Brain imaging in classic nonketotic hyperglycinemia: Quantitative analysis and relation to phenotype
Funding information Brodyn s Friends Fund, Grant/Award Number: NA; CU Nonketotic Hyperglycinemia Fund; Joseph's Goal Fund; Hope for NKH Fund; Les Petits Bourdons; Lucy s BEElievers Fund; Madi s Mission to Find a Cure for NKH Fund; NKH Crusaders Fund; Smiles for Miles NKH Research Fund
Abstract
Patients with severe nonketotic hyperglycinemia (NKH) have absent psychomotor development and intractable epilepsy, whereas attenuated patients have variable psychomotor development and absent or treatable epilepsy; differences in brain magnetic resonance imaging (MRI) between phenotypes have not been reported. In a retrospective cross-sectional study, we reviewed 38 MRI studies from 24 molecularly proven NKH patients, and 2 transient NKH patients. Quantitative analyses included corpus callosum size, apparent diffusion coefficient, automated brain volumetric analysis, and glycine/creatine ratio by spectroscopy. All patients age <3 months had restricted diffusion in the posterior limb of the internal capsule, anterior brainstem, posterior tegmental tracts, and cerebellum, not present in transient NKH. In older infants, the pattern evolved and included generalized diffusion restriction in the supratentorial white matter, which quantitatively peaked between 3 and 12 months. No patient had absent corpus callosum or gyral malformation. The corpus callosum was relatively short in severe compared to attenuated phenotypes, and thin in severe cases only. The corpus callosum growth rate differed by severity; age-matched Z-scores of thickness worsened in severe cases only. Cerebral volume was decreased in the hippocampus, globus pallidus, cerebral cortex, thalamus, and cerebellum. Severe patients had greatest glycine/creatine ratios. In this study, no brain malformations were identified. The growth failure of the corpus callosum is worse in severe NKH, whereas the diffusion restriction pattern, reflecting microspongiosis, does not discriminate by phenotypic severity. NKH is therefore a disorder of brain growth best recognized in the corpus callosum, whereas spongiosis is not prognostic.
1 INTRODUCTION
Nonketotic hyperglycinemia (NKH) (OMIM 605899) is an autosomal recessive neurometabolic disorder characterized by deficient activity of the glycine cleavage enzyme. Glycine is elevated in plasma, in brain, and cerebrospinal fluid (CSF) with an increased ratio of cerebrospinal fluid:plasma glycine.1 Patients with classic NKH have mutations in the GLDC gene encoding the P-protein (EC 1.4.4.2) or in the AMT gene encoding the T-protein (EC 2.1.2.10),2, 3 while patients with variant NKH have mutations in genes involved in the biogenesis of lipoate, its cofactor.
Patients with the severe form of NKH typically present in the first week of life with lethargy progressing to coma, myoclonic epilepsy often with a burst suppression pattern on EEG, and apnea requiring ventilation.4 Patients spontaneously regain ventilatory drive but develop profound developmental delay, spasticity, and intractable epilepsy requiring multiple anticonvulsant medications. Patients with the attenuated form of NKH carry at least one mutation that confers residual enzyme activity.3 They can present similarly in the neonatal period or later in infancy.3-6 These patients make developmental progress but still have mild to severe developmental delay, have either no seizures or a seizure disorder that responds to anticonvulsant treatment. Many exhibit chorea and hyperactivity.
Data on neuroimaging in NKH have been limited to case reports and to three limited series using computed tomography (CT) and magnetic resonance imaging (MRI).7-9 A few patients with brain malformations such as hypoplastic corpus callosum or a retrocerebellar cyst with hydrocephalus were noted.7, 8, 10, 11 Development of progressive atrophy and hypointensity of white matter, which was interpreted as delayed myelination, was noted in a series of 10 patients.9 Restricted diffusion in the posterior limb of the internal capsule (PLIC) and the brainstem was noted on diffusion weighted imaging (DWI) in five of six patients in one series and in case reports of neonates.8, 12-18 In follow-up of a single patient, diffusion restriction apparently resolved in late infancy.15 Magnetic resonance spectroscopy (MRS) showed elevated glycine in a few case reports.17, 19, 20
We present in a retrospective cross-sectional study a systematic review of brain imaging findings and their evolution over time using modern MRI techniques in patients with classic NKH who were well characterized as to genotype and phenotype. We delineated typical imaging patterns and performed several quantitative analyses and compared the results of attenuated and severe phenotypes.
2 METHODS
2.1 Patients
Written informed consent was obtained from all participants or their guardians. This retrospective analysis was approved by the local IRB (COMIRB# 05-0790). All subjects were identified from a natural history study of classic NKH. The clinical, biochemical, and molecular details for all but seven patients (#9, 10, 12, 13, 17, 18, 24) were previously published.3 All diagnoses were verified biochemically and pathogenic mutations were identified in the GLDC or AMT gene in a clinical laboratory through sequencing and exonic copy number variant analysis. Patients were classified into severe or attenuated outcome, and further subdivided into attenuated poor (developmental quotient DQ < 20), attenuated intermediate (DQ 20-50), attenuated mild (DQ > 50), or attenuated not otherwise specified (NOS, if the DQ was not determined).3 Original brain MRI studies retrieved from the medical record were reviewed. Since examinations were from multiple institutions, imaging protocols differed. Inclusion criteria required MRI studies with axial and sagittal T1, axial T2 weighted imaging, and axial DWI. Although not required for inclusion, MRS data were reviewed when available.
2.2 MRI analysis
For qualitative analysis, two pediatric radiologists reviewed, in consensus, the MRI studies. The radiologists were blinded to genotype and phenotype during the analysis. Anatomic imaging was evaluated for corpus callosum structure and thickness, gyral malformation, maturity of white matter myelination, and volume of gray and white matter. DWI was reviewed for the presence of restricted diffusion as signal hyperintensity on trace DWI and signal hypointensity on apparent diffusion coefficient (ADC) in eight locations: (a) PLIC, (b) corona radiata, (c) perirolandic white matter, (d) brainstem, (e) central tegmental tracts (CTT), (f) cerebellum, (g) optic radiations, and (h) diffuse centrum semiovale.
Quantitative analysis of the corpus callosum was performed by measuring on a midline sagittal image: anteroposterior diameter (APD), length of the corpus callosum, genu thickness, body thickness, isthmus thickness, splenium thickness, and the frontooccipital diameter (FOD) to calculate the ratio APD/FOD as described21 (Supporting Information Figure S1A). For quantitative evaluation of white matter diffusion restriction, the ADC values were determined from a manually drawn 5 mm diameter circular region of interest (ROI) in bilateral parietal juxtacortical white matter in the posterior centrum semiovale (Figure S1B). The ADC values were measured in all 38 patient examinations and in 108 controls between the ages of 1 day and 36 months. All patients with available high resolution T1 images obtained at ≥1 year of age were sent for automated brain volumetric analysis using NeuroQuant (CorTechs Labs, San Diego, California) version 2.0, and volumes of intracranial structures provided on the general morphometry report were recorded. High resolution T1 imaging from 12 age-matched normal controls per patient was similarly processed. When available, the ratio of glycine to creatine on intermediate (135-144 ms) or long (270-288 ms) echo time MRS was calculated.
2.3 Statistical analyses
Using the published equation of the distribution of normal values, all patient measurements of the corpus callosum were normalized for age and gender, and a standardized score (Z-score) was calculated.21 Raw data were plotted with corresponding normal 3rd, 50th, and 97th percentile curves. Multiple measurements from the same patient were considered independently in the analysis. Normalized values between the two severity groups were compared using two-sample t tests. Linear models with an interaction term between age ≤3 months and >3 months, and severity classification group were used to test if differences between the latter were modified by the former. Linear models were also used to determine the rate of change in Z-score per year with an interaction term between time and severity classification. All hypothesis tests were two-sided and significance was set at 0.05. R version 3.1.1 software (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/) was used.
The ADC values as a relation to age were modeled using a Gompertz function written as Y = asymptote.exp(−delay.exp(−speed(t))), where Y is the ADC value in 10−6 mm2/s and t is age in months, and the best fit to this equation was derived by computer modeling from 108 control subjects using a Gauss-Newton algorithm in Statistical Analysis Software (SAS) package version 9.4 (Cary, North Carolina), evaluating the convergence of the sum of squares of residuals to a stable minimum. Best fit parameters of the asymptote, the delay, and the speed were derived for the right and the left side. Using this equation, the mean and SD for each time point were calculated. This allowed conversion of patient ADC values into age-adjusted Z-scores relative to the age-adjusted mean and SD in the equation: Z-ADCpatient/age(t) = (ADCpatient/age(t) − meanADCcontrol/age(t))/StdADCcontrol/age(t).Thus, Z-ADCpatient.age(t) stands for the number of SDs of the NKH patient from the mean of normal controls matched for age at age t. Comparisons of these Z-scores were done by age and by severity using either a t test, or an analysis of variance (ANOVA) was done using IBM SPSS version 24 (New York, New York).
After evaluation of normality of distribution, comparison between two groups was done by two-sided student t test or by Mann-Whitney U test, and relation between two variables explored by Pearson correlation or by Spearman rank correlation for variables that are normally distributed or not respectively.
3 RESULTS
3.1 Patients
There were 38 MRI studies from 24 patients (1 patient each with 5 sequential studies, 4 sequential studies, and 3 studies, 5 patients with 2 studies, and 16 with one study) with adequate sequences for analysis. The patient characteristics are listed in Supporting Information Table S1. Age at time of imaging ranged from 2 days through 17 years (median 184 days). Disease phenotype was severe in 12 patients and attenuated in 12 patients. Three subjects with neonatal demise had exonic copy number variants on both alleles, a genotype that consistently predicts severe outcome. At time of first imaging, the median age was lower in severe patients (median 4.5 days, range 2-299 days) than in attenuated patients (median 313 days, range 6 days-17 years, Mann-Whitney U test P < 0.001).
The neonatal brain MRI was retrieved and reviewed from two patients with transient NKH. They presented with neonatal epileptic encephalopathy with elevated CSF glycine, which spontaneously resolved in the first year of life. No mutations were identified in the AMT and GLDC genes. Details of the case reports are listed in Supporting Information.
3.2 Qualitative MRI findings
The qualitative MRI findings are summarized in Table 1 (details in Table S2 including technical details of the reviewed scans). Neither callosal agenesis nor gyral malformation was present in any patient. The subjective evaluation of corpus callosum thickness was difficult before 1 month of age. After age 1 month, the corpus callosum was abnormally thin in all 12 exams in severely affected patients, whereas it was normal in 11/12 patients with attenuated phenotype.
| Age | ≤3 months | 3-13 months | >13 months |
|---|---|---|---|
| Number of scans | 17 | 11 | 10 |
| Perirolandic | 5 (29%) | 9 (82%) | 0 (0%) |
| Corona radiata | 14 (82%) | 9 (82%) | 0 (0%) |
| PLIC | 17 (100%) | 9 (82%) | 2 (20%) |
| Brainstem | 15 (88%) | 3 (27%) | 1 (10%) |
| Central tegmental tracts | 15 (88%) | 2 (18%) | 1 (10%) |
| Cerebellum | 17 (100%) | 5 (45%) | 2 (20%) |
| Optic radiations | 6 (35%) | 10 (91%) | 3 (30%) |
| Centrum semiovale | 1 (6%) | 11 (100%) | 0 (0%) |
| Diffuse white matter | 2 (12%) | 11 (100%) | 0 (0%) |
- PLIC, posterior limb of the internal capsule.
A typical pattern of restricted diffusion evolving with age was identified (Figure 1). In all scans at age <3 months, there was restricted diffusion in the PLIC, brainstem, CTT, and cerebellum. The diffusion restriction often extended to perirolandic white matter, and few involved the optic radiation. From age 3 months on, the diffusion restriction faded in the brain-stem, CTT, and cerebellum, but persisted in the corona radiata and perirolandic white matter up to 13 months of age, with PLIC involvement up to age 15 months (10/13 scans). In all scans performed between 3 and 13 months of age (five attenuated, five severe patients), a consistent pattern of signal hyperintensity on trace diffusion images with iso- or mild signal hypointensity on ADC maps was seen throughout the supratentorial white matter. This pattern was not seen in any patient younger than 3 months. At more than 1 year of age, all attenuated NKH patients had normal findings, whereas 3/5 available scans of severely affected patients had persistent diffusion restriction.
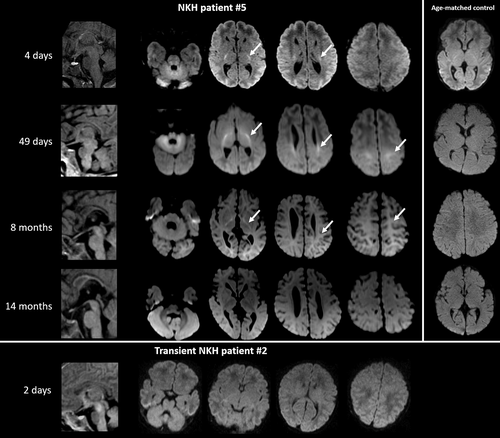
The white matter appeared hypomyelinated in 4/8 patients with attenuated phenotype imaged before age 6 months. Myelination was delayed on every exam performed before 3 years of age in all 12 patients with a severe phenotype, including absence of the PLIC T1 bright spot on 11 neonatal exams. Myelination appeared normal in a single exam in a severe phenotype patient at age 5 years.
In the two patients with transient NKH, brain MRI obtained at 2 and 3 days of life did not show diffusion restriction in any brain area, different from that observed in all patients with classic NKH (Figure 1).
3.3 Quantitative analysis: corpus callosum measurements
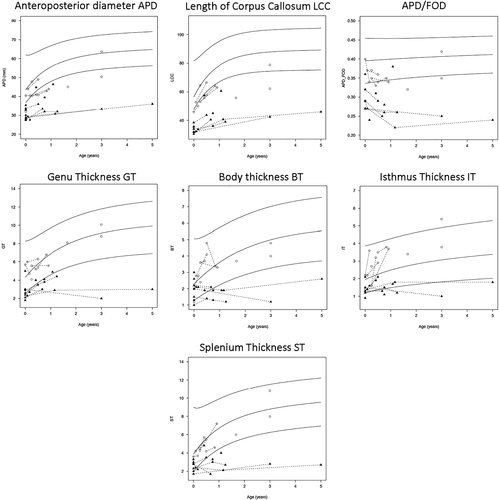
Both the traced length of the corpus callosum and the APD expressed as normalized Z-scores compared to age-matched control values were short for all patients with NKH but were significantly shorter for patients with severe NKH than for patients with attenuated NKH (Figure 2 and Table 2). Even when controlling for head size, the APD over the occipitofrontal length (APD/OFD) had a mean Z-score of −3.9 SD in severe NKH compared to −1.3 SD in attenuated NKH.
| Parameter | Attenuated (n = 15) | Severe (n = 20) | P = value | |
|---|---|---|---|---|
| A. Normalized scoresa | ||||
| APD | −1.7 ± 1.5 | −5.6 ± 2.1 | <0.0001 | |
| LCC | −2.5 ± 1.8 | −8.2 ± 2.6 | <0.0001 | |
| GT | 0 ± 0.7 | −2.2 ± 1.1 | <0.0001 | |
| IT | 0.1 ± 1 | −1.8 ± 1.5 | <0.0001 | |
| ST | −0.3 ± 0.8 | −2.3 ± 1.5 | <0.0001 | |
| APD/OFD | −1.3 ± 1.5 | −3.9 ± 1.6 | <0.0001 | |
| B. Slope of normalized scoresb | Difference | |||
| APD | 0.2 (−0.2, 0.6) | −0.5 (−1.2, 0.3) | −0.6 (−1.5, 0.2) | 0.1178 |
| LCC | 0.1 (−0.4, 0.6) | 0.4 (−0.5, 1.3) | 0.3 (−0.7, 1.4) | 0.5003 |
| GT | 0 (−0.2, 0.2) | −0.6 (−0.9, −0.3) | −0.6 (−0.9, −0.2) | 0.0015 |
| BT | 0.1 (−0.2, 0.3) | −0.8 (−1.2, −0.3) | −0.8 (−1.3, −0.4) | 0.0014 |
| IT | 0.2 (0, 0.4) | −0.3 (−0.7, 0) | −0.6 (−1, −0.2) | 0.0069 |
| ST | 0.1 (−0.1, 0.3) | −0.9 (−1.3, −0.6) | −1 (−1.4, −0.6) | 0.0000 |
| APD/OFD | 0.4 (0.2, 0.7) | −0.7 (−1.2, −0.3) | −1.1 (−1.7, −0.6) | 0.0001 |
| C. Age stratified normalized scores by severityc | ||||
| Attenuated (n = 15) | Severe (n = 20) | |||
| <3 months | >3 months | <3 months | >3 months | |
| APD | −1.4 ± 1.5 | −2 ± 1.5 | −5.5 ± 1.9 | −5.8 ± 2.5 |
| LCC | −2.6 ± 2.5 | −2.4 ± 1.3 | −8.9 ± 2.2 | −7.5 ± 3 |
| GT | 0 ± 0.9 | −0.1 ± 0.5 | −1.7 ± 1 | −2.7 ± 1 |
| BT | −0.2 ± 1.1 | 0.3 ± 0.9 | −0.7 ± 1 | −2.9 ± 1.1 |
| IT | 0.2 ± 1.1 | 1.4 ± 0.6 | −1.5 ± 0.9 | −1.9 ± 1.1 |
| ST | −0.4 ± 0.3 | −0.2 ± 1 | −1.3 ± 0.6 | −3.3 ± 1.5 |
| APD/OFD | −1.4 ± 1.2 | −1.2 ± 1.8 | −3.4 ± 1.1 | −4.5 ± 1.8 |
- a The length of the corpus callosum was measured as the anteroposterior diameter (APD) and the traced length of the corpus callosum (LCC), and related to the head size measured by the occipitofrontal length (OFD). The thickness of the corpus callosum was measured at various locations, thickness at the genu (GT), body (BT), isthmus (IT), and splenium (ST). The values are calculated as age adjusted normalized values based on the published norm curves and expressed as normalized Z-values. The length of the corpus callosum was low for all NKH children but significantly shorter for severe NKH children than for attenuated NKH children. The thickness was decreased in severe NKH children but not in attenuated NKH children, a difference that was significant.
- b The rate of change per year in the parameter is given for the normalized Z-score as a function of time. The slope is given as mean and the 95% confidence interval. The difference in the slope is given between the severe and attenuated NKH and the significance value from the interaction term in the linear model.
- c Comparison of the corpus callosum measurements of young infants age <3 months (n = 16) vs older infants (n = 20) and comparing the difference between those affected with severe NKH compared to those affected with attenuated NKH, providing the mean and SD. The difference in BT between the severe group and the attenuated group was significantly larger by, difference (95% CI): −2.7 (−4.1, −1.3), in the older subjects compared with the younger subjects; P = 0.0006. The difference in IT between the severe group and the attenuated group was significantly larger by, −1.7 (−3.1, −0.4), in the older subjects compared with the younger subjects; P = 0.0110. The difference in ST between the severe group and the attenuated group was significantly larger by, −2.2 (−3.6, −0.7), in the older subjects compared with the younger subjects; P = 0.0043.
The mean thickness values of the corpus callosum at all four locations (genu, body, isthmus, and splenium) were decreased in children with severe NKH but not in children with attenuated NKH, a statistically significant difference. We evaluated the predictive value of this difference in the first month of life for severity of outcome. When placing the cutoff at the lowest level observed in attenuated NKH, the best predictors were thinner genu (cutoff 3 mm) which identified 7/10 severe NKH patients, short APD (cutoff 32 mm) which identified 6/10 severe NKH patients, and shorter traced length (35.5 mm) which identified 6/10 severe NKH patients. Other parameters identified less than half of the severe NKH patients.
Corpus callosum growth rates differed between phenotypes (Table 2(A)(B)). The normalized Z-scores worsened over time in the severe NKH patients resulting in a negative slope, but not in attenuated NKH. This difference in growth rate was significant for the thickness of the corpus callosum measurements but not for the length. Due to this reduced growth, the corpus callosum was thinner in the group of severe subjects age 3 months or older than for the subjects age <3 months, when comparing age adjusted Z-scores (Table 2(C)).
3.4 Quantitative analysis: DWI
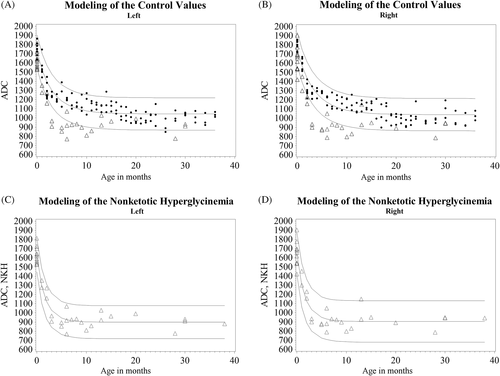
The ADC values of normal controls decreased with age approaching a stable value with no apparent laterality difference. A least squares nonlinear regression modeling for a Gompertz curve using Gauss-Newton algorithm converged on stable minimal sum of least squares after 13 iterations with an F-value of 6618 and 6784 (left, right) and P-values <0.001. This modeling is shown for controls in Figure 3A,B and in 32 patient images obtained at an age <36 months in Figure 3C,D, and provided a better fit than logarithmic or quadratic approximations. They showed that NKH patients compared to controls had a significantly lower asymptote (mean ± SD): (904.4 ± 26.4 vs 1038.7 ± 12.6 right; and 897.9 ± 21.2 vs 1043.5 ± 12.0 left), a shorter delay (−0.60 ± 0.03 vs −0.50 ± 0.02 right; and −0.60 ± 0.03 vs −0.50 ± 0.02 left), and a faster speed (0.60 ± 0.07 vs 0.76 ± 0.02 right and 0.061 ± 0.05 vs 0.76 ± 0.02 left) (Table S3). This reflects a more rapidly decreasing ADC that stabilizes at an earlier age and to a lower value than in controls.
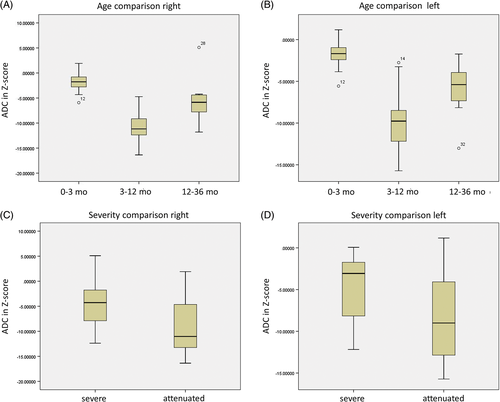
As expected for a symmetric condition, the right and left normalized Z-values were strongly intercorrelated (Pearson r2 = 0.938, P < 0.001), and decreased with age (Spearman ρ = −0.657 and ρ = −0.556 right and left respectively, both P < 0.001). However, reviewing the graph and the qualitative impression, there appear to be three age categories: 0 to 3 months, 3 to 12 months, and greater than 12 months. When comparing the age-adjusted Z-values, the difference between these categories was highly significant (ANOVA P < 0.001 both sides) (Figure 4 and Table S4). The youngest category 0 to 3 months showed the least mean diffusion restriction (ADC Z = −1.89 right and Z = −0.173); the infantile range 3 to 12 months had the strongest mean diffusion restriction (ADC Z = −10.66 right and Z = −9.61 left); and then became less restricted after 1 year of age (mean Z = −5.27 right and Z = −6.10 left), with significant category differences on post hoc Tukey analysis. Analysis by phenotype comparing severe NKH to attenuated NKH showed a significant difference (t test P < 0.03) with severe NKH have less mean diffusion restriction (ADC Z = −4.76 right and Z = −4.55 left) compared to attenuated NKH (ADC Z = −8.84 right and Z = −8.30 left; t test P < 0.03 both sides).
3.5 Volumetric analysis
Reliable volumetric analysis was only possible in four patients due to delay in myelination in others (Table S5). Volumes were normal in the most mildly affected patient, except for the pallidum. In the three patients with either severe NKH or intermediate attenuated NKH, volumetric analysis shows decreased volume in the globus pallidus and hippocampus and less frequently in the cerebral cortex, thalamus, and cerebellum, with concomitant increased volume of the lateral ventricles. The white matter volume appeared normal in these four scans.
3.6 Magnetic resonance spectroscopy
Glycine peaks were detected on MRS with intermediate or long echo times in five patients with a severe phenotype and in four patients with an attenuated phenotype (Table S6). The glycine/creatine ratio in the white matter tended to be lower in the attenuated patients than in the severe patients. Limited number of scans with MRS data and inconsistency in the methods used did not allow further analysis including of the consistency of this finding, the evolution over time, or quantitative comparison. The glycine/creatine peak of cases reported in the literature (14 scans from 6 patients)12, 19, 20, 22, 23 is listed in Table S7. In cases where values in both the white matter and the gray matter were recorded, the glycine/creatine ratio was larger in the white matter.
4 DISCUSSION
4.1 The corpus callosum is not absent but small with lack of growth that differs by phenotype
Brain malformation is the most widely reported neuroimaging finding, reported only in severely affected patients.3, 4, 10, 11, 24 Hydrocephalus was reported in 7% to 9% of severely affected patients,3, 4, 10, 11, 25 and is also noted in the mouse model of NKH.26 Gyral malformation was reported only once from a CT scan and, as noted by the author, was not adequately described,7 was not present in large series3, 4, 8, 9 and appears unlikely to be a part of classic NKH. Agenesis of the corpus callosum was noted only in a CT scan series, where the corpus callosum is frequently difficult to visualize in neonates.7 Agenesis of the corpus callosum was not identified in our cohort or in any of the reported brain MRI series or case reports, nor in any of the autopsy cases,27-34 except as caudal shortening.32 Rather, we found that the corpus callosum was present but short and thin, similar to published cases.3, 4, 8, 18, 28 It is possible that a very thin corpus callosum in a neonate could have been previously misconstrued as absent. Indeed, critical review of cases from our past outcome study,3 which was based on clinical radiology reports, confirmed this misinterpretation in each case where we could retrieve the images (cases #1, 3, 6, and 8). A survey on metab-L, a world-wide e-mail list of metabolic physicians, did not identify any case with callosal agenesis. Thus, there remains only the finding of aqueduct stenosis presenting with hydrocephalus in severely affected patients, which has been well documented in the literature and is similar to the mouse model.3, 4, 10, 11, 24-26
At birth, the corpus callosum is shorter and thinner in severe than in attenuated NKH, and the growth rate is worse in severe NKH, thus relating corpus callosum growth to severity of clinical outcome. The growth rate, or lack thereof, of the corpus callosum may be one of the first objective measures relating brain growth with outcome, possibly preceding volume loss in other areas. This distinction can be a predictive parameter in neonates for outcome with a fair sensitivity of 60% to 70% predicting severe outcome. In infancy, this growth failure enhances the distinction, and subjectively no patient with severe disease imaged after 1 month of age had a normal corpus callosum.
4.2 Progressive brain atrophy evolves in specific brain areas
Past brain MRI series in NKH noted moderate to severe brain atrophy in patients older than 20 months.8, 9, 18, 35 Our volumetric analyses localize this volume loss to specific gray matter structures including globus pallidus, hippocampus, cerebral cortex, and thalamus, and in severe cases the cerebellum. The hippocampal volume loss was not associated with a typical gliosis pattern as seen with prolonged seizures, and we thus surmise it relates more to the NKH disorder rather than the consequence of continued seizures. The pallidal volume loss may relate to the choreatic movements, and was most notable in a patient with attenuated NKH with pronounced choreatic movements. In past literature, cerebellar volume loss was noted on pathology,28 atrophy of basal ganglia in two patients, and diffusion restriction in globus pallidus in a single patient.8 The expected decrease in T2 signal intensity with myelination did not occur in several supratentorial white matter tracts and was interpreted as delayed myelination.9 The high signal intensity of the white matter on T2 images resulted in failure of volumetric analysis, which could introduce bias. Indeed, some patients had subjectively decreased white matter volume (eg, Figure 1 at 14 months).
4.3 An evolving pattern of diffusion restriction is diagnostically useful but does not relate to phenotype
A novel observation was the evolving pattern of diffusion restriction during the first 2 years. In the neonate, diffusion restriction was consistently present in the corticospinal tracts, specifically the PLIC, brainstem, and cerebellar white matter, as previously noted.8, 12-18 After 3 months, this pattern of diffusion restriction evolves, fading infratentorially but becoming more conspicuous in the corona radiata and perirolandic white matter, which then fades in later infancy. In addition, there is global diffusion restriction in the cerebral white matter most notable between 3 and 15 months, decreasing in the second year of life, but which, on quantitative analysis, never completely disappears. Diffuse involvement of the corticospinal tracts and cerebral white matter was noted once before,16 and regression of diffusion restriction in the second year was also noted once.15
The consistency of the diffusion restriction pattern on brain imaging has diagnostic value in neonates and young infants. Elevated plasma glycine is a marker with limited specificity, and elevated CSF glycine can be observed in neonates with a variety of causes,36, 37 Elevated glycine can be seen on MRS in the presence of hemorrhage.38 The pattern in NKH neonates should not be confused with that of hypoxic ischemic injury, where diffusion restriction in the PLIC is not typically accompanied by diffusion restriction in the brainstem and cerebellum, thus differentiating these two entities. Restricted diffusion was not present in our two cases of transient NKH, and two previously reported cases had normal brain MRI,39, 40 or showed abnormalities in other locations not consistent with NKH.41 Thus, recognition of the specific diffusion restriction pattern in a neonate indicates that the hyperglycinemia is not of an acquired cause but due to the genetic disorder NKH. Confirmation by genetic testing should be pursued by sequencing and exonic copy number analysis of GLDC and AMT.2
4.4 Relation with pathology findings
Previous authors related the white matter diffusion restriction and increased T2 signal in NKH patients to the presence of microcystic spongiosis, which resolved around age 17 months, suspected secondary to enlargement of these microcysts.15 The restriction of proton diffusion in any direction is detectable by DWI when the axis is 14 to 16 μm.15 On pathology, neonates with classic NKH show a normal amount of myelin but with microvacuolation of variable sizes, with a maximum size of 300 μm, most prominently affecting the cerebellar white matter, corticospinal tracts, optic tracts, and optic nerves, and least severe in the posterior columns and anterior limb of the internal capsule.27, 29, 31, 33, 34 In older children with NKH, at autopsy spongiosis persisted at 15, 24, and 36 months, and even at 17 years of age,28, 34, 42 consistent with the persistence of decreased ADC values on quantitative analysis.
In NKH microspongiosis, the axon appears normal but the myelin lamellae are split along the intraperiod line by separation of the myelin sheets in the extracellular space, and more so on the outer site of the compact myelin.27, 30 The microcysts do not stain with glial fibrillary acid protein; there is variable astroglial reaction, and no change in the number of oligodendroglial cells.27, 28, 30-32 Splitting at the intraperiod line implies dysfunction of maintaining contact between the extracellular membranes of opposing folds of the oligodendrocyte, which can be due to lack of the function of proteins of the outer oligodendrocyte membrane, changes in the special lipids of the outer myelin membrane such as galactocerebrosides and cholesterol esters, or due to lack of removal of the intervening water, electrolytes and osmolytes between the outer membrane sheet layers by astrocytes lining the myelin.18, 43 Interestingly, the evolution of the restricted diffusion over time closely matches the onset of myelination on T1-weighted imaging. This suggests that the spongiosis may correspond to the development of the cell membrane components that cause T1 shortening on MRI, which include galactocerebrosides and cholesterols.44 The glycine cleavage enzyme is only expressed in astrocytes of the cerebrum, cerebellum and, to a lesser extent, the brain-stem.45 In the chloride channelopathy CLCN2 disorder, dysfunction of astrocyte water and electrolyte management results in myelin splitting at the intraperiod line and a similar diffuse increase in diffusion restriction over the entire cerebral white matter as is noted in NKH in late infancy.43 These patients often have preserved cognitive function illustrating that spongiosis is not necessarily related to cognitive dysfunction. Indeed, despite the prominence of spongiosis on neuropathology and the corresponding diffusion restriction on neuroimaging, this feature was consistently present in all categories of NKH regardless of severity and neurodevelopmental outcome. This raises the question whether the spongiosis is an epiphenomenon.
In contrast, the size and growth rate of the corpus callosum related to outcome, differing significantly between severe and attenuated NKH. Patients with severe NKH developed regions of brain atrophy over time, whereas the more mildly affected patient with attenuated NKH did not. The corpus callosum reflects the largest structure of the white matter, and its growth failure illustrates how the disease process affects the growth and function of the white matter. The pathophysiology of this process is currently largely unknown but factors that potentially can affect white matter growth pertinent to NKH include abnormalities in folate metabolism identified in mouse models of NKH,26 and persistent epilepsy in severe NKH.
4.5 Brain glycine levels on MRS relate well to measured postmortem levels
On short echo time (TE 35 ms) MRS the glycine signal at 3.55 ppm coincides with myoinositol, but at intermediate echo time (TE = 135 ms), glycine is recognized at 3.6 ppm without overlap. Glycine concentrations obtained by relating the MRS peak to that of an external glycine standard present in the MR instrument showed values between 4.0 and 8.0 mmol/L, which correlated within 15% with chemical quantification of glycine levels in the brain (4.4-6.6 mmol/L) at postmortem 2 days later.46 Glycine has two protons whereas creatine has three protons to make up the NMR spectrum peak, and using an estimated creatine concentration of 5 mmol/L, glycine brain levels were estimated at between 2.3 and 7.4 mmol/L.22 Glycine levels measured at autopsy in the brain have ranged in the frontal cortex between 4.1 and 5.89 mmol/L (controls 1.24-1.77 mmol/L); in the occipital cortex between 3.55 and 6.79 mmol/L (controls 1.57 and 1.66 mmol/L), and in cerebellum between 8.1 and 15.4 mmol/L (controls 1.67 and 2.13 mmol/L).1, 28, 47 Thus, cerebral glycine levels are increased 3- to 9-fold in NKH over controls. This is reflected in the glycine signal on MRS, but the variable instrument settings did not allow quantitative relationship in this retrospective study and review. Some authors pointed to a relation of the glycine/creatine ratio with CSF glycine levels,22, 23 or the clinical state,22 but no systematic or controlled studies have been done, and the relation of cerebral glycine with age in NKH has not been examined. In patients with attenuated NKH, the glycine peak can be difficult to see on MRS, hence appearing to be of lower amount, consistent with our findings.48, 49 A small lactate peak rarely observed on MRS was not identified in our study.22, 50
4.6 Study limitations
Several limitations inherent to this type of study are worth mentioning. As a retrospective review study of clinically obtained MRI images, the MRI sequences and protocols differed between institutions, although the impact on the accuracy of ADC values was likely less than 5%.51 Brain imaging as used for clinical analysis cannot identify microscopic malformations of cortical development, which can therefore not be excluded. The growth rate was developed from a composite of multiple patients rather than from longitudinal tracking of individual subjects. However, the data from several patients with available longitudinal data were consistent with the findings. The high signal intensity of T2 resulted in multiple failures of automated volumetric analysis which can result in biased interpretation of the data in particular to white matter volume. Indeed, subjectively some patients had white matter volume loss on long-term follow up. It is possible that this white matter volume loss is more prevalent in patients where the white matter is hyperintense and volumetric analysis was not possible. A future prospective longitudinal study with standardized instrument settings is optimal to provide a quantitative within-patient growth rate of the corpus callosum and other brain structures, while quantitatively monitoring the diffusion restriction, and quantitative assessment of glycine levels on MRS.
5 CONCLUSION
No brain malformations were identified in this study. Previous studies have documented only obstructive hydrocephalus. Lack of growth of the corpus callosum as reflected in shorter length and decreased thickness is common, and this growth failure is directly related to the severity of the clinical phenotype. Following the growth of the corpus callosum may serve as an early indicator of clinical success for new therapeutic measures currently under development, before atrophy becomes evident. Diffusion restriction is consistently present in a specific pattern, which evolves over the first 2 years of life, but does not relate to phenotype. Recognition of this diffusion restriction is helpful for accurate diagnosis, particularly in the first 3 months of life, and aids to distinguish from transient NKH.
ACKNOWLEDGMENTS
This study was supported by donations to J.L.K.V.H. and C.R.C. from the following organizations: NKH Crusaders Fund, Joseph's Goal Fund, CU Nonketotic Hyperglycinemia Fund, Les Petits Bourdons, Brodyn's Friends Fund, Smiles for Miles NKH Research Fund, Hope for NKH Fund, Lucy's BEElievers Fund, and Madi's Mission to Find a Cure for NKH Fund. These funding sources had no role or influence in the study design, the collection and interpretation of the data, or the writing of the manuscript.
CONFLICTS OF INTEREST
There are no conflicts of interest or disclosures for any author. J.L.K.V.H. has received reimbursement for attending a symposium related to NKH by Lucane Pharma, who had no involvement with or influence over this study.
ETHICS
This study was approved by the Colorado Multiple Institutional Review Board and all patients signed an informed consent. No animals were used in this study.
AUTHOR CONTRIBUTIONS
N.V.S and L.Z.F. designed the study, contributed to data acquisition and analysis, figure preparation, and revised the manuscript for intellectual content. J.B.H and S.B.W. and C.R.C contributed to acquisition and analysis of data, and revised the manuscript for intellectual content. C.L. and S.T. conducted statistical analysis, figure preparation, and revised the manuscript for intellectual content. J.L.K.V.H. designed the study, contributed to data acquisition and analysis and figure preparation, drafted the manuscript for intellectual content, study coordination and corresponding author.




