycemic event required special education in later life. Pre-NBS patients who did not experience hypoglycemic events all attained normal educational levels. Of the two NBS patients who had experienced hypoglycemic events, one required special educational services and one died at the age of 7 months.
To assess whether the effect of an early diagnosis by NBS had equal effect in severely and mildly affected patients, we divided both cohorts into “severe” and “mild” enzymatic phenotypes based on the results of the LC-FAO flux analysis in fibroblasts. LC-FAO flux <10% of healthy values was considered a severe impairment.10 All patients in this group were homozygous for mutations predicted to result in truncation of the protein. An LC-FAO flux >10% of control values was considered relatively mild. In the pre-NBS group, LC-FAO flux values were available in 16 of the 23 patients, four of whom were considered severe. Of those four, three patients had experienced at least one hypoglycemic event, and the remaining patient was diagnosed within the first week of life because of sibling screening. Of the pre-NBS patients who were considered mild based on LC-FAO flux, 7 out of 12 (58%) experienced hypoglycemic events. Of the 37 NBS patients, LC-FAO flux was available in 28, and only two (7%) were considered severe. Both severe patients developed hypoglycemic events in contrast to none of the mild patients.
3.2.2 Cardiomyopathy and rhythm disturbances
Cardiomyopathy was observed in five (22%) pre-NBS patients during infancy and in four (11%) NBS patients (P < 0.29) (Figure 3B). Three out of five (60%) pre-NBS patients with cardiomyopathy during infancy completely recovered within a year after starting LCT restriction and MCT supplementation. One experienced a relapse with hypertrophic cardiomyopathy at the age of 14 years induced by fasting. The median age of detection of cardiomyopathy was 0.1 (0-0.3) years for pre-NBS patients and 0.6 (0.2-9; P < 0.06) years for NBS patients (Figure 4). Rhythm disturbances were reported in six (26%) pre-NBS patients and in two (5%; P < 0.05) NBS patients; all except three pre-NBS patients had documented cardiomyopathy. Cardiomyopathy was not observed in any of the mild patients based on LC-FAO flux. Rhythm disturbances were observed in all severe patients and two mild pre-NBS patients.
3.2.3 Myopathy and myopathy-related complications
Fourteen out of 23 (61%) pre-NBS patients and 3 out of 37 (8%; P < 0.0001) NBS patients met the criteria for myopathy (Figure 3C). The median age of onset of myopathy was 14.5 (6-37) years in pre-NBS patients and 5 (1-9; P < 0.13) years for the NBS patients (Figure 4). The four severe pre-NBS patients all met the criteria for myopathy as well as 8 out of 12 (67%) mild pre-NBS patients. One out of two severe NBS patients met the criteria for myopathy as well as one out of 26 mild NBS patients. Rhabdomyolysis was documented in 11 (48%) pre-NBS patients and 4 (11%; P < 0.003) NBS patients (Figure 3C). The four severe pre-NBS patients all experienced rhabdomyolysis events, as did 6 out of 12 (50%) mild pre-NBS patients. Both severe NBS patients experienced rhabdomyolysis events, as did 2 out of 26 (8%) mild NBS patients. Complications related to myopathy were all significantly more prevalent in pre-NBS patients than those in NBS patients (Figure S2).
3.2.4 Treatment
An overview of dietary treatment is given in Table S3. Details on most patients have been reported previously.9
4 DISCUSSION
This is the first study that compares genetic, biochemical, and outcome-related clinical characteristics of a nationwide cohort of VLCADD patients identified by NBS and patients identified before the addition of VLCADD to NBS. We demonstrate several important differences between both groups in genetic and biochemical characteristics. In addition, a clear beneficial outcome on some, but not all, of the clinical characteristics is observed, despite longitudinal follow-up.
Patients identified by NBS generally have higher levels of enzyme activity and a higher overall LC-FAO flux in cultured fibroblasts than pre-NBS patients, which suggests that NBS leads to the identification of milder genetic and biochemical phenotypes in the majority of patients (93%). A better clinical outcome was shown in patients with an LC-FAO flux >10% identified by NBS compared to that in patients identified pre-NBS. This is largely the result of prevention of cerebral damage due to hypoglycemia. However, NBS did not prevent hypoglycemic events and cardiac complications in patients with an LC-FAO flux <10%, indicating that current therapeutic options are ineffective in these patients (Figure 3).6, 19 Finally, although myopathy-related complications in pre-NBS patients are significantly more prevalent than those in NBS patients, these complications manifested mostly during adolescence, and as the oldest patients identified by NBS in the Netherlands are now 10 years of age, we cannot yet assess the impact of NBS on myopathy.
The benefits of early detection come at the cost of detection of milder phenotypes or even asymptomatic individuals with VLCADD. Similar effects have been reported for almost all disorders introduced in NBS programs, including medium-chain acyl-CoA dehydrogenase deficiency (MCADD), another disorder of FAO.20-24 NBS for MCADD resulted in the identification of two to three times more newborns with MCADD than was expected from the estimated disease prevalence before its addition to NBS, which was largely due to identification of very mild phenotypes.20, 24
This study underlines the importance of functional assays to follow-up on positive screening results given the poor correlation between acylcarnitine and clinical outcome. The genotype-phenotype correlation has been investigated previously with poor clinical outcome in patients with homozygous frameshift mutations or mutations that cause deletion, in agreement with our cohort.25 The correlation between protein function and missense mutations had been tested using expression studies in Escherichia coli and molecular modeling, including some observed in our cohort.26, 27 However, these assays do not take into account the interaction with other enzymes involved in lcFAO and many individuals picked up by NBS carry compound heterozygous mutations, limiting the translation of these models to clinical practice.
The most apparent beneficial effect of NBS on outcome in VLCADD patients is the prevention of hypoglycemic events in patients with residual enzyme activity. NBS did not prevent hypoglycemic events in patients with low residual enzyme activity, which is consistent with VLCADD cohorts in the United States and Australia.19, 28 Comparison of cohort data before and after initiation of screening is challenging due to the difference in duration of follow-up, in our case 47 vs 11 years and the likelihood of missed pre-NBS cases. However, if one takes into account that it took 32 years to accumulate seven hypoglycemic events in pre-NBS patients with some residual enzyme activity, it is unlikely that after 11 years of screening not a single event has been reported in the NBS patients. It is also important to note that it is difficult to take into account the role of environmental factors that can induce catabolism which may have changed over time.
The prevalence of cardiac disease in our study is low, which is also in line with previous reports.19 The yield of NBS on cardiac symptoms appears to be minimal, as there was no difference in prevalence between pre-NBS and NBS patients. Strikingly, patients with LC-FAO flux >10% did not develop cardiac symptoms. Since cardiomyopathy and arrhythmias are, however, also inducible by catabolic situations in later life, NBS may have a preventive effect.
Myopathy-related symptoms were observed in almost all pre-NBS patients and in merely three NBS patients. However, as the highest prevalence of myopathy in pre-NBS patients is found during adolescence (Figure 4), a period of life that has not been reached by Dutch NBS patients, long-term follow-up is needed to study the impact of NBS on the frequency of myopathy in VLCADD.
With the rapidly expanding NBS programs worldwide, it is important to realize that the benefit of NBS is not always easy to prove and that there is no generally accepted guideline on how to monitor outcome and hence fully benefit from NBS programs.29-31 It is even more complicated to substantiate the decision to discontinue screening, as was recently done in New Zealand for carnitine uptake disorders.32 Although most NBS programs use the criteria from Wilson and Jungner33 and the adaptations from the World Health Organization34 for inclusion of disorders in the NBS panel, no clear standardized long-term clinical follow-up system exists, despite the fact that the adapted criteria clearly state “the objectives of screening should be defined at the outset” and “program evaluation should be planned from the outset”.34 Our study underlines the need for definition and implementation of such evaluation programs that incorporate clinically relevant effects and possibly even psychological outcomes of screening over time. The psychological burden posed to parents of a child with a FAO disorder, including those whose child is mildly affected or even asymptomatic, is immense. Feeding problems and fear of metabolic crisis are common in this group and have a negative impact on family life.35 Furthermore, given the rarity of most disorders international collaboration is crucial before disorders are implemented, or discontinued from screening programs.
In conclusion, NBS for VLCADD has a clear beneficial effect on the prevention of hypoglycemic events in patients with some residual enzyme activity. However, for VLCADD patients with very low residual enzyme activity, NBS does not prevent hypoglycemia or cardiac complications. Furthermore, it is too soon to establish the effect of NBS on myopathy-related complications. Hence, long-term clinical follow-up studies are needed to evaluate and enhance the beneficial effect of NBS on VLCADD. These studies should be implemented as part of NBS programs.
ACKNOWLEDGMENTS
First, we would like to thank all the patients and their families for participating in our study. Second, we would like to thank Jos Ruiter for his help with enzymatic analyses and Astrid Verhoef, Sanna Kulik, Ellen Blok and Debbie Spiekman for their help in coordinating clinical evaluation in the Dutch Fatty Acid Oxidation Expertise Center. This work was supported by grants from ZonMW and Metakids.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
G.V. conceived the project and gained funding. J.C.B., R.H.H., S.F., F.A.W., and G.V. designed the study protocol. P.J.C.I.S. and E.D. were responsible for NBS of patients. A.M.B., M.L., T.G.J.D., M.W., M.V., M.F.M., E.R.G., and P.M.H. collected data, treated, and referred patients to the Dutch expertise center. J.C.B. and G.V. coordinated the data collection and clinical evaluation of patients in the Dutch expertise center. S.F., H.R.W., M.G.M.S.V., and R.J.A.W. were responsible for diagnostic laboratory assays. W.L.P., I.C., and M.S. were responsible for neurological and motor developmental assessment. I.L.K. was responsible for dietary analysis. M.L.P.-R. gave statistical advice. J.C.B. executed statistical analysis and created the figures. J.C.B. and G.V. wrote the first draft of the manuscript. All authors critically reviewed the manuscript.




