Humulus lupulus – a story that begs to be told. A review
Abstract
The hop cones of the female plant of the common hop species Humulus lupulus L. are grown almost exclusively for the brewing industry. Only the cones of the female plants are able to secrete the fine yellow resinous powder (i.e. lupulin glands). It is in these lupulin glands that the main brewing principles of hops, the resins and essential oils, are synthesized and accumulated. Hops are of interest to the brewer since they impart the typical bitter taste and aroma to beer and are responsible for the perceived hop character. In addition to the comfortable bitterness and the refreshing hoppy aroma delivered by hops, the hop acids also contribute to the overall microbial stability of beer. Another benefit of the hop resins is that they help enhance and stabilize beer foam and promote foam lacing. In an attempt to understand these contributions, the very complex nature of the chemical composition of hops is reviewed. First, a general overview of the hop chemistry and nomenclature is presented. Then, the different hop resins found in the lupulin glands of the hop cones are discussed in detail. The major hop bitter acids (α- and β-acids) and the latest findings on the absolute configuration of the cis and trans iso-α-acids are discussed. Special attention is given to the hard resins; the known δ-resin is reviewed and the ε-resin is introduced. Recent data on the bittering potential and the antimicrobial properties of both hard resin fractions are disclosed. Attention is also given to the numerous essential oil constituents as well as their contributions to beer aroma. In addition to the aroma contribution of the well-known essential oil compounds, a number of recently identified sulfur compounds and their impact on beer aroma are reviewed. The hop polyphenols and their potential health benefits are also addressed. Subsequently, the importance of hops in brewing is examined and the contributions of hops to beer quality are explained. Finally, the beer and hop market of the last century, as well as the new trends in brewing, are discussed in detail. Hop research is an ever growing field of central importance to the brewing industry, even in areas that are not traditionally associated with hops and brewing. This article attempts to give a general overview of the different areas of hop research while assessing the latest advances in hop science and their impact on brewing. Copyright © 2014 The Institute of Brewing & Distilling
Introduction
Brewing science is an extremely broad field dedicated to the study of all aspects of beer and its production process. It spans many disciplines and applications of interest to the practical brewer. At its most simple it encompasses the study of malt, yeast and hops (Humulus lupulus L.). Through research and collaboration, a number of major breakthroughs have been achieved in the brewing field. Research in brewing plays a predominant role in the brewing industry in part because it provides information about the properties and composition of the materials used. By understanding and then applying this knowledge to practice, brewers may tailor new beers in which specific properties are enhanced or suppressed for specific purposes.
Compared with the large quantities of malt required in beer production, the amount of hops needed is significantly smaller. This minor ingredient has, however, a crucial impact on beer quality and, thus, hops are of paramount importance for beer brewing. The complex chemistry associated with hop substances and their function in brewing has been the subject of extensive investigation for over a century. Yet, surprisingly, greater understanding of the chemical character of the hop compounds is still needed. Recent findings have not only helped strengthen the existing research but also have improved our knowledge of hops. Hop research covers all matters related to hops, from breeding and growing to harvesting and drying as well as their chemistry and their impact on beer quality. As a result of progress in analytical techniques, our knowledge of hops has advanced tremendously, leading to improvements and new product development in the hop and brewing industries. Although a considerable amount of research has been done on a great variety of hop constituents and their function in brewing, much remains a matter of debate, in particular, the chemical background of hoppy aroma 1-4 and the influence of hop polyphenols on the organoleptic properties of beer 5-7. Ultimately, the brewer and brewing chemists understand what the hop substances can do; however, to some extent, it remains unclear how they do it. It is intended in the following to evaluate and review the available literature on this subject and, thus, provide the current status of hop research.
Hop botany
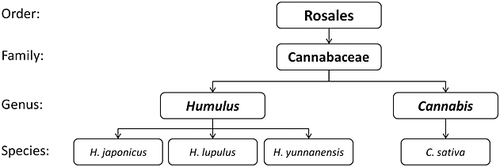
The genus Humulus is made up of dioecious, perennial, climbing vines 8. This genus belongs to the Cannabaceae family of the Urticales order which in 2003 was incorporated into the natural order of Rosales 9. The only other genus in the family is Cannabis (see Fig. 1) represented solely by C. sativa (i.e. Indian hemp, marijuana or hashish) 10. For years it was believe that the genus Humulus was only represented by two species, the ‘common hop’, Humulus lupulus L., and the ‘Japanese hop’, H. japonicus Zieb. et Zucc 11. In 1936, the species Humulus yunnanensis Hu was first described; however, it remained a relatively unknown species thought to have originated at high elevations in the Yunnan province of southern China. Previous to Small's study, it had been almost universally unappreciated and identified as H. lupulus. It was in 1978 that Small identified H. yunnanensis as a species of its own 12. Currently more is known of the H. japonicus species; this is an annual plant indigenous in China, Japan and the neighbouring islands. For brewing purposes it is of no value as its hops are devoid of lupulin glands 11. H. japonicus is widely cultivated as a strong climber, often used in gardens as a decorative, leafy screen 8.
Hop cultivation
The hop cones of the female plant of the common hop species Humulus lupulus L. are grown almost exclusively for the brewing industry. About 97% of worldwide cultivated hops are destined for brewing purposes. The world hop production is dominated by Germany and the USA. The hop production of both countries accounts for about 75–80% of the total hop output 13. Successful cultivation of the hop plant requires optimal growth conditions, especially with respect to the length of daylight, summer temperature, annual rainfall and soil fertility 14. The hop plant is grown throughout most moderate climate regions of the world; these are located between latitudes 35° and 55° of the Northern and Southern Hemispheres 8. More than 60% of the hop area under cultivation is located in Germany and the USA 13. The largest hop growing areas include the Hallertau region in Germany and the states of Washington, Oregon and Idaho in the USA. Other hop growing countries are the Czech Republic, Poland, Slovenia, England, Ukraine, China, South Africa, Australia and New Zealand.
Hop cones
The inflorescences of the female plants form the hop cones (strobile) 11 used by the brewing industry. The hop cone consists of stipular petal-like structures called ‘bracts’ and ‘bracteoles’ around a central axis or strig. At the base of the bracteoles, the lupulin glands are formed as the hop ripens. Only the cones of the female plants are able to secrete the fine yellow resinous powder known as lupulin glands. It is in these lupulin glands that the main brewing principles of hops, the resins and essential oils, are synthesized and accumulated 8, 10. In 1821, Ives assigned the name ‘lupulin’ to this yellow powder; he was the first one to observe that it is in the lupulin where the bitter and aromatic substances of hops are stored 15. The lupulin glands are only weakly attached to the hop cones, therefore, hops have to be handled carefully to avoid losing the valuable hop components 14.
In most commercial hop growing areas worldwide the seed content in hops is regulated. Male hops are physically removed from the hop fields to avoid the fertilization of the female plants and, thus, the production of seeds. In most parts of the world, seeds are considered by brewers to be undesirable. It is believed that oxidation of the seed fatty acids produce off-flavours in beer 16. Further, it has been proven that seedless hops are generally richer in essential oils and resins than seeded ones (i.e. higher brewing value) 10. However, male plants are essential in hop breeding programmes to develop new varieties through controlled hybridization.
Hop harvesting and drying
In late summer or early autumn, when the hop cones have ripened and the resin content is highest, the hops are harvested. At harvest the moisture present in hops is about 75–80%; at such high moisture level not only do the hop compounds change rapidly but they also go mouldy quickly. Thus, the hop cones are carefully dried in the oast house or kiln at temperatures between 60–75 °C to reduce the moisture content to about 10% 17. After cooling and conditioning the hops are compressed and packed into bales. These bales are stored cold until required for use, either sale or processing into hop products.
Chemical composition of hops
Whole hop cones comprise several components, such as resins, essential oils, proteins, polyphenols, lipids, waxes, cellulose and amino acids 10, 18. The typical overall average chemical composition of fresh dried hop cones is shown in Table 1. The leafy nature of the hop petals ensures the presence of ubiquitous substances such as proteins, carbohydrates and polyphenols 8, 19. The brewing value of hops is primarily attributed to the precursors of the flavour- and bitter-active compounds found in the resins secreted by the lupulin glands. The hop essential oils are also important to the brewer as they provide flavour and aroma characteristics to the beer.
Hop chemistry
Hop resin nomenclature
It is in the lupulin glands of the female hop cone that the main brewing principles, the resins and the essential oils, are synthesized 10. The characteristic resins of hops include many substances; Hayduck originally separated the hop resins into α-, β- and γ-fractions on the basis of their solubility in different solvents and their ability to form a precipitate with lead acetate 20. Over the years, the hop resin nomenclature constantly changed and already in 1897 the term γ-resin had become obsolete, and ever since this fraction has been more commonly referred to as hard resins 21. In 1957, the situation was clarified by joint proposals of the European Brewery Convention (EBC) and the American Society of Brewing Chemists (ASBC) 22. It was then revised in 1969 by the Nomenclature Sub-committee of the Hops Liaison Committee 23 and has not changed since then.
Hop resins
Total resins
Several resin extraction methods were developed over the years, but all seemed to be flawed and further proved the difficulty of achieving a total purification of the resins 24. Currently, the method most commonly used for fractionation of hop resins is a modified version of Wöllmer's protocol 25-27. In Fig. 2 the composition and most recent nomenclature of the hop resins are shown. Depending on the hop variety and growing conditions, the total resin content can range between 15 and 30% of the total weight of the dried hops 28. The total resin is defined as the fraction soluble in diethyl ether and cold methanol. The requirement that the total resins should be soluble in cold methanol is designed to exclude the hop waxes, which will slowly crystallize from cold methanol 29. For the extraction of the total resin, cold methanol is specified because the hot solvent dissolves or disperses waxes 22.
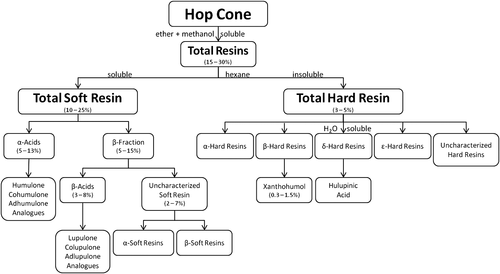
The hop total resins can be further divided into soft and hard resins. Compared with the soft resins, the hard resin content in whole hop cones is low; it ranges between 3 and 5%, while the soft resins comprise 10–25% of the total weight of dried hops 28. The amounts of soft and hard resins recovered by further fractionation of the total resins are 90 and 10%, respectively 30, 31. These values may vary as the total resin composition is dependent on the hop variety and hop product. The chemical and physical properties of the soft and hard resins differ from one another. A soft-resin enriched extract purified from cultivar (cv.) Hallertauer Taurus (H. Taurus) type 90 pellets is shown in Fig. 3(A). The soft resin extract usually displays a distinct yellow hue. The soft resins are able to produce a thick, viscous, very dense fluid, whose consistency is comparable to that of honey. The dark green powder shown in Fig. 3(B) is the hard resin extract prepared from cv. H. Taurus CO2 spent hop material (i.e. hop powder remaining from the supercritical CO2 extraction). Depending on the hop variety and hop product, the colour of the purified extracts may vary. When preparing the resin rich extracts, the varietal characteristics are revealed through the extract's colour. However, the consistency of the extracts is not affected by the hop variety or hop product used.

Soft resins
To date, it is generally accepted that the total soft resin is soluble in hexane and that the bulk of the brewing and bittering value of the hop is found in this fraction. The total soft resins consist of α-acids and the β-fraction (i.e. β-acids and uncharacterized soft resins). The term ‘resin’ is used as the base available in spite of the recognition that the two main groups of constituents (i.e. α-acids and β-acids) are crystalline and not resinous in nature 22. The α-acids can be readily separated from the total soft resins by their ability to form an insoluble lead salt with lead(II) acetate in methanol 10. Prior to 1952, the α-acid fraction of hops was believed to consist almost entirely of a single component, known as humulone 32. Rigby and Bethune used countercurrent distribution to establish that the α-acids are, in fact, mixtures of homologues and analogues. In addition to humulone, two other substances are present in substantial amounts, cohumulone and adhumulone 33, 34. Trace amounts of two other α-acids, prehumulone and posthumulone, are also found in hops. The composition of the α-acid fraction varies greatly among the hop varieties.
α-Acids
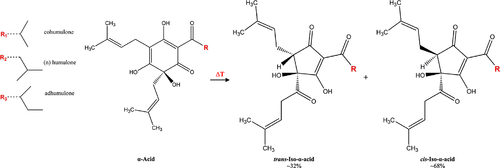
It is well established that the α-acids are by far the most important constituents of the hop resins. Upon addition of hops to the wort kettle, the α-acids are extracted and thermally isomerized during the boiling process into the more water soluble and bitter iso-α-acids (Fig. 4). Only traces of the α-acids survive into the finished beer; the largest loss of α-acids occurs when the hops are added to the wort kettle. Each of the α-acids undergoes isomerization to produce the corresponding iso compound. The major constituents comprising the iso-α-acids are isohumulone, isocohumulone and isoadhumulone. Each α-acid yields in fact two iso-α-acids as these occur as diastereoisomers in their cis and trans forms 35. Although the isomerization process has been known since the late 1920s 36 and has been extensively studied by many research groups over the years 37-47, the configuration of the iso-α-acids had remained speculative. In a recent study 48, the absolute configuration of the cis and trans iso-α-acids was determined by X-ray crystallography. The configuration of (−)-humulone was determined as (6S), thus the conserved stereocentre during isomerization is C4, while cis and trans iso-α-acids differ stereochemically at C5.
In further studies on the α-acid composition, traces of other α-acids were found in hops. In 1955, Verzele identified a fourth component, prehumulone, by partition chromatography. Verzele also established that the component eluting last in the chromatographic separation of the hop α-acids was posthumulone 49, 50. As previously mentioned, the composition of the α-acids is characteristic of the hop variety; however it is further dependent on the harvesting time. It was determined by Verzele that, independent of the hop variety, the levels of prehumulone and posthumulone are higher when the hops are harvested late 14.
β-Fraction
β-Acids
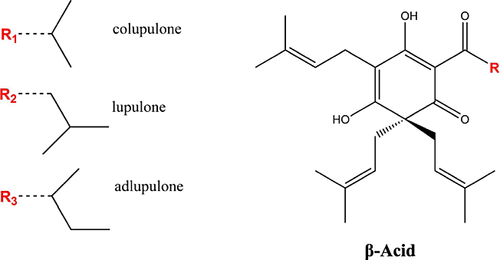
The β-fraction of the total soft resins can be further divided into β-acids and uncharacterized soft resins. The less acidic β-acids can be isolated from hops by removing the stronger α-acids first with lead(II) acetate 51. Lupulone, a β-acid constituent, was first isolated from hops by Lermer in 1863 52, 53. Compared with the α-acids, the β-acids have been subjected to less extensive studies. Up until the 1950s, humulone and lupulone were the only known hop acids. It was later established that, like the α-acids, the β-acids are a mixture of analogues. The compounds in the β-acid series bear the same relation as the α-acid constituents and, thus, these are termed analogously. The β-acid group is composed of lupulone and four congeners: colupulone, adlupulone, prelupulone and postlupulone (see Fig. 5). In 1956, it was established by Howard and Tatchell that the β-acids are always richer in colupulone than the α-acids are in cohumulone 54.
The β-acids show poor solubility in water 52, and while the α-acids undergo isomerization during wort boiling, the β-acids do not. The wort properties (i.e. low pH) do not favour β-acid solubility and as a result only trace amounts are transferred into beer. Previous to the studies of Haseleu et al. 55, 56 the β-acids were believed to be lost in the brewing process and, thus, did not contribute to beer bitterness. Haseleu et al. were able to identify a number of bitter tasting β-acid transformation products that are generated during wort boiling, thereby proving that, in addition to the α-acids, the β-acids are potential bitter taste precursors present in the hop soft resins.
Uncharacterized soft resins
The uncharacterized soft resins is considered a non-specific hop fraction 29. This fraction consists of the portion of the total soft resins remaining after the α-acids have been precipitated and the β-acids are allowed to crystallize out 10. Hitherto, the constituents of the uncharacterized soft resins have not been characterized as any specific compound. Essential oil components and hop wax are also found in this fraction. This is possible since the hop oils are soluble in ether and light petroleum. Although most of the hop wax is removed, the separation of hop wax from the cold methanolic solution is slow and normally incomplete; as a result, traces of wax occur in this fraction 10. The uncharacterized soft resins can be further divided into α-soft resins and β-soft resins. These terms are reserved for substances that may later be identified as having arisen from the α-acids or the β-acids, respectively 22. The brewing value of the uncharacterized soft resins remains unknown.
Hard resins
By definition the hard resin is the portion of the total resin that is soluble in methanol and diethyl ether but insoluble in hexane and low boiling paraffinic hydrocarbons 29. It is generally accepted that the hard resins arise by oxidation of the soft resin; however, it is neither well defined nor proven conclusively what the hard resins consist of. In 1956, Schild and Raum found hard resins in hops at the earliest stage of development 57; therefore it is necessary to differentiate between the native hard resin of hops and that which arises by autoxidation of the soft resin during kilning and storage 10. Unfortunately, hitherto, no distinction has been made between the deterioration or oxidation products and the natural hard resin constituents of fresh hops 32. However, given that the soft resins are prone to oxidation, this results in a challenging task.
As hops age during storage, the percentage of soft resin falls while that of the hard resin increases 58. In 1964, Ashurst et al. 59 inconclusively addressed the question of what constituents are responsible for the bittering ability of stored hops when the α-acids have been transformed into other substances. At that point, it had been recognized for many years that the resins of the hop undergo several changes during storage: the α- and β-acids become oxidized, the products still being analytically classed as soft resins, while further oxidation results in gradual transformation to hard resins. Thus, the α- and β-acids decrease continually during storage while the amount of uncharacterized soft resin increases at first and then decreases as the hard resin steadily increases 59, 60. While some authors 19, 58 automatically considered oxidized soft resins to be hard resins, others 32, 59-61 considered the uncharacterized soft resins in old hops to be the intermediate deterioration products of the α- or β-acids which, upon further oxidation, will ultimately turn into hard resins. It is believed that these intermediate deterioration products have brewing value, but the exact role of the oxidation products, be it oxidized soft resins or hard resins, in the brewing process is not fully understood 32, 60.
α-Hard resin and β-hard resin
Hitherto, the complex nature of the total hard resin has not been conclusively characterized. In the early 1960s, Burton and Stevens proposed two fractions derived from the total hard resin, the α-hard resin and the β-hard resin fraction 58. The α-hard resin fraction represents a small part of the hard resins capable of forming an insoluble lead salt when treated with a lead acetate solution 58. Using ion-exchange chromatography it was possible to perform further fractionation of the α-hard resin. Some of the collected fractions of the α-hard resin were believed to be α-acids that did not completely precipitate earlier in the fractionation 10. However the term α-hard resin does not imply that all the material is derived from the α-acids but that, like the α-acids, it gives an insoluble lead salt 58. The β-hard resin is the major portion of the total hard resin; unlike the α-hard resin, this fraction fails to give an insoluble lead salt 10, 58. Xanthohumol accounts for the bulk of the β-hard resin. With the exception of xanthohumol, the precise chemical identity of the hard resins is not well understood 8.
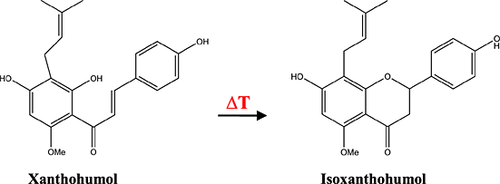
Xanthohumol
Xanthohumol is the most abundant prenylated chalcone present in the hop lupulin glands and naturally found in the hard resins. Many of the native hard resins are made up of prenylflavonoids 8. Xanthohumol was first isolated from hops by Power, Tutin and Rogerson in 1913 62. Xanthohumol is the only known naturally occurring methylated hop resin 10. In the last decade, on account of the wide range of potential health benefits, xanthohumol has been the source of numerous investigations 63-65. Although xanthohumol is the major compound in hop hard resins, only trace amounts are found in beer as it is lost in significant quantities in the conventional brewing process. During the brewing process, the thermal isomerization of chalcones into flavones takes place and xanthohumol is cyclized to isoxanthohumol (see Fig. 6). Attempts to increase the amount of xanthohumol present in the finished beer have been made by several brewing scientists. To achieve this, brewing trials using xanthohumol enriched extracts were carried out 66-69.
δ-Hard resin
In 1952, Walker et al. 70 found a water-soluble portion of the hard resin that also had a bitter character. They termed this portion of the total hard resin the δ-resin. An aqueous solution of the δ-resin was described to be intensely bitter yet the taste was pleasant. In their study, Walker et al. collected evidence to determine that the δ-resin is produced by oxidation of a non-water-soluble constituent of the hop resins. Additionally, data from preliminary chromatographic examination of the δ-resin indicate that it is not homogeneous 70. In a later study, Abson et al. 71 developed a method for estimating the δ-resin content in hops. In their experiments, they observed that this fraction accumulates during the storage of hops. However, they could not find any direct relationship between the percentage decrease in the α-soft resin (i.e. α-acids) and the amount of δ-resin accumulated during the same period of time. From this observation it could be inferred that the δ-resin is not the sole product of changes occurring in the α-soft resin. In the analysed hop samples, they found δ-resin contents varying from 0.6% to about 4%. From their results, it could be concluded that the δ-resin content in hops, just like most hop constituents, is variety dependent. Further, the formation of δ-resin upon storage varies among the different hop varieties 71.
Jackson and Walker 72 were able to separate the total δ-resin into several non-crystalline fractions. In their preliminary fractionation of the δ-resin by column chromatography, they isolated six fractions (δI–δVI). Small-scale experiments indicated that these were not single substances. Moreover, they collected evidence to determine that in terms of chemical reaction the fractions were qualitatively similar. All fractions exhibited the same association of chemical functions: unsaturation, enolic/phenolic acidity and carbonyl (ketone) activity. From the crude δ-resin, two major fractions were recovered, δII (66.3%) and δIII (22.5%). In an attempt to fully characterize both fractions, further chromatography was performed. Based on its solubility in benzene and light petroleum, a significant portion of δII is believed to be residues of the soft resin. Contrary to δII, fraction δIII showed the same solubility characteristics as the hard resins. Fractionation of δIII by partition between immiscible solvents yielded four subfractions (δIII A, δIII EA, δIII B and δIII E). It was further observed that neither the total δ-resin nor the fractions isolated from the crude δ-resin are totally soluble in water. However, when using any solvent to suspend the fractions, water was required to achieve complete solubility. Jackson and Walker were not able to identify any pure compounds in the δ-resin. However, from the recorded observations on the similarity in chemical behaviour of the fractions, it was possible for them to conclude that some basic type of structure predominates amongst the δ-resin components 72.
In a recent study, 11 fractions were recovered from the total δ-resin. The isolated fractions could be distinguished based on their physical properties and their solubility. The more polar fractions were recovered as thick resins while the non-polar fractions were collected as brittle powdery material. The fractions could be further differentiated based on their bittering potential as well as their antimicrobial properties. The more non-polar fractions (i.e. δ 9–δ 11) proved to be more active than the polar fractions. No pure compounds were isolated from any of the recovered fractions 73.
Bausch et al. 74 also conducted research on the δ-resin of hops. In their studies, they were able to quantify the δ-resin in fresh green hops. However, they observed that not all substances present in the δ-resin extracted from aged hops were found in that of fresh hops. The δ-resin was found in all hop fractions. Small amounts of crude δ-resin were isolated from raw α-acids and pure α-acids, 2.3 and 1.4%, respectively. In their trials, the hop fractions were artificially aged by subjecting the samples to extreme temperatures and high moisture levels. When the raw α-acids and pure α-acids were separately heated at 70 °C for 8 h (90% moisture level), the δ-resin increased to 32 and 40.6%, respectively. The δ-resin content in the β-fraction of hops was 1.15%; after forced aging this increased to 8.3%. Upon forced aging of the β-fraction, the δ-resin slightly increased, as opposed to the α-acids, where large amounts of δ-resin were produced. The δ-resin content in the hard resin of hops was 22.3%; upon accelerated aging it increased to 23%, thus suggesting that δ-resin found in the hard resins is relatively stable. Moreover, no further oxidation products were generated from the δ-resin. Finally, in picking trials of 1962, Bausch et al. monitored the δ-resin content and composition of hops during a 6 week period. Some substances were no longer found in the δ-resin of all samples harvested a month or more after the first sample was harvested. It was also observed that the δ-resin content of the hop cones significantly decreased during drying 74.
Hulupones
A new group of bitter substances was identified by Spetsig et al. in the late 1950s. It was observed that the β-acids are readily oxidized to the strong bitter-tasting hulupones. It was also established that, for the conversion of β-acids to hulupones to take place, an oxidizing agent was required 75. When these substances were examined by reversed-phase chromatography, three peaks that elute before the α-acids were detected 75-77. Like the humulones and lupulones, the hulupones also consist of a series of analogues. The names cohulupone, hulupone and adhulupone were assigned to the individual compounds; the prefixes have the same significance as in the humulone and lupulone series. Hulupone concentrations have been reported to be between 0.5 and 3.0% of the weight of the dried cones. In contrast to the hard resins, no hulupones were detected in fresh hops 76, 78. When the course of the hulupones was monitored during the brewing process, it was found that the bulk of the hulupones is usually retained in the spent hops that remain after wort boiling. However a considerable fraction is transferred to the wort in its unchanged form, that is, the hulupones in wort exist in the form of salts 75, 76, 78. At least in the 1960s, it appeared that the hulupones commonly contributed to a little over 5% of the bittering principles in beer 78. Owing to the complex nature of the substances, these three compounds are challenging to classify. As mentioned previously, it is generally accepted that the hard resins arise by oxidation of the soft resins. Although hulupones are autoxidation products of the β-acids, when present in hops, these are soluble in all organic acids, and therefore components of the soft resin 78.
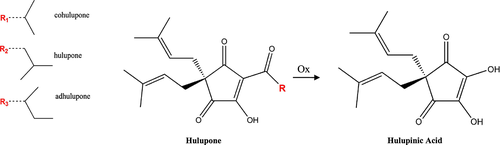
Hulupinic acid
Hulupones are degradation products of the β-acids, yet these can only be considered to be an intermediate oxidation state. Further oxidation of the hulupones will result in the non-bitter hulupinic acid (see Fig. 7) 79, 80. In 1964, Burton and Stevens isolated a crystalline product from the α-hard resin and suggested the name hulupinic acid for this compound 58, 79. When a solution of cohulupone in ethanol was heated under reflux while a stream of oxygen was passed through it, it was possible to isolate hulupinic acid after 3 days. Surprisingly, autoxidation of hulupone and cohulupone in an ethanolic solution gave the same hulupinic acid 79; exactly the same transformation product was obtained from adhulupone by similar treatment 81. From the evidence gathered, it was possible to state that the acyl side chain (i.e. R group) must have been removed and possibly replaced by a hydroxyl group during the oxidation process 14, 79. It was also determined that the amount of hulupinic acid increased with the age of the hops. Nevertheless, at the time, the highest detected hulupinic acid concentration in aged hops was <0.05% 58, 79. The solubility of the hulupinic acid was tested in water and it was established to be approximately 1 g/L at pH 4.0 and it increased to 2 g/L at pH 5.0 58. Based on this information, it is expected to find low amounts of hulupinic acid in the finished beers. Although hulupinic acid is the ultimate oxidation product of the β-acids and moreover this compound was isolated from the α-hard resin, owing to its solubility in water, hulupinic acid is classified as a component of the δ-resin 58.
ε-Hard resin
Previous to a recent study 73, no data have been reported in the literature regarding the ε-resin of the total hard resin. In this study, a portion of the hard resin which was not soluble in water yet had a bitter character was found. This portion of the hard resin was termed the ε-resin 73, 82, 83. It was found that the ε-resin accounts for up to 80% of the total hard resin composition; however, like most hop constituents, this is variety dependent 83. Like the total δ-resin, the ε-resin is not homogenous. When further fractionation of the ε-resin was done, 11 crude fractions were recovered. In the current study, a method was developed for the large-scale production of the ε-extract 84. Upon characterization of the ε-resin enriched extract, compounds that are usually found in the commercially available xanthohumol extracts were also identified in the produced extract. Highly polar compounds were not detected in the ε-extract; moreover, the xanthohumol and isoxanthohumol content in the ε-extract was found to be significantly lower. It was also established that the ε-resin composition is variety dependant, that is, some compounds are only found in certain hop varieties 85.
The bitter intensities of the δ-resin, ε-resin and total hard resin were tested and compared. To do this, each hop resin was separately suspended at its natural concentration ratio in 5% aqueous ethanol. Subsequently, to simulate a beer-like matrix, the pH of all solutions was adjusted to 4.4 with trace amounts of aqueous formic acid (1% in water). The trained panellists were asked to rate the bitter intensity of all samples on a six-point sensory scale ranging from not detectable (0) to extreme 5. The ε-resin was considered by the panellists to be six times more bitter than the δ-resin. The bitter intensity conferred by the ε-resin was comparable to that delivered by the total hard resin (data not shown). The results collected from the tasting sessions revealed that, at least in a water matrix, the ε-resin can deliver a perceivable bitterness 85. However, it must be borne in mind that the sensory bitterness is relative to the matrix.
The 11 isolated fractions from the total ε-resin could be distinguished based on their physical properties and their solubility. Unlike the isolated δ-resin fractions, all ε fractions were collected as powdery material. From the crude ε-resin, two major fractions were recovered: ε 10 (31.3%) and ε 11 (22.9%). In this study, the bittering potential and the antimicrobial properties of the 11 ε fractions were assessed. From the collected data it was possible to classify the fractions based on their activity. The highly polar fractions ε 1 and ε 2 showed no activity. Generally, there was a good relationship among the bittering potential and the inhibitory activity of the ε fractions. As the fractions became more non-polar, the bitter intensity as well as the antimicrobial activity of the ε fractions increased 82.
Almost 100 fractions were recovered by additional purification and fractionation of the ε-resin. In addition, it was possible to isolate over 20 pure compounds from the ε-resin 73, 83. As before, sensory evaluations and microbiology tests were conducted with these fractions. From the collected data it was possible to classify the subfractions and pure compounds based on their bittering intensity as well as their inhibitory effect. The bitter intensity scale ranged from high bitterness to low bitterness. In a similar manner the antimicrobial activity scale was broken down into three levels, ranging from high inhibition to no inhibition. Multidimensional scaling was used to determine the similarities among the subfractions, as a result, eight clusters were generated (data not shown) 73. Ultimately, through the screening and selection of the hop fractions, it is possible to tailor brews to comply with the consumer's requests without compromising the microbial stability of the beer.
Hop essential oils
Like the hop resins, the essential oils are secondary metabolites of the hop plant secreted in the lupulin glands. By definition, the hop oil fraction is the portion of the hop material that is volatile. These volatile aroma compounds are considered ‘essential’ since they give hops their characteristic smell. While the hop resins give beer its bitterness, the essential oils give beer aroma and flavour. Dried hops contain 0.5–3.0% of essential oil 29, 86, 87; it has been reported that this relatively small volatile fraction is a complex mixture of over 200 components. While only 200 compounds had been conclusively identified in the late 1990s, capillary gas chromatography analysis revealed up to 400 peaks in a chromatogram recorded at high detector sensitivity 51. By the beginning of the century, the total number of identified and chemically characterized compounds found in hops was 440. In a more recent study, comprehensive multidimensional gas chromatography (GC × GC) with flame ionization detection was applied; from the collected data, it was suggested that there may be over 1000 different compounds in the hop oil fraction 88.
Almost 200 years ago, it was independently recognized by Loiseleur-Deslongchamps 89 and Hanin 90 that hop cones are very aromatic. Loiseleur-Deslongchamps described the hop aroma to be garlic-like. A few years later, the first attempts to characterize the hop oil were made by Payen and Chevallier. The volatile oils were first distilled from the hop cones and the distillate was further separated into two fractions 91. However, the first systematic examination of the essential oil of hops was done by Chapman from 1895 to 1929 92-98. It is now known that the content and composition of the total essential oil of hops are affected by numerous factors such as hop variety, growth conditions, time of picking (ripeness), drying conditions, aerial oxidation, age and storage conditions 99. There is evidence to suggest that the yield may also be slightly influenced by seasonal effects 91.
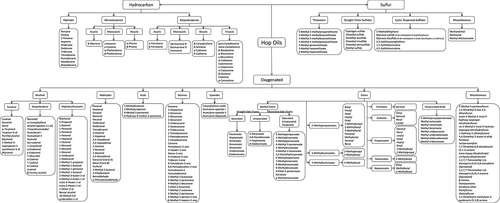
As of 1980, the chemical composition of the hop essential oils has been conventionally described under three main chemical groups, the hydrocarbons, the oxygenated compounds and the sulfur-containing components 100. It has been established that the hop essential oil profile depends on the hop variety. In particular, the amounts of hydrocarbons and oxygenated compounds vary according to the hop variety and age 101. In the late 1950s, Howard and Slater 99 recognized that, independent of the country in which the hops were grown, there was a varietal uniformity in the general pattern observed in the composition of the hydrocarbon fraction. A decade later, Likens and Nickerson 101, 102 and Buttery and Ling 103 confirmed that the essential oil of several hop varieties displayed good varietal uniformity in their chemical composition. Likens and Nickerson established that certain components or ratios of components in the hop oil are highly specific for the individual varieties.
In 1990, Kenny 104 published a key for the varietal characterization of hops based on six ratios calculated from the amounts of 10 essential oil components. Other varietal identification models, based on the hop oil analysis and composition, were proposed by Kralj et al. 105, Kovačevič and Kač 106, 107 and Perpète et al. 108. From their collected data they were also able to propose hop cultivar markers. It was only recently that differences in the essential oil composition within the individual varieties were observed. In some varieties, certain components are affected by the environmental conditions in which they were grown, in which they were grown; in particular country of origin. These oil compounds will either be present in higher amounts or not present at all. Additionally, year to year variations in the hop oil composition may be observed within the same variety 105, 106, 109. Van Opstaele et al. studied the impact of the varietal character of the fractions associated with the floral note 2 and the spicy (or herbal) note 3. Independent of the variety the hop oil fractions were extracted from these fractions deliver similar flavour characters to beer: floral or spicy. The chemical composition of these hop oil fractions is, however, variety dependent.
Hydrocarbons
In Fig. 8, a general overview of some of the known hop oil compounds is shown. The contribution of most of these compounds continues to be controversial. The bulk of the hop oil is made up of hydrocarbons and oxygenated components. The hydrocarbons can be classified into three groups: aliphatic hydrocarbons, monoterpenes and sesquiterpenes 100. The hydrocarbon group is very volatile (i.e. lower boiling fraction) and will readily oxidize and polymerize 92. The solubility of hydrocarbons in water, wort and beer is very low; additionally, most of these compounds are lost by evaporation during the boiling process; as a result only trace amounts are found in beer 110, 111.
β-Myrcene
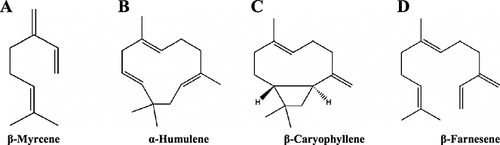
Of the hydrocarbon fraction, the most important and abundant monoterpene is β-myrcene (Fig. 9A); it may account for 30–60% of the total oil content 99, 111-113. In 1895, β-myrcene was first obtained from bay oil by Power and Kleber. In 1903, Chapman recognized that the properties of one of the isolated hop oil compounds were the same as those of the acyclic terpene β-myrcene. By directly comparing the properties of the unknown compound with those of β-myrcene extracted from oil of bay, it was possible to conclude that β-myrcene is also found in the hop oil fraction 95. β-Myrcene is the main component responsible for imparting the pungent smell to fresh hops 51. Other monoterpenes present in the hop oil fraction in significantly lower amounts are ocimene, β-pinene, limonene and ρ-cymene, among others 10.
Sesquiterpenes
In the hydrocarbon fraction, the major components of the sesquiterpene group are α-humulene, β-caryophyllene and β-farnesene. These sesquiterpenes have higher boiling points than the monoterpenes. This high-boiling group is dominated by α-humulene and β-caryophyllene 114. β-Myrcene plus these two hydrocarbons make 80–90% of the total essential oil in hops 95. α-Humulene, the most abundant sesquiterpene found in hops, was one of the first compounds to be identified in hop oil; the structure of the compound is shown in Fig. 9(B) 92. The second most important sesquiterpene found in hops is β-caryophyllene (see Fig. 9C) 92. Although it was suspected as early as 1895 that β-caryophyllene could be present in the essential oil fraction of hops, it was not until 1949 when this component was conclusively identified in hop oil by Šorm et al. 115. When a typical hop oil chromatogram is examined, it can be observed that much of the material is accounted for in three elution bands. The substance responsible for the first peak is β-myrcene; β-caryophyllene elutes much later and shortly after, the peak for α-humulene appears 86. In Fig. 9D, the chemical structure of β-farnesene, an acyclic sesquiterpene, is shown. This compound was first isolated by Šorm et al. from Žatec (Saaz) hops 115, 116. Unlike β-myrcene and α-humulene and β-caryophyllene; β-farnesene is only found in certain hop varieties and usually in low amounts 99, 117.
Howard and Slater investigated hop oil production during the ripening period. To do this, samples were picked at defined intervals during the ripening of hops and the essential oil fraction was examined 118. The essential oil fraction was isolated from these hop samples by steam distillation and analysed by gas chromatography. A portion of the hop oil extract was divided into two fractions by adsorption chromatography and then analysed separately by gas chromatography. From their results it was concluded that the total oil content rises steadily during ripening 99. It was observed that, for most varieties, the oil develops at a later stage than the resins 118. Stevens et al. 119 isolated the essential oils using steam distillation and analysed them by gas chromatography. They were the first to report that the essential oils continue to be synthesized until harvest. They also observed that the production of the essential oil components present in ripe hops does not start before resin development is completed 119. Since many hops were picked when resin development was complete but before oil production had ceased 120, the hop quality in earlier times cannot be compared with the current one. From the picking trials, it was seen that the proportion of β-myrcene rose rapidly throughout the ripening period. The β-myrcene content increased from an almost negligible figure to 36%. The proportions of β-farnesene and β-caryophyllene remained comparatively constant. Unexpectedly, the α-humulene content of hops fell significantly from 79 to 42% 118. From the collected data it could also be concluded that the β-myrcene content is directly proportional to the proportion of cohumulone and colupulone found in the hop resins 99. In another study, Rigby and Bethune isolated the hop essential oils by steam distillation and separated them into different fractions by countercurrent distribution. By means of the chromatostrip and chromaplate technique 121 as well as gas chromatography 122, they collected data to show that hops with high cohumulone had high β-myrcene; conversely, hops with high humulone had a high α-humulene content 122, 123. It was also recognized by Howard and Slater that a high β-myrcene proportion generally accompanied a low α-humulene content. Based on these conclusions, there was enough evidence to suggest that there may be a common intermediate in the biosynthesis of β-myrcene and the α-acids and β-acids 99.
A later study by Murphey and Probasco 124 confirmed the findings of Howard and Slater. It further proved that the harvest point can considerably affect the aroma quality of hops. In all hop varieties studied, the total essential oil content continued to increase throughout the sampling period. The higher total oil content is primarily due to β-myrcene synthesis. The β-myrcene content in most late harvested samples increased from negligible amounts to almost 50% of the total oil content. The β-caryophyllene and α-humulene concentrations of all late-stage harvested samples decreased by more than half of the early stage content. Recently, the effect of the time of harvest and location on the hop essential oil composition of two American aroma hop varieties were compared 125. Contrary to previous studies where the increase in the total oil content was primarily attributed to β-myrcene synthesis, Sharp and Shellhammer were able to determine other hop compounds responsible for the increase in the oil quantity. This increase was strongly correlated with α-pinene, β-pinene, limonene, methyl heptanoate and linalool.
Oxygenated compounds
The hydrocarbon fraction of hop oil is quantitatively eluted from silica gel by light petroleum; a second fraction containing the oxygenated compounds will elute once ether is applied. Although the oxygenated fraction is found in substantially lower amounts [ca. 30% of the total essential oil 126], the composition of this fraction is more complex than the hydrocarbon one 86. In the oxygenated fraction a large number of components are found; however, most of these are present below their odour threshold concentrations. Normally, the oxygenated compounds of the hop oil comprise two major portions that are termed the ‘volatile’ and ‘non-volatile’ portions. The former consists of a complex mixture of compounds with boiling points lower than that of α-humulene. Since the boiling point of the non-volatile fraction is higher than that of α-humulene in early hop oil examination, this portion was more challenging to analyse 99. However, these higher boiling substances are of interest to the brewer since these will most likely be retained in the wort after boiling and they may eventually find their way into the finished beer 117. The total oxygenated fraction is a complex mixture of alcohols, aldehydes, acids, ketones, epoxides and esters. In 1981, Sharpe and Laws reported 60 aldehydes or ketones, 70 esters, 50 alcohols, 25 acids and 30 oxygen heterocyclic compounds in hop oil 100; since then, the list has significantly increased.
It was observed by Howard and Slater that, as hops aged, the oil became richer in oxygenated components at the expense of the hydrocarbons. In addition, oxidation resulted in non-volatile oxygenated compounds at the expense of the hydrocarbons, and as a result the proportion of β-myrcene decreased. Finally, non-volatile oxygenated compounds were produced with a concomitant loss of some of the volatile ones 99. The effects of autoxidation of β-myrcene were studied in detail by Dieckmann and Palamand 127. It was reported that autoxidation proceeds in four reaction classes: cyclization, oxidation, disproportionation and polymerization. Over 40 compounds were found to be produced from the autoxidation of β-myrcene.
Linalool
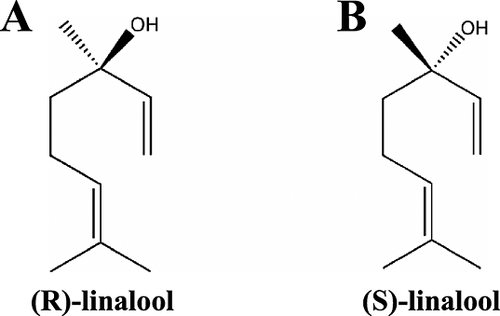
The alcohols of the essential oil fraction can be classified into three groups: terpene alcohols, sesquiterpene alcohols and aliphatic/aromatic alcohols 100. The major constituents of the alcohol fraction are 2-methylbutanol and linalool with lower amounts of geraniol, nerolidol, nerol and terpineol 10. The most abundant terpene alcohol is linalool, and like many essential oil components, it was first identified in 1903 by Chapman 95. Linalool is considered an important hop aroma indicator substance in beer 111. The contribution of linalool to the hop aroma profile is dependent on the hop treatment. It is unlikely that linalool will make a contribution to the aroma profile of the beer if the wort is traditionally hopped (i.e. beginning of the boil). The contribution of linalool to hop aroma in beer is more perceivable when a late hop addition is done. Only minor linalool contributions are detected in dry hopped beers 128. Linalool is a hydration product of β-myrcene 128; it is also a chiral compound; therefore there are two stereoisomers: (R)-(−)-linalool and (S)-(+)-linalool (see Fig. 10A and B). Both enatiomeric forms are found in the hop essential oil fraction. It has been shown that (R)-linalool is more flavour-active and, of the total linalool content, the (R)-form is usually present in hops between 92 and 94% 129.
Linalool is usually considered to be responsible for the floral character in beer. The odour threshold concentration of linalool in water was first reported by Guadagni et al. to be 6 ppb (i.e. 6 µg/L) 130; however in beer this value increases to 10 ppb 111. Sensory threshold values are highly dependent on the olfactory properties and the structures of the molecules, but also on the matrix in which they are tested 131. The composition of the beer matrix (i.e. lower pH and sugar profile) plays a key role in the determination of thresholds as it exerts an effect on the presence or absence of sensation of the hop compounds 132. In addition to the testing matrix, other odour-active substances can also interact and cause strong changes in the odour thresholds of these compounds 131. The data found in the available literature for the flavour threshold values of linalool in beer vary significantly. In 1975, Meilgaard reported a flavour threshold in beer of 80 ppb and described it as having an aniseed and terpenoid flavour 133. A few years later, Peacock and Deinzer reported a flavour threshold value of 27 ppb and characterized it as floral and citrusy 134, 135. More recently a value of 8 ppb was reported by Kaltner et al. 136. Independent of the threshold, linalool is one of the few hop oil components that is universally accepted as being flavour-active in beer 128.
Other oxygenated compounds
Jahnsen 114 suggested that a whole series of aldehydes occurs in hop essential oils. It was shown that aldehydes such as 1-hexanal and (Z)-3-hexenal are responsible for delivering the green and grassy notes to beer 137. Among the acids, it has been reported that only a few free acids are present in hop oil 100. The fatty acids of hop such as 2-methylbutyric acid are linked with a cheesy aroma 138. The first ketone to be characterized by Šorm et al. in 1947 was undecan-2-one 116. It was first identified in 1928 by Chapman and termed luparone 97; this term is no longer used. This compound, also known as methyl nonyl ketone, was found in the late 1950s to be the most abundant oxygenated component in most hop oils 122. It has been reported that this hop oil component gives a floral flavour to beer 139. Many other ketones were later reported to be present in hops by Jahnsen 126 and Guadagni 130, among others.
The oxygenated hop sesquiterpenoids, in particular humulene epoxide II, have been associated with the spicy or herbal hop character 140. Efforts have been made to unravel the nature of this spicy note. However, owing to the very complex chemical composition of the spicy hop character and the lack of reference compounds to verify the analytical data, many of the compounds responsible for this hop character remain unknown. Eyres et al. 141 and Nielsen 142 were able to tentatively identify 14-hydroxy-β-caryophyllene and caryophylla-3,8-13-dien-5-β-ol, respectively, as highly odour-active compounds responsible for the spicy character of hops. More recently, Van Opstaele et al. 3 were able to associate another 22 oxygenated sesquiterpenes to the spicy fraction of hops. Among these 22 constituents, eight compounds were tentatively identified as α-humulene or β-caryophyllene oxidation products. The precise identity of 10 of these compounds remains unknown. Of the 12 tentatively identified oxygenated hop sesquiterpenoids, humulene epoxide II appeared to be the most predominant constituent, followed by (−)-caryophyllene oxide. Other tentatively identified compounds associated with the spicy or herbal character of hops are humuladienone, caryolan-1-ol, globulol, viridiflorol, 10-epi-α-cadinol and τ-cadinol 3. Goiris et al. 143 determined the flavour threshold of the enriched sesquiterpenoid hop oil fraction in beer to be 5 ppb; however, an addition of 20 ppb was usually preferred by the panellists.
The total ester fraction represents a complex mixture having a wide boiling range 86. The esters are probably the most important of all the hop oil components owing to their contribution to hop aroma and flavour 100. It was independently reported by Wright and Connery 144 and others that esters of hop oil are the constituents more closely associated with quality. It was also noted that oils with high ester content, low acid and low volatile reducing power had a more delicate and pleasant aroma 121. Naya and Kotake reported that the β-myrcene-rich varieties show high contents of esters 145. Many esters such as 2-methylpropyl isobutyrate are known for their fruity and floral notes 138.
Sulfur compounds
The essential oil of hops contains only trace amounts of sulfur compounds. However, since these have potent aromas and low odour thresholds they may easily influence the overall flavour of beer 146. The volatile organosulfur substances afford the most detrimental hop flavours to beer 147. These compounds have very low sensory thresholds; values can be as low as 0.1 ppb 148. In general, these components impart undesirable sulfury, cooked vegetable, musty, cabbage-like and onion-like flavours to beer 100, 148. The methyl thioesters impart unpleasant cheesy, cooked vegetable and sulfury aromas. In addition to these aromas, the methylthiomethyl thioesters also impart onion-like and garlic-like odours. The rubbery and garlic-like off-flavours in beer are imparted by 2,3,5-trithiahexane and 3,3-dimethylallyl methyl sulfide, respectively 148. Many studies have shown that a broad range of odours can be produced by the sulfur compounds; however, most of them are considered to be undesirable off-flavours. The thioester responsible for the truffle aroma is S-methyl-2-methyl thiobutanoate 149. Green and fruity notes are associated with S-methyl thiohexanoate; this substance has also been reported to produce a pineapple odour 150. Another aroma delivered by the organosulfur compounds is that of roasted meat; this note is imparted by 2-methyl-3-furanethiol 147. Another interesting sulfur containing class is S-methylthiomethyl thioesters, which represents a new class that has not been isolated from any other natural source 151. Recently, an S-cysteine conjugate, S-3(1-hydroxyhexyl) cysteine, was identified for the first time in Cascade hops. The exact aroma character of this compound has not been conclusively defined 152.
4-Mercapto-4-methylpentan-2-one
Of particular interest to the brewer and brewing chemist has been the intense muscat-like (i.e. grape) character found in some beers. These muscat-like characteristics were attributed to 4-mercapto-4-methylpentan-2-one (4MMP). This compound can only be found in specific hop varieties and has been shown to have an extremely low threshold value in beer 1.5 ppt (i.e. 1.5 ng/L) 137. It has also been documented that 4MMP produces aromas reminiscent of blackcurrant and cat urine 109, 153. The growing location and conditions have the most noticeable effect on the levels of thioesters and sulfur compounds 151. Interestingly, 4MMP was detected in hop cultivars grown in the USA, Australia and New Zealand. This compound was, however, not found to be present in the same cultivar grown in Europe 109.
Hop polyphenols
Polyphenols are another secondary metabolite of the hop plant; hop polyphenols comprise up to 4% of the total weight of dried hop cones. Similar to most hop constituents, the level of polyphenols is variety dependant. These are to be found mostly in the hop cone petals and strig and, with the exception of the prenylflavonoids (e.g. xanthohumol), not in the lupulin glands 8. The polyphenol fraction represents a vast class of compounds with widely varying structural characteristics 154. Chemically, these are substances that are built up by multiple phenol units. Although they are a very heterogeneous group of substances, the polyphenols share a common structural element: an aromatic ring with at least two hydroxyl groups 155. The composition of the polyphenols depends on the hop variety, cultivation area, harvesting technique and degree of aging 154. It has been reported that aged hops contain higher levels of polyphenols than fresh ones 156. It has also been shown that certain polyphenolic substances are unique to a specific hop variety 157.
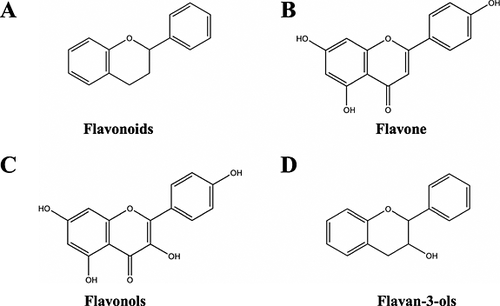
Analysing hop samples by high-performance liquid chromatography–diode array detection, Forster et al. found over 100 compounds in the polyphenol fraction of hops 158. Hop polyphenols can be classified into flavonols (e.g. quercetin), flavan-3-ols (i.e. flavanols or flavanoids, e.g. catechin), phenolic carboxylic acids (e.g. ferulic acid), and other polyphenolic compounds (e.g. prenylflavonoids and multifidol glucosides) (see Fig. 11) 159. Flavonols (Fig.12C) and flavan-3-ols (Fig. 12D) are derived from the structure of flavone (see Fig. 12B) and as such are classified as flavonoids (Fig. 12A), a subgroup of polyphenols 155. Some polyphenols are unique to hops; that is, they have not yet been found in any other natural source. Some of these polyphenolic substances hitherto found only in hops are multifidol glucosides and prenylflavonoids such as xanthohumol, desmethylxanthohumol, 6-prenylnaringenin and 8-prenylnaringenin 159, 160.
In general, aroma hops contain a higher amount of low molecular weight polyphenols than bittering hops 161. The reason for this is that an increase in α-acid content can only be obtained at the expense of the polyphenol content 162. Most polyphenols in wort are derived from malt; however, about 20–30% of the polyphenols found in the wort come from the hop material 139. The importance of the hop polyphenols in the brewing process is due not only to their contribution to flavour but also to their role in the production, by protein–polyphenol interaction, of non-biological haze, which limits the shelf-life of bottled beers 10. Since hop polyphenols can be very easily oxidized, they act as strong antioxidants 155. They do this by reacting as free radicals scavengers, by inhibiting lipoxygenases, or by acting as metal chelators 163. Low-molecular-weight polyphenols are natural antioxidants and account to a great extent for the reducing power of wort, thereby protecting beer against oxidation and improving the taste stability. Higher-molecular-weight polyphenols contribute to the colour of beer and to haze formation 154.
Flavonoids
Flavonols
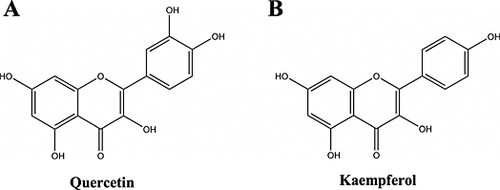
Approximately 20% of the total hop polyphenols consist of low-molecular-weight substances or monomer substances such as the phenolic carboxylic acids as well as the flavonoids and their glycosides (Table 2). Hop flavonoids consist mainly of catechins and their polymers, proanthocyanidins, quercetin (Fig. 13A) and kaempferol (Fig. 13B) 164. The two protonated aglycones, quercetin and kaempferol, do not occur in free forms in hops but only in glycosidically bound forms (i.e. bound to sugars) 155, 165. The more hydrophilic protonated quercetin glycosides elute earlier than protonated kaempferol glycosides when the glycosidic residues are the same. The glycosides of quercetin elute earlier because of the extra hydroxyl group on the flavonoid unit 165. Of the known flavonols, quercetin has the highest antioxidative potential 164. Later eluting glycosides, such as kaempferol, are more prominent in aroma hops 165. Hitherto, the most suitable polyphenols for cultivar differentiation are the quercetin and kaempferol glycosides; the other phenolic components are less cultivar specific 155.
| Substances and substance groups | Amount (%) |
|---|---|
| Phenolic carboxylic acids | |
| Benzoic acid derivatives | <0.01 |
| Cinnamic acid derivatives | 0.01–0.03 |
| Flavonoids | |
| Xanthohumol | 0.20–1.70 |
| 8-,6-Prenylnaringenin | <0.01 |
| Quercetin | 0.05–0.23 |
| Kaempferol | 0.02–0.24 |
| Catechins and epicatechins | 0.03–0.30 |
| Oligomeric proanthocyanidins | 0.20–1.30 |
| Acylphloroglucinol derivatives (multifidols) | 0.05–0.50 |
| Higher molecular substances | |
| Catechin tanning agents and tannins | 2.00–7.00 |
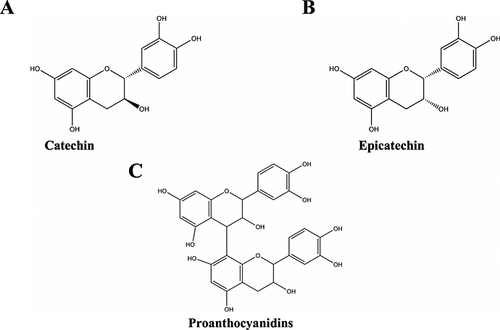
Flavan-3-ols
The polyphenol solubility in wort and beer is not the same for all compounds. As expected, the hydrophilic groups of substances such as hydroxybenzoin, hydroxycinnamic acids, flavan-3-ols and proanthocyanidins have a high solubility. The prenylflavonoids (e.g. xanthohumol) are more difficult to bring into solution. However the flavonoids have an intermediate solubility 161. A portion of the hop polyphenolic material is composed of water soluble substances such as catechin and epicatechin flavan-3-ols (Fig. 14A and B, respectively). These are the monomeric building blocks of dimers, trimers and higher polymeric structures sometimes having as many as 20 or more basic units. These polymers, known as proanthocyanidins or condensed tannins, may also contain gallocatechin and epigallocatechin as extension units 166. Chemically, molecules that consist of up to eight monomer units (oligomers) are referred to as proanthocyanidins. Tannins (polymers), however, consist of a higher number of monomers 111. Proanthocyanidins (see Fig. 14C) are flavan-3-ol oligomers and polymers that give anthocyanidins upon acid depolymerization reactions 167. These are the most reactive substances of the polyphenol fraction 139. While the proanthocyanidins may contain catechin and epicatechin as monomers or as terminal and extension units, gallocatechin and epigallocatechin were only found to be subunits but not terminal units of the polymers 166. Hop proanthocyanidins have received special attention in the brewing industry because they contribute to haze formation 168. As previously mentioned, the proanthocyanidins or tannins are a group of water soluble polyphenolic compounds 139. In beer these proanthocyanidins will slowly react with the proteins present to form a non-biological haze. These insoluble precipitates will ultimately limit the shelf-life of bottled beers 29.
Other polyphenolic compounds
Prenylflavonoids
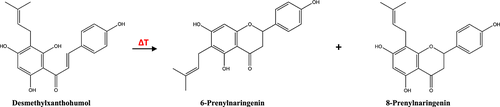
The major component of the prenylflavonoids in fresh hops is the chalcone xanthohumol. A smaller amount of desmethylxanthohumol is also found in the lupulin glands of hops. The prenylchalcones, xanthohumol and desmethylxanthohumol, are naturally present in hops and they are precursors of the isomeric flavanones 169. During the brewing process, these prenylchalcones are largely converted into their isomeric flavanones: isoxanthohumol and prenylnaringenins, respectively 67. While xanthohumol can only cyclize to isoxanthohumol (see above Fig. 6), desmethylxanthohumol gives a mixture of 6-prenylnaringenin and the 1:1 racemate of (±) 8-prenylnaringenin (Fig. 15) 29, 170, 171. The estrogenic racemate of (±)8-prenylnaringenin is often referred to simply as 8-prenylnaringenin or ‘hopein’. The term hopein was coined in 2001 by De Keukeleire. Of the prenylnaringenins, the most important one is 8-prenylnaringenin. This compound was reported as the most active (in vitro) phytoestrogen known in the plant kingdom 172. Phytoestrogens are a plant form of the oestrogen hormone and these can help prevent cardiovascular diseases and cancer 159. Of the other prenylflavonoids found in hops, 6-prenylnaringenin displayed only very weak estrogenic activity. Moreover, while isoxanthohumol was only weakly active, xanthohumol proved to be completely inactive in terms of estrogenic activity 172. Additional health benefits of the estrogenic properties of hops were reviewed by Chadwick et al. 171. It was not until 1988 that the chemical structure of 8-prenylnaringenin, the principal estrogenic component of hops, was for the first time correctly established by Hänsel and Schulz 170. The presence of the estrogenic properties of hops had long been known. However the report by Milligan et al. may be regarded as the beginning of the modern, unambiguous understanding of the in vitro estrogenic activity of hops 172.
Multifidol glucosides
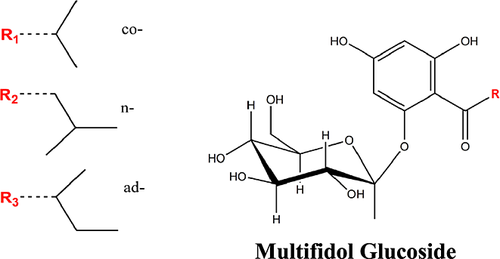
The naturally occurring acylphloroglucinol 1-[(2-methylbutyryl)-phloroglucinyl]-β-d-glucopyranoside, commonly known by its trivial name ‘multifidol glucoside’, was first isolated from the latex of Jatropha multifida L. The term ‘multifidol’ is used to refer just to the aglycon. This compound is known to be immunologically active and have curative properties 173. Multifidols do not occur in the free form but are, like many other polyphenols, glycosidically bound 159. Recently four monoacylphloroglucinol-glucopyranosides and one aglycon were isolated from hops. The occurrence in hop cones of all these compounds but one was first reported in 2005 174, 175. Similar to the α-acids and β-acids of hops, multifidols are acylphloroglucinol derivatives with different side chains. The identified compounds were named according to the nomenclature of the hop bitter acids. Based on the structure of the multifidol glucosides homologue, the prefix ‘co-’, ‘n-’ or ‘ad-’ is assigned (structures shown in Fig. 16). The isolated hop multifidol glucosides demonstrate anti-inflammatory activity in vitro 174.
Resveratrol
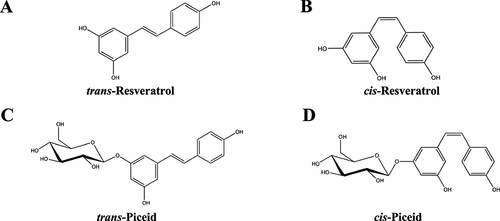
In 2005, Callemien et al. 176 mentioned for the first time the presence of three cardioprotective stilbenes (a class of phenolic compounds) in hops: trans-resveratrol, trans-piceid and cis-piceid (Fig. 17A–D, respectively). cis-Resveratrol (see Fig. 17B) was absent in the fresh hop cones and pellets of all tested hop varieties 177. However, evidence was gathered to prove that cis-resveratrol is generated from cis-piceid (the resveratrol glucoside) during storage 178, 179. Similar to many hop constituents, the stilbene content in hops is affected by the variety, harvest year and geographical origin, among other factors. Except for very high oxygen-sensitive hop varieties, the lower the α-acid content, the higher the resveratrol potential 177. It is well known that the biologically active ingredient of red wine is resveratrol. Recently, the cardioprotective effects of wine have been attributed to this compound 180, 181. Compared with grapes, the concentration in hops is low; up to 2 mg/kg of trans-resveratrol has been found in fresh hop cones 182. trans-Resveratrol is much more hydrophobic than other hop polyphenols 176, and thus difficult to transfer to the finished beer. However its importance should not be disregarded since trans-resveratrol has been linked to various health benefits. As a phenolic compound, resveratrol contributes to the antioxidant potential and thereby may play a role in the prevention of human cardiovascular diseases. Resveratrol has also been shown to modulate the metabolism of lipids, and to inhibit the oxidation of low-density lipoproteins and the aggregation of platelets. Moreover, as a phytoestrogen, resveratrol may provide cardiovascular protection. This compound also possesses anti-inflammatory and anticancer properties 181. A thorough review of the health benefits of resveratrol was published in 2006 by Baur and Sinclair 183. The presence of trans-resveratrol and cis-resveratrol as well as trans-piceid and cis-piceid was confirmed in beer. Of 110 analysed commercial beers, trans-resveratrol was found to be the most abundant stilbene in beer. The highest trans-resveratrol concentration detected in beer was 66.74 µg/mL. Although resveratrol is a naturally occurring phenolic phytoalexin with potential health benefits, these effects depend on the ingested amount and the bioavailability of the compounds 184.
Polyphenols in beer
Hop polyphenols can provide both bitterness and astringency, depending on their degree of polymerization. It has been reported by Peleg et al. 185 that, in a water matrix, as the degree of polymerization increases, maximum bitterness intensity is perceived and total duration decreases whereas astringency increases. Peleg et al. also noticed that the monomers were significantly higher in bitterness than the dimers, which were significantly higher than the trimers 185. In a recent study 186, brewing trials were conducted using a polyphenol-rich extract derived from CO2 spent hop material. It was shown that the bitterness produced by the polyphenols interacts with the bitterness delivered by the iso-α-acids. For a beer with low levels of polyphenols, the quality and temporal intensity of bitterness was similar, but not identical, to that from iso-α-acids. As the levels of polyphenols were increased, the produced bitterness was harsher and more astringent. At the highest tested levels the bitterness was considered to be harsh, medicinal or metallic. In this study it was found that beers with 200 mg/L of polyphenols were rated as more bitter than beers with 10 mg/L iso-α-acids 186. Another beer quality aspect that should not be disregarded is mouthfeel. In 1995 Forster et al. 158 recognized that hop polyphenols have a positive impact on the fullness of beer. Before 2007 no conclusive study had been conducted regarding the influence of hop polyphenols on the mouthfeel of beer. In 2007, Aerts et al. 5 filed an application for a US Patent (0254063 A1). Their data not only confirmed that hop polyphenols positively enhance the mouthfeel of beer but also that the impact on mouthfeel is subject to varietal differences 5. Recently Goiris et al. 6 summarized these findings and focused on the contribution of the different hop polyphenol classes to the fullness of beer. Their results showed that the prenylated flavonoid-rich extract as well as the flavonol glycoside-rich extract positively enhance the mouthfeel of beer. The addition of a proanthocyanidin rich extract to beer had no perceivable impact on the mouthfeel but caused unwanted astringency. Of the three hop polyphenol-rich extracts, beers with added flavonol glycosides were preferred by the panellists 6.
Hop varieties
At its most simple, hop varieties have traditionally been classified, based on their chemical composition, as ‘bittering hops’ and ‘aroma hops’. This distinction is important as the brewer may benefit from proper selection of particular hop varieties to add subtle tastes and flavours to the beers. Recent proposals suggest a more refined classification of hop varieties. On the basis of α-acid content, the bittering hops are further subdivided into ‘bittering hops’, ‘high alpha’ and ‘super high alpha’ varieties. Based on the hop oil composition, aroma hops are classified as ‘fine aroma hops’ and ‘aroma hops’. At the time of harvest, the long-term average α-acid content in the bittering varieties and aroma varieties is in the range of 10–20 and 2–10%, respectively. Some of the most important traded bittering hop varieties on the world market are Hallertauer Magnum, Hallertauer Taurus, Herkules, Galena, Nugget, Millennium and CTZ (Columbus, Tomahawk, Zeus). The representative aroma hop varieties are Hallertauer Perle, Hallertauer Tradition, Spalter Select, Hallertauer Mittelfrüh, Hersbruck Hersbrucker, Tettnang Tettnanger, Saaz and Cascade 87.
Varietal acreage in Germany
In 2011, Germany became the world's leading hop producer by volume displacing the USA. The 2008, 2009 and 2012 crops caused the greatest α-acid surplus the world has ever experienced. As a result of this historic hop glut, hop acreages were forced to decline in nearly all countries. In recent years, Germany has shouldered much of the brunt of the needed global hop acreage reduction and hop production 187. While in most countries the hop acreage continues to decline, in 2011, the USA offset this trend by significantly expanding the hop acreage (from 3,736 to 5,123 ha) assigned to aroma varieties 188.
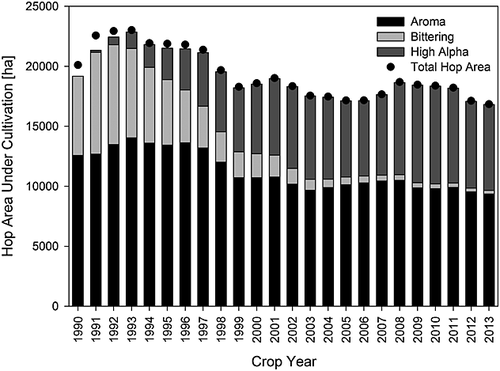
Since 1990, the total hop area under cultivation in Germany has remained between 16,000 and 23,000 ha. The largest hop cultivated areas (>22,500 ha) occurred from 1991 to 1993; conversely, between 2003 and 2007 the area under cultivation did not surpass 18,000 ha. In Fig. 18 the breakdown of the cultivated hop categories in the overall hop acreage is shown. The total acreage planted with aroma hop varieties has remained relatively constant. It represents between 50 and 60% of the total hop cultivated area in Germany. The occasional decline in area allotted for aroma hops is a consequence of fields not being cultivated rather than farmers switching to other hop varieties. The acreage for traditional bittering varieties (e.g. Northern Brewer) has suffered a significant decrease over the years. In the early 1990s, 30% of the total area was planted with bittering varieties; currently, <2% is used for growing bittering hops. The reduction in the area designated for traditional bittering hops resulted as a consequence of the introduction of the high-alpha varieties (e.g. Hallertau Magnum). The decline of the cultivated area with bittering hops was parallel to the increase of acreage used for high-alpha hops. Currently, about 55% of the land is planted with aroma hops and 40% of the acreage is used to cultivate high-alpha varieties. Of the remaining 5%, <2% is assigned to the bittering varieties.
Flavour hops
Recently certain hop varieties have gained increasing popularity in the hop and brewing industry. These varieties are referred to as ‘flavour hops’. Currently, the term is not uniformly used but mostly refers to varieties originating in the USA, Australia and New Zealand. Recently, Germany and other countries have also started cultivating these varieties. The term was coined about 20 years ago by the president of the American Association of Brewers, Charles N. Papazian 155. Flavour hops describe varieties that are designed to impart bold tastes as well as distinct aroma and flavour characteristics to beer. The flavour profile of these varieties is commonly related to tropical and fruity notes. Unlike aroma and bittering hops, flavour hops are not associated with their α-acid content nor their yield. Owing to the complexity, hitherto, there has been no conclusive scientific definition of flavour hops. However it has been suggested that a flavour hop variety can be both an aroma and a bittering hop. Moreover, hop varieties that have been traditionally classified as aroma or bittering varieties could now be reclassified as flavour hops. Four flavour hop selections that have been recently released for cultivation in Germany are Polaris, Hallertauer Blanc, Mandarina Bavaria and Hüll Melon 189. The most popular flavour hops in the USA are Cascade, Simcoe, Centennial and Citra. Galaxy, Ella (formerly known as Stella), Summer, Topaz and Vic Secret are the flavour hop varieties cultivated in Australia 190. Nelson Sauvin, Kazbek and Aramis are being grown in New Zealand, the Czech Republic and France, respectively 188. By increasing the range of hop varieties available to the brewer, the brewing industry is provided with endless creative possibilities and hence the global beer portfolio can be expanded.
Hops and brewing
In the past 100 years global beer production increased sevenfold (Fig. 19). In 1911, 273 million hectolitres (hL) of beer were produced; by 2013 world beer production had reached a new record of 1960 million hL. In the last four decades (1970–2010) global beer production tripled, from 630 million to 1846 million hL 191. In general, world beer production has experienced a very steady increase except for three representative periods, in which owing to the political and economic situation, beer production and consumption significantly decreased. First, the 1914–1918 war ravaged Europe, altered the political map and had a severe effect on the beer market. During this period, beer consumption worldwide was more than halved. Shortly afterwards, American brewers were affected by the Prohibition Act of 1919, which stayed in force until 1933. Beer production in the USA reached a low of 3.6 million hL in 1926. Finally, Europe was again thrown into turmoil during the 1939–1945 war, and beer consumption in Germany fell from 50 million hL in 1939 to 5.8 million hL in 1949 192, 193. Since 1948, world beer production has experienced a steady increase.
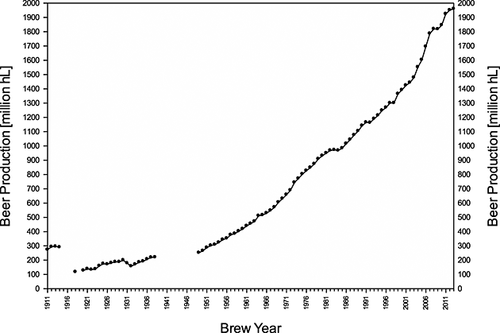
The hop grower knows that every hop season is unique and different from all the other ones, and that year-to-year fluctuations are normal 194. The size of the world hop crops partly reflects the demand of brewers as well as the events surrounding the hop harvest (detailed data in Table 3). A particular example is the very large world crop in 1905, which resulted in enormous surpluses and a corresponding decline in prices below actual production costs. This, in turn, caused a reduction in acreage and consequent recovery in prices during 1908–1910. Such cycles were not new to the hop industry and have been experienced many times (Fig. 20) 192. Another factor that forces hop growers to adjust acreage is the introduction of production quotas 194. Until 1930, acreage under cultivation was significantly larger than total hop production volume. Except for the 1963 hop crop, as of 1948 a clear trend may be observed: smaller areas under cultivation achieve a higher hop production yield. This change may be attributed to the introduction of more productive hop varieties with a higher α-acid content. In 2013, with approximately 46,000 ha, the hop industry had the smallest acreage under cultivation since 1955. Although the area under cultivation continues to shrink, the varieties being grown are delivering increasingly high yields in terms of both quantity and alpha. In 2013, the total volume of hop production is estimated to be 81,300 metric tons (t) with an estimated alpha yield of 8,000 t 195. Since 2008 record levels of high alpha yields have been achieved, above 10,000 t. In 2009, the hop crop provided an unparalleled boom; the alpha production was 10,952 t 196. This higher alpha yield per hectare requires an acreage reduction and possibly variety replacements in order to avoid a crop surplus and ultimately a market imbalance.
| Crop year | Hop area under cultivation (ha)a | Hop production (t)b | Beer production (million hL)c | Crop year | Hop area under cultivation (ha) | Hop production (t) | Beer production (million hL) | Crop year | Hop area under cultivation (ha) | Hop production (t) | Hop α production (t) | Beer production (million hL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1880 | 89,000 | 68,600 | — | 1924 | 50,482 | 67,975 | 159 | 1969 | 67,291 | 94,876 | 5276 | 605 |
| 1881 | 89,000 | 68,800 | — | 1925 | 55,322 | 58,755 | 174 | 1970 | 70,666 | 102,589 | 6038 | 630 |
| 1882 | 89,063 | 43,000 | — | 1926 | 64,199 | 58,115 | 171 | 1971 | 75,042 | 96,050 | 5377 | 658 |
| 1883 | 103,641 | 81,200 | — | 1927 | 78,639 | 72,190 | 180 | 1972 | 78,015 | 105,013 | 6170 | 689 |
| 1884 | 115,402 | 80,400 | — | 1928 | 81,122 | 67,815 | 187 | 1973 | 81,329 | 118,301 | 7469 | 743 |
| 1885 | 119,513 | 87,950 | — | 1929 | 78,558 | 83,425 | 187 | 1974 | 82,037 | 111,176 | 6631 | 771 |
| 1886 | 120,798 | 86,850 | — | 1930 | 57,788 | 61,215 | 199 | 1975 | 80,584 | 113,503 | 7234 | 802 |
| 1887 | 115,800 | 77,750 | — | 1931 | 50,397 | 48,485 | 178 | 1976 | 78,197 | 107,533 | 6012 | 826 |
| 1888 | 116,900 | 69,250 | — | 1932 | 41,816 | 41,960 | 157 | 1977 | 78,563 | 116,892 | 7049 | 848 |
| 1889 | 114,000 | 103,400 | — | 1933 | 47,806 | 51,870 | 170 | 1978 | 77,599 | 109,440 | 6456 | 874 |
| 1890 | 114,730 | 65,000 | — | 1934 | 52,548 | 55,400 | 185 | 1979 | 79,733 | 121,867 | 7142 | 909 |
| 1891 | 115,739 | 79,300 | — | 1935 | 55,391 | 61,610 | 191 | 1980 | 86,926 | 120,611 | 7269 | 931 |
| 1892 | 114,958 | 80,850 | — | 1936 | 53,945 | 59,116 | 206 | 1981 | 94,739 | 131,017 | 8049 | 950 |
| 1893 | 117,411 | 73,050 | — | 1937 | 53,792 | 63,358 | 218 | 1982 | 97,462 | 146,115 | 8471 | 968 |
| 1894 | 117,000 | 112,400 | — | 1938 | 53,017 | 59,846 | 220 | 1983 | 95,665 | 132,742 | 7540 | 972 |
| 1895 | 115,000 | 106,800 | — | 1939 | — | — | — | 1984 | 92,821 | 128,728 | 8175 | 968 |
| 1896 | 112,589 | 89,750 | — | 1940 | — | — | — | 1985 | 86,855 | 124,050 | 7056 | 984 |
| 1897 | 105,685 | 83,650 | — | 1941 | — | — | — | 1986 | 84,220 | 112,466 | 7199 | 1016 |
| 1898 | 100,461 | 74,300 | — | 1942 | — | — | — | 1987 | 87,393 | 118,341 | 8080 | 1044 |
| 1899 | 110,415 | 107,650 | 254 | 1943 | — | — | — | 1988 | 89,875 | 117,363 | 7276 | 1076 |
| 1900 | 106,758 | 77,400 | — | 1944 | — | — | — | 1989 | 90,177 | 118,551 | 7290 | 1104 |
| 1901 | 107,684 | 91,300 | — | 1945 | — | — | — | 1990 | 91,271 | 114,416 | 6864 | 1142 |
| 1902 | 108,240 | 75,550 | 261 | 1946 | — | — | — | 1991 | 91,409 | 130,060 | 8612 | 1165 |
| 1903 | 107,241 | 77,900 | 261 | 1947 | — | — | — | 1992 | 91,835 | 122,379 | 7537 | 1163 |
| 1904 | 113,304 | 77,750 | 263 | 1948 | 41,749 | 49,481 | — | 1993 | 91,121 | 137,275 | 9099 | 1190 |
| 1905 | 115,401 | 123,900 | 263 | 1949 | 44,569 | 53,449 | 251 | 1994 | 86,786 | 121,323 | 6907 | 1214 |
| 1906 | 116,176 | 80,300 | — | 1950 | 48,272 | 68,157 | 265 | 1995 | 81,466 | 126,686 | 7831 | 1248 |
| 1907 | 115,978 | 95,800 | — | 1951 | 50,110 | 73,297 | 287 | 1996 | 76,967 | 124,379 | 9300 | 1269 |
| 1908 | 115,106 | 101,350 | — | 1952 | 50,257 | 67,141 | 302 | 1997 | 70,290 | 112,192 | 8773 | 1300 |
| 1909 | 97,379 | 46,200 | — | 1953 | 47,742 | 68,921 | 308 | 1998 | 60,111 | 94,610 | 7245 | 1301 |
| 1910 | 94,761 | 79,850 | — | 1954 | 48,194 | 64,605 | 323 | 1999 | 57,427 | 95,450 | 7393 | 1365 |
| 1911 | 93,832 | 67,400 | 273 | 1955 | 45,818 | 63,394 | 343 | 2000 | 58,991 | 96,715 | 8294 | 1392 |
| 1912 | 98,026 | 98,650 | 293 | 1956 | 52,200 | 58,049 | 352 | 2001 | 58,903 | 99,214 | 8646 | 1424 |
| 1913 | 101,103 | 75,400 | 295 | 1957 | 54,522 | 66,693 | 377 | 2002 | 56,237 | 100,932 | 8749 | 1443 |
| 1914 | 89,397 | 97,250 | 290 | 1958 | 63,346 | 81,196 | 385 | 2003 | 53,500 | 87,056 | 6722 | 1479 |
| 1915 | 78,492 | 67,300 | — | 1959 | 65,258 | 83,356 | 402 | 2004 | 50,693 | 92,266 | 8103 | 1552 |
| 1916 | 66,414 | 57,800 | — | 1960 | 63,956 | 81,333 | 418 | 2005 | 50,273 | 94,385 | 7903 | 1603 |
| 1917 | 46,546 | 42,550 | — | 1961 | 62,494 | 68,458 | 439 | 2006 | 49,466 | 85,585 | 7103 | 1696 |
| 1918 | 40,516 | 20,800 | 117 | 1962 | 66,410 | 80,943 | 455 | 2007 | 50,455 | 91,584 | 7663 | 1787 |
| 1919 | 40,516 | 37,700 | — | 1963 | 96,790 | 91,834 | 471 | 2008 | 57,297 | 111,175 | 10,242 | 1819 |
| 1920 | 48,312 | 56,200 | 127 | 1964 | 70,967 | 93,066 | 510 | 2009 | 56,747 | 113,669 | 10,952 | 1818 |
| 1921 | 52,955 | 40,750 | 138 | 1965 | 71,494 | 92,001 | 515 | 2010 | 52,156 | 99,879 | 9475 | 1846 |
| 1922 | 51,277 | 53,700 | 133 | 1966 | 71,600 | 94,371 | 529 | 2011 | 48,528 | 100,604 | 10,348 | 1925 |
| 1923 | 47,842 | 35,400 | 137 | 1967 | 70,895 | 94,127 | 547 | 2012 | 46,971 | 89,090 | 9139 | 1951 |
| 1968 | 68,203 | 91,909 | 570 | 2013d | 46,300 | 81,300 | 8000 | 1960 |
- a ha, Hectare = 10,000 m2;
- b t, metric ton = 1,000 kg;
- c million hL = 100,000,000 L;
- d 2013 = estimated data.
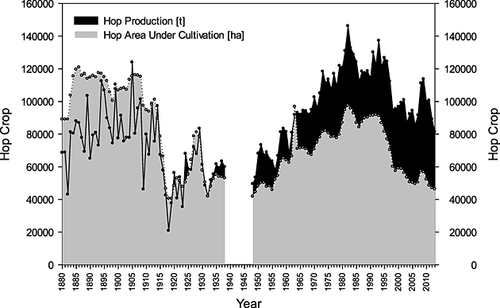
The growing interest in bittering compounds and the analytical properties of hops on the part of the brewing industry prompted Joh. Barth & Sohn in 1970 to attempt to list the respective hop producing areas according to alpha values. The α-acid production represents the actual hop supply available to the brewers 194. In the hop market, a key indicator is the average hopping rate, which unfortunately has undergone a steady decline since it was first recorded. Since 1970 the hopping rate has fallen by nearly 60% (Fig. 21). In the last four decades from 1970 to 2010, the average α-acid addition in beer has decreased from 9.6 to 4.1 g α/hL 191. From 1970 to 1980, the hopping rate dropped almost by 2 g α/hL, from 9.6 to 7.8 g α/hL. When looking at the world market critically, it may be inferred that, owing to the alpha deficit before 1980 and the resulting increase in hop prices, as well as the better utilization of the bittering substances, a reduction of the alpha rate became possible worldwide 197. Moreover, today China, not the USA, is the world's biggest beer producer. Unfortunately beer in these new beer-producing countries is less heavily hopped. The reduction of beer bitterness around the world is the result of changing tastes in today's consumer society. These new trends partly explain the decline in the hopping rate 194. In addition to lowering the bitterness levels in mainstream beers, the hopping rate has decreased owing to the use of hops with higher α-acid levels and the increased use of more sophisticated hop products that achieve higher utilization levels 193. Another factor is the modernization of the brewing industry worldwide 198. The decline in the hopping rate from 1980 to 1990 was not as drastic as that in the decade before or in the two following decades. In 1990 the hopping rate was 6.9 g α/hL, almost 1 g α/hL less than in 1980. By 2000, the hop addition was 5.6 g α/hL and by 2010 it was 4.1 g α/hL. The reduction was 1.3 and 1.5 g α/hL, respectively. In 2012, the hopping rate increased to 4.3 g α/hL and it is expected to continue to increase. This recent increase in the hopping rate is primarily attributed to the growing craft brewing sector.
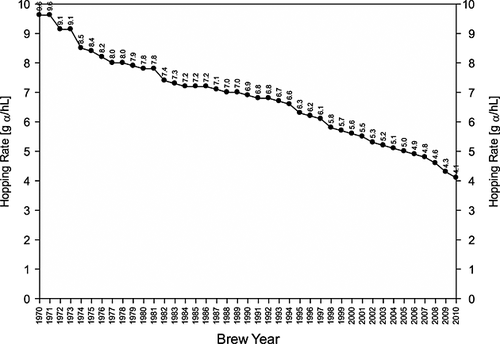
Hop contributions to beer
In 1891, Moritz and Morris 199 gave five reasons for adding hops to beer: (a) to impart the distinctive bitter taste and aroma (i.e. hoppy character); (b) to precipitate certain nitrogenous constituents of the wort (i.e. break formation); (c) to act as a filter aid in the hop back (i.e. clarify the wort); (d) to confer antibacterial properties to beer; and (e) to assist in the sterilization of the wort. A century later, Hughes and Simpson 200 extended the list by including two other benefits: (f) to enhance and stabilize beer foam; and (g) to promote foam lacing. As mentioned above, the brewing value of hops is primarily attributed to the presence of resins, essential oils and polyphenols.
Bitterness
Many complex reactions occur during the boiling of wort with hops in the brewery. In addition to the transformations involving carbohydrates, proteins, polyphenols and various polymeric substances, the hop bitter principles are extracted, thereby undergoing intricate transformations and interactions with other constituents 201. Bitterness is one of the most readily discerned sensory attributes of beer as far as the consumer is concerned 202. Over the years, the chemical changes in the hop resins during wort boiling have been thoroughly studied and therefore adequately profiled. These studies were crucial since the chemistry of hop extraction during wort boiling determines to a great extent the flavour and the quality of the finished beer 201. By far the most important chemical conversion occurring during wort boiling is the thermal isomerization of the α-acids into the more soluble and bitter iso-α-acids. The α-acids yield two iso-α-acids, as these occur as diastereoisomers in their cis and trans forms 35. For a given pair of cis- and trans-isomers, the cis-component is significantly more bitter than its trans-counterpart. It has also been established that the isohumulones are more bitter than the isocohumulones 202. Of the six major iso-α-acids present in beer, the most bitter compound is cis-isohumulone and the least bitter one is trans-isocohumulone 203. As mentioned previously, the characteristic beer bitter taste is attributed to components formed from the hop resins during wort boiling, mainly the iso-α-acids. Over 85% of the perceived pleasant bitterness in beer is delivered by the iso-α-acids 204. However, other hop substances, such as hop polyphenols and oxidation products of the β-acids (i.e. hulupones) also contribute to beer bitterness.
Aroma
In addition to giving the pleasant bitter taste, hops impart a characteristic ‘hoppy’ aroma to beer. The hop chemistry associated with the hoppy character of beer is very complex. However, since the advent and utilization of gas chromatography, much has been learned regarding the composition of the hop oil fraction and its contribution to beer. It is known that, after the vigorous boiling of hops in the kettle, very little hop oil constituents will remain in the wort in their native form. Most of the essential oil compounds are highly volatile and poorly soluble in wort; however, their presence in beer depends on the chemical properties of the specific essential oil component and the hopping technology used. After boiling for 90 min, 85–95% of the essential oil in hops has evaporated from the kettle and the rest has polymerized to form resinous substances. As a result, the hop oil components are hardly retained in the wort in their native form. Further, during the fermentation process there is a considerable loss or transformation of the hop oils retained in the wort 4, 205. These hop oil components disappear to a large extent by adsorption on yeast and the filter mass 206, 207. A detailed review of the biotransformations of hop-derived aroma compounds by yeast upon fermentation was published in 2012 by Praet et al. 208. Some hop oil constituents survive unchanged through to the finished beer. In 1960 Harold et al. 209 were the first to demonstrate the occurrence of hop oil in beer. After examining the volatile portion Harold et al. were able to clearly show the presence in beer of β-myrcene, methyl nonyl ketone and α-humulene, as well as a number of unidentified hop oil constituents 209.
No one single component of the hop oil fraction is responsible for the hoppy character in beer. The combination of many compounds is necessary for the characteristic hop aroma. In 1963 Guadagni et al. suggested that this contribution is due to the additive or synergistic effects of many components (i.e. hop oil compounds and volatile fermentation products) 210. In 1966 Hashimoto 211 and Buttery and Ling 212 suggested that the volatile hop components with sufficient solubility in water to be retained in boiling wort may cause the hoppy aroma in beer. They further established that the hoppy aroma is not derived directly from the hop oil itself, but from certain volatile water soluble compounds that are produced partly from the hop resins, as decomposition products 207. It was later shown that terpene and sesquiterpene hydrocarbons, the major components of hop oil, are rarely found in beer. Therefore, these are not considered to be responsible for hoppy flavours in kettle-hopped beer. On the other hand, the oxidation products of these hydrocarbons are commonly found in beer and have been suggested to contribute to the hoppy character 134, 138, 213, 214. The aromas derived from the hop essential oils are usually described in terms of fruity, floral, citrus, grassy and spicy (or herbal) notes. To the brewer, these differences in the hoppy character are important since they help profile the many existing beer styles.
Microbial stability
Hop bitter acids exhibit an inhibitory effect on bacteria; thus they play a major role in enhancing the preservative properties of beer. It was shown as early as 1888 that hops contribute to the microbial stability of beer and protect beer from infections 20. Thereafter the antibacterial activity of the hop compounds and the hop resistance of lactic acid bacteria have been extensively investigated. In 1937, Shimwell discovered that the antibiotic properties and bacteriostatic power of hops are restricted to Gram-positive bacteria, while the Gram-negative species were mostly unaffected by the hop acids 215. It was later found that hop acids are also mildly antifungal (on selected fungi species) 216 and antiprotozoal 217. Among the Gram-positive bacteria some species of lactic acid bacteria are less sensitive (i.e. more resistant) to hop compounds and are able to grow in beer. Shimwell reported that the antiseptic potency of the hop acids increases at lower pH 215. Shimwell later proposed that the antibacterial activity of the hop constituents is associated with permeability changes of the bacterial cell wall 218. Almost 35 years later, Teuber and Schmalreck 219 showed that hop compounds and their derivatives cause leakage of the cell plasma membrane in the aerobic organism Bacillus subtilis. This results in complete inhibition of the active transport of sugars and amino acids. Subsequent inhibition of the respiratory chain and of protein, RNA and DNA synthesis occurs as a secondary event. However, this organism cannot grow in beer and beer spoilage bacteria differ from B. subtilis in the physiology of their cell membranes 220. Therefore this cannot be considered the mechanism by which hop bitter acids inhibit the growth of beer spoiling bacteria.
Another major breakthrough in understanding the preservative value of the hop bitter acids occurred in the 1990s. In the early 1990s, Simpson established one of the molecular mechanisms by which beer spoilage bacteria are inhibited 221, 222. In an earlier study Simpson and Smith showed that the undissociated forms of hop compounds and hop-derived compounds are mainly responsible for the antibacterial activity. The ionized forms of such compounds have a negligible effect on lactic acid bacteria 223. The practical implication of this finding is that small variations in beer pH cause large changes in the antibacterial activity of the hop bitter acids in beer 220. It had been known for some time that the antibacterial activity of certain hop compounds and their derivatives is influenced by the pH of the test medium 218, 224. However, the relationship was first placed on a quantitative basis by Simpson and Smith 223. They established the dependence of the inhibitory effect of hops on the pH of the medium; low pH favours antibacterial activity but high pH reduces it 220. The antibacterial activity of trans-isohumulone is enhanced by the addition of monovalent cations (e.g. K+, Na+, NH4+, Rb+, Li+). However the presence of divalent cations (e.g. Mg2+, Mn2+, Ni2+, Ca2+) reduces the antibacterial activity of trans-isohumulone 223.
It was also observed by Simpson that trans-isohumulone causes an increase in proton permeability, thus suggesting that it acts as an ionophore of the mobile-carrier type 221. Ionophores are antimicrobial agents that increase the permeability of cell membranes to specific ions. Hop compounds as ionophores inhibit the growth of Gram-positive bacteria by catalysing the exchange of protons (i.e. H+) for cellular divalent cations, such as Mn2+, in an electroneutral process. Changes in the intracellular pH are induced by ionophores 225. This reduction in intracellular pH leads to inhibition of nutrient transport and starvation of the affected organisms 220. In Lactobacillus species, trans-isohumulone dissipates effectively the transmembrane pH gradient of non-growing cells and reduces their ability to accumulate l-[U-14C]leucine; further it causes leakage of the accumulated leucine. Conversely, the membrane potential is only dissipated to a smaller extent 221. Some components of the cell plasma membrane may be responsible for conferring hop resistance on beer spoiling lactic acid bacteria. Such components could act to restrict the ability of trans-isohumulone to conduct protons or divalent cations across the plasma membranes of the bacteria or could increase the rate at which protons are expelled from the cell interior 222. A clear complication in hop inhibition research is the complexity of the chemical composition of hops. This is due not only to compound variation on account of consecutive chemical conversions but also to the variations of the natural product 223. It has been shown that resistance to hop bitter acids is a unique character associated only with beer spoilage lactic acid bacteria. The expected cross resistance to other proton ionophores could not be detected. The lack of cross resistance to other antibacterial agents suggests that the mechanism of resistance includes features that discriminate on the basis of molecular structure rather than, or in addition to, physicochemical properties. Cross resistance between different hop acids suggests that the most important structural feature in this respect is the β-triketone group 226.
Beer is a very unfavourable medium for the growth of most Gram-positive microorganisms owing to the presence of alcohol, hop compounds, high carbon dioxide level, relatively low pH, extremely reduced oxygen content and the presence of only trace amounts of nutrient substances such as glucose, maltose and maltotriose 227. Therefore, in order for bacteria to survive and grow in beer, several mechanisms are involved in their resistance to the adverse conditions of the hostile environment. Of particular importance is the resistance to hops as this is a prerequisite for lactic acid bacteria to be able to grow in beer, thereby causing beer spoilage. Ultimately, hop resistance of lactic acid bacteria requires multiple defence mechanisms 228. Following the studies of Simpson, several mechanisms involved in the hop resistance of lactobacilli were proposed.
Sami et al. 229 were the first to identify horA as one of the genes responsible for hop resistance. They used horA as a genetic marker and developed a rapid and accurate polymerase chain reaction method for the prediction of beer spoiling lactobacilli 230. The putative protein product of horA, HorA, has an ATP-binding (adenosine-5′-triphosphate) domain, a consensus sequence for an ATP-binding cassette transporter reported to be located in the cytoplasm, and six hydrophobic regions that are large enough to span the plasma membrane. The HorA protein acts as a multidrug resistance transporter that excretes the hop compounds into the outer medium. Therefore, the bacteriostatic effect of the iso-α-acids may be counteracted by the HorA protein, which mediates cellular extrusion of iso-α-acids or other toxic molecules that accumulate intracellularly in the presence of the hop compounds. From the collected data, Sami et al. suggested that a multidrug resistance pump contributes to the resistance mechanisms to hop compounds of lactic acid bacteria 229.
The horA gene was shown by Sami et al. 229 to function as a primary multidrug transporter; this implies that the gene is dependent on ATP-binding. A few years later, Suzuki et al. 231 found another defence mechanism that is not mediated by the horA gene. Upon biochemical characterization of a hop resistant Lactobacillus brevis strain two conclusions were drawn: first, that the second hop resistance mechanism is mediated by proton motive force (PMF)-dependent multidrug transporter and second that this defence mechanism may be induced upon lactic acid bacteria. The genetic marker found to mediate the horA-independent hop resistance mechanism is ORF5 232. The ORF5 identified encodes a protein with putative 11–12 transmembrane domains, a feature that is consistent with the previously reported PMF-dependent multidrug transporters 233. Similar to the horA properties, the presence or absence of ORF5 was highly correlated with the beer spoilage ability of L. brevis strains 232. Two other genetic markers that mediate hop resistance in lactic acid bacteria were found. The first one is horB, which is a regulator that modulates the transcription of genes that encode multidrug transporters; the other is the horC gene, which encodes a PMF-dependent multidrug transporter 234, 235. From all the collected data it was possible for Suzuki et al. to suggest that the beer spoilage ability of lactic acid bacteria is acquired by horizontal transfer of hop resistance genes 233.
Other putative hop resistance mechanisms include the contribution of the hitA (hop-inducible cation transporter) gene, which encodes for HitA, a divalent cation transporter. This gene has been shown to be present in most beer spoiling lactobacilli 236. It was also hypothesized that genes responsible for the production of teichoic acid in the cell wall also contribute to hop resistance in beer spoiling lactic acid bacteria strains 237. The proton translocating, membrane-bound ATPase was suggested to ameliorate the protonophoric actions of hop bitter acids by pumping out the intracellular protons in an ATP-dependent manner 238. The hop resistance of beer spoiling lactic acid bacteria is a complex phenomenon yet to be fully understood. The collected evidence indicates that cell walls and cytoplasmic membranes play an important role in defending beer spoiling lactic acid bacteria from the bactericidal effects of the hop bitter acids 239.
In a more recent study, Haakensen et al. 240 confirmed that lactic acid bacteria species that harbour the horA gene are more likely to spoil beer than those that do not. The horA gene has been conclusively shown to function as a multidrug transporter 241. Therefore, the presence of the horA gene is a significant predictor to assess the beer spoilage ability of lactobacilli isolates. Throughout the years, three other putative beer spoilage associated genes have been suggested; these are hitA, horC and ORF5 233, 235, 236, 242, 243. Recently, it was found that hitA and horC are not predictive of an organism's ability to grow in beer. However the presence of hitA, horC or both in addition to horA is indicative of the ability of a microorganism to grow rapidly in beer. The horA and hitA genes code for primary- and secondary-type multidrug transporters, respectively 244. The primary-active, ATP-binding cassette multidrug transporters are dependent on ATP-binding/hydrolysis, whereas secondary-active multidrug transporters are coupled to the proton motive force that exists across the cytoplasmic membrane 245. In another study by Haakensen et al. 241 Pediococcus isolates were screened for beer spoilage genes. Two genes, bsrA and bsrB (beer spoilage related) were highly correlated with the beer spoilage ability and hop resistance of Pediococcus isolates. These bsrA and bsrB genes were not found in any Lactobacillus isolates, regardless of whether they were able to grow in beer or not. This makes them the first genetic markers capable of differentiating between beer spoilage lactobacilli and pediococci 246. Like horA, bsrA and bsrB are both primary-type ATP-binding cassette multidrug resistance genes 241.
The mechanisms of hop resistance of beer spoiling Lactobacillus brevis have also been studied on the proteomic level. The gathered evidence suggests that the stress caused to the bacteria by hop compounds is not only associated with proton motive force depletion but it also implies that there is a limitation of divalent cations. More specifically, hop compounds inhibit the bacteria's metabolism by reducing the intracellular manganese concentration, thereby causing a conflictive regulation of the energy metabolism 247. The specific role of the single hop resistance mechanisms described in the literature is not fully understood, particularly since none of the defence mechanisms confers hop resistance in the absence of other (unknown) mechanisms of hop resistance. This suggests that the resistance of lactobacilli isolates towards hop compounds requires multiple interdependent resistance mechanisms 248, 249.
Foam
Hop acids not only contribute to beer bitterness, but also play an essential role in achieving beer foam formation and stability. The role of hops for beer foam is to stabilize the foam complexes 250. It is well recognized that foam is an important quality factor in the beer production process. According to MEBAK (Mitteleuropäischen Brautechnischen Analysenkommision) standards, a good foam stability for a ‘Vollbier’ (full beer; 11.0–15.9°P) is a head retention time between 220 and 300 s 251. The foam suppressor–enhancer properties of the hop acids have long been known. In 1976, the first observations on the role of the iso-α-acids of hops in the structure of beer foam were documented by Fly and Chicoye 252. Following their studies, numerous researchers 202, 253-258 have looked into the effect of different hop compounds on foam properties. It has been revealed that hydrophobicity is the key factor in determining the effectiveness of a hop compound to stabilize beer foam. These hydrophobic compounds have the tendency to leave the beer and concentrate in the foam, thereby increasing foam stability 259.
It has been known for a number of years that the bitter iso-α-acids, derived from the α-acids of hops, are a major contributor to the improvement in beer foam stability 255. It was further observed by Diffor et al. that, of the iso-α-acid analogues, isohumulone provides a more stable foam than isocohumulone 253. This difference is attributed to the lower solubility of isohumulone at beer pH, which results in its greater tendency to associate with other foam-forming constituents in the beer. This, however, does not mean that isocohumulone has a negative effect on foam, but rather that isohumulone provides a more stable foam 260. Other natural but minor hop components such as isoadprehumulone, dihydroisohumulone, tetrahydroisocohumulone and tetrahydroisohumulone have been suggested to promote foam stability and lacing 255.
Cling
As beer is being consumed, the texture of the foam changes from being liquid to almost solid; this solid or dry foam can adhere to the glass surface. The ability of the foam to adhere to the glass wall is known as foam ‘cling’ or ‘lacing’ 261. In 1964, Glenister and Segel 262 raised the importance of the primary and secondary cling. The primary cling is created once the initial foam head is allowed to collapse. The secondary cling is the pattern that is formed when the consumer, at intervals, removes the beer from the glass. From their observations, they concluded that the differences between commercial beers as to their secondary cling are frequently more striking than to those of primary cling. It is well documented that hops contribute in a positive manner to foam cling. While beer foam is enhanced by the presence of hop substances, lacing will not occur unless hop compounds are present 202, 263. To date, there are no defined standards for cling measurements. Further it may be supposed that the perception of what constitutes good lacing is a subjective matter 259, yet this foam quality aspect cannot be disregarded.
Summary
With a view to increasing the understanding of the nature of hops, research has focused on the chemical composition of hops and their brewing properties. Plentiful research has been conducted on identifying and elucidating the structures of specific hop compounds and their impact on beer quality. Although much is known about hops and their function in brewing, new aspects continue to be revealed. Extensive international research continues to be actively conducted by respected scientists and institutions in countries all around the world. In these new aspects of hop research, while much has been achieved, much more remains to be done to resolve outstanding issues. It must be borne in mind that any progress at all in these new aspects is pioneering. Hop science has provided brewers with one of the main ways of meeting the abiding need to understand the nature of hops. While hop research has been changing and progressing as times change, to the brewing scientist, the profound mystery of hops remains revering and reinvigorating. Humulus lupulus has a complex story yet to be fully understood but, undeniably, one worth telling and retelling.




