Quality of pilsner malt and roasted malt during storage
Abstract
Malt is usually expected to be stable during 12 months of storage. However, in practice many brewers notice changes in malt aroma during storage. The oxidative stabilities of pilsner malt and roasted malt were evaluated during a 12 month storage at different temperatures (10 and 20 °C) and water activities (0.231 and 0.432). The radical content in malt kernels was measured by electron spin resonance spectroscopy and the volatile profile of the resulting sweet worts was measured by head-space analysis followed by GC-MS analysis. The storage of malt resulted in oxidative reactions and a large change of the volatile profile of the resulting worts. Roasted malt was much more unstable than pilsner malt, as illustrated by a higher initial radical intensity, larger radical decay during storage and a larger change in the volatile profile of the wort with increased amounts of lipid oxidation products. For both roasted malt and pilsner malt, good correlations were found between radical decay and changes in the volatile profile of the wort, where high temperature and high water activity resulted in the largest changes. During the 12 months of storage, the sugar extract of the wort made from the malts remained constant and was not affected by the chemical changes. This study suggests that chemical changes occurring in malts during less than 12 months of storage may potentially affect the aroma of beer, and that water activity and storage temperature should both be kept low in order to maintain a high malt quality. Copyright © 2014 The Institute of Brewing & Distilling
Introduction
Beer quality is highly influenced by malt quality 1, 2. It is generally accepted that malt has good storage stability when kept in a dry environment and most malts are accepted to have a storage stability of 12 months. In practice, however, many brewers notice changes in malt aroma during storage, but very few scientific studies have examined the flavour and oxidative stabilities of malt during storage. The effect of storage on malts has been investigated in relation to wort filtration rates 3, where longer storage times were found to have a positive effect, most likely explained by the decay of a thiol oxidizing enzyme 4-6. Lipid oxidation is a known cause of off flavours in beer 1 and the levels of the lipid oxidizing enzyme lipoxygenase (LOX) have been studied during malt storage 7. Selected volatiles were studied in relation to storage and it was observed that Strecker aldehydes and N-heterocyclic compounds increased slightly in concentration within the first few months and then decreased to concentrations below the initial content, and interestingly similar variations in malt and beer aromas were identified 2. In a recent study it was found that sweet worts made from roasted malts with colours above 33 EBC units were very susceptible to a loss of volatiles when exposed to mild heating, whereas the profile of volatile compounds of wort made from pilsner malt was not affected by the heating 6.
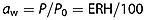 (1)
(1)The purpose of the current study was to gain an understanding of chemical changes and oxidative stability of pilsner malt and roasted malt during storage. Also the influence of temperature (10 and 20 °C) and water activity (0.231 and 0.432 being equal to 23.1 and 43.2% ERH) was tested. The observations were compared with the volatile profile of wort produced from malt after 0, 6 and 12 months of storage. The study was based on pilsner and roasted malts derived from the same batch of malt, and all variations between pilsner malt and roasted malt can be ascribed to the differences in roasting. A detailed study of the influence of water activity on radical formation was also carried out on pilsner malt at 20 °C using a range of water activities between 0.113 and 0.910.
Materials and methods
Malt
Fresh pale ale malt [two-row spring barley (Hordeum vulgare) harvest 2010] was kindly provided by Danish Malting Group, Vordingborg, Denmark.
Roasting
Pilsner malt was roasted by distributing it in a single layer on a cloth on a baking plate and heating it in an oven at 190 °C for 50 min.
Storage of pilsner malt at 10 different water activities
Approximately 50 g aliquots of fresh pilsner malt were placed in 10 containers and the water activities were adjusted with the presence of saturated aqueous salt solutions: lithium chloride (aw 0.113), potassium acetate (aw = 0.231), magnesium chloride (aw = 0.331), potassium carbonate (aw = 0.432), magnesium nitrate (aw = 0.529), sodium nitrate (aw = 0.693), sodium chloride (aw = 0.756), ammonium sulphate (aw = 0.817), potassium chloride (aw = 0.859) and barium chloride (aw = 0.910) 13. The malt was stored in a thermostated cabinet at 20 °C.
Storage of pilsner malt and roasted malt
Pilsner malt and roasted malt were stored in the dark at 10 or 20 °C at a water activity (aw) of 0.231 or 0.432 (Table 1). The water activity of the malt was adjusted with saturated salt solutions of potassium acetate (aw = 0.231) and potassium carbonate (aw = 0.432). Water activity may be influenced by temperature; however, no significant difference was found between 10 and 20 °C for these two salts 13.
| Colour (EBC) | pH | Filtration Time (min) | Yield (mL) | °Brix | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | aw | Temperature (°C) | 0 months | 6 months | 12 months | 0 months | 6 months | 12 months | 0 months | 6 months | 12 months | 0 months | 6 months | 12 months | 0 months | 6 months | 12 months |
| P1 | 0.231 | 20 | 5.6 ± 0.2a | 5.6 ± 0.5a | 6.00 ± 0.01c | 6.03 ± 0.08bc | 34.5 ± 6.4ab | 34 ± 1b | 49 ± 13ab | 325.0 ± 8.5a | 335 ± 12a | 310 ± 31a | 13.46 ± 0.00a | 13.46 ± 0.00ab | 13.46 ± 0.00abcd | ||
| P2 | 0.432 | 20 | 5.6 ± 0.1a | 5.4 ± 0.2a | 5.6 ± 0.0a | 6.01 ± 0.01abc | 5.99 ± 0.01c | 6.06 ± 0.07bc | 39 ± 10ab | 42 ± 5ab | 332 ± 8a | 322 ± 3a | 13.46 ± 0.00abcd | 13.45 ± 0.00d | |||
| P3 | 0.231 | 10 | 5.4 ± 0.1a | 5.3 ± 0.2a | 6.02 ± 0.01bc | 6.09 ± 0.03ab | 46 ± 1ab | 48 ± 9ab | 328 ± 0a | 315 ± 7a | 13.46 ± 0.00ab | 13.46 ± 0.00abc | |||||
| P4 | 0.432 | 10 | 5.3 ± 0.2a | 5.8 ± 0.3a | 6.10 ± 0.01ab | 6.15 ± 0.00a | |||||||||||
| R1 | 0.231 | 20 | 104.5 ± 5.1b | 103.7 ± 3.1b | 4.97 ± 0.01d | 5.06 ± 0.02a | 33.5 ± 16.2b | 31 ± 13b | *69a | 194.5 ± 21.9a | 215 ± 4a | 124 ± 86b | 13.38 ± 0.01a | 13.38 ± 0.00a | 13.39 ± 0.00a | ||
| R2 | 0.432 | 20 | 114.7 ± 6.9 a | 104.0 ± 0.0b | 103.9 ± 1.5b | 5.03 ± 0.01ab | 4.96 ± 0.03d | 5.00 ± 0.03bcd | 20 ± 1b | 26 ± 16b | 228 ± 1a | 219 ± 9a | 13.37 ± 0.00ab | 13.38 ± 0.00ab | |||
| R3 | 0.231 | 10 | 104.4 ± 1.2b | 103.9 ± 6.8b | 4.99 ± 0.01bcd | 5.02 ± 0.01abc | 16 ± 2b | 19 ± 1b | 223 ± 4a | 226 ± 2a | 13.36 ± 0.02b | 13.38 ± 0.00ab | |||||
| R4 | 0.432 | 10 | 100.6 ± 2.2b | 104.4 ± x 1.0b | 4.99 ± 0.01cd | 5.02 ± 0.02abc | 15 ± 1b | 17 ± 3b | 223 ± 1a | 226 ± 2a | 13.37 ± 0.00ab | 13.37 ± 0.01ab | |||||
- Values are given as means ± standard deviation (n = 2). Letters indicate the statistical difference of samples, and the levels bearing different letters are significantly different (p < 0.05).
- * Single determination as filtration of the second determination came to a complete stop.
ESR spectroscopy
Samples were taken from both experiments for ESR spectroscopy analysis throughout the 12 months of storage. Radical intensities were measured directly on the malt kernels and the method was developed with inspiration from Cortes et al. 10, Kaneda et al. 11 and Tokai et al. 12. The malt kernel was placed in the centre of the cavity using a cylindrical thin-walled 702-PQ-7 clear-fused quartz tube (Wilmad Glass Company Inc., Nuena, NJ, USA). The weight of each kernel was measured before ESR analysis and the peak to peak height of the resulting ESR signal was divided by the weight of the kernel. Each result consisted of an average of 10 measurements. ESR spectra were recorded at room temperature with a Miniscope MS 200 X-band spectrometer (Magnettech, Berlin, Germany) using the following settings: microwave power, 10 mW; sweep width, 73.8 G, sweep time 30 s; number of passes, 2; modulation, 2000 mG; and microwave attenuation, 10 mW. Pilsner malt was recorded at gain 700 and roasted malt was recorded at gain 100.
Mashing
Mashings of the malts were carried out after 0, 6 and 12 months of storage according to Analytica EBC 4.5.1 ‘Extract of Malt: Congress Mash’ 14, with the modifications described by Frederiksen et al. 15. The colour of the fresh worts was measured spectrophotometrically according to Analytica EBC 8.3 ‘Colour’ 14 and sugar content was determined in °Brix values, using a refractometer (Analytic Jena, Jena, Germany). Wort filtration time was noted as the time where no more liquid appeared on the top of the filter cake, and the extract yield was measured 45 min after filtration start. The worts were stored at −20 °C and thawed before volatile analysis.
Volatiles
Dynamic head-space analysis was carried out in triplicate using 5 mL wort and 0.25 mL 4-methyl-1-pentanol (5 mg/L) added as the internal standard. The volatile compounds were collected on a Tenax-TA trap (Buchem bv, Apeldoorn, The Netherlands). The trap contained 250 mg of Tenax-TA with mesh size 60/80 and a density of 0.37 g/mL (Buchem bv, Apeldoorn, The Netherlands). The samples were equilibrated to 30 ± 1 °C for 5 min in a circulating water bath and then purged with nitrogen (200 mL/min) for 30 min. Analysis was carried out in triplicate.
The trapped volatiles were desorbed using an automatic thermal desorption unit (ATD 400, Perkin Elmer, Norwalk, CT, USA). Primary desorption was carried out by heating the trap to 250 °C with a flow (60 mL/min) of carrier gas (H2) for 15 min. The stripped volatiles were trapped on a Tenax TA cold trap (30 mg held at 5 °C), which was subsequently heated at 300 °C for 4 min (secondary desorption, outlet split 1:10). This allowed for rapid transfer of volatiles to a gas chromatograph–mass spectrometer (GC-MS, 7890A GC-system interfaced with a 5975C VL MSD with Triple-Axis detector from Agilent Technologies, Palo Alto, CA, USA) through a heated (225 °C) transfer line. Separation of volatiles was carried out on a DB-Wax capillary column 30 m long × 0.25 mm internal diameter, 0.5 µm film thickness. The column pressure was held constant at 2.4 psi, resulting in an initial flow rate of approximately 1.2 mL/min using hydrogen as carrier gas. The column temperature programme was: 10 min at 40 °C, from 40 to 240 °C at 8 °C/min, and finally 5 min at 240 °C. The mass spectrometer was operating in the electron ionization mode at 70 eV. Mass-to-charge ratios between 15 and 300 were scanned. Volatile compounds were identified by probability-based matching of their mass spectra with those of a commercial database (Wiley 275.L, HP product no. G1035A). The software program MSD Chemstation (Version E.02.00, Agilent Technologies, Palo Alto, CA, USA) was used for data analysis. Relative amounts are presented as areas based on single ions (target ions) divided by the peak area of internal standard.
Multivariate data analysis
Multivariate data analysis was applied to the relative areas of the identified volatiles analysed by GC-MS to visualize the influence from storage, temperature and water activity. The algorithms principal component analysis (PCA) and partial least squares–discriminant regression analysis were used. Multivariate data analysis was performed using Latentix software (LatentiXTM 2.0, Latent5, Copenhagen, Denmark, www.latentix.com). Analyses were carried out on the relative peak areas and data was autoscaled and fully cross-validated.
Statistical data analysis
Statistical analysis was carried out as one-way ANOVA using the software SASjmp 9, SAS Institute Inc., USA. Storage day and sample were included as fixed effects. The differences in radical intensities between treatments (temperature and water activity) were evaluated by including the average radical intensity of the malt and treatment as fixed effects and storage day as random effect.
Results
Storage of pilsner malt at different water activities
Temperature and water activity have a large influence on the storage stability of foods and the effects of these factors were investigated during malt storage. An initial study was set up where pilsner malt was stored at 20 °C in closed boxes with atmospheres where the water activities were kept fixed at 10 different levels from 0.113 to 0.910.
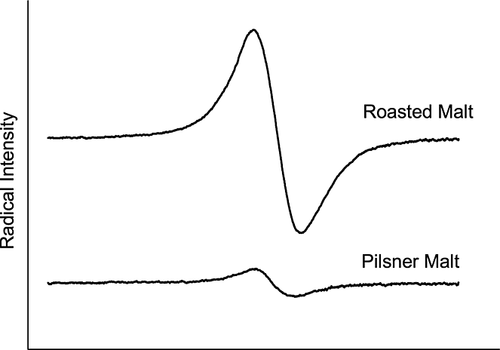
Radical intensity was measured directly on the malt kernels using ESR spectroscopy during the storage. Each result is based on an average of the peak to peak height of the ESR spectrum of 10 malt kernels divided by the weight of each kernel (Fig. 1, pilsner malt). After 1 month of storage visual mould appeared on malt stored at the high water activities, 0.817 and 0.910. After 2 months of storage mould also appeared on malt stored at a water activity of 0.756, and after 3 months mould appeared on malt stored at a water activity of 0.693 as well. Malt samples were excluded from the study after visible mould formation appeared. Malt with a water activity at 0.529 and below remained uninfected by mould throughout the 12 months of storage in agreement with previous findings 16, 17. The initial water activity of the malt kernels was low (on average 0.15). The radical content decreased in the malt samples and eventually stabilized at different levels during the storage period (Fig. 2, A). The decrease in the radical content during the first months of storage is probably caused by increased mobility of the kernel components, thus allowing chemical reactions to take place. Radicals observed in the malt kernels are probably highly reactive, but the entrapment in the physically locked matrix of the dry kernels prevent their decay. The increased mobility in the malt kernels owing to the higher water activity enables the radicals to react and their increased activity can be detected as a radical decay as observed by ESR spectroscopy. A drop in radical content in malt kernels is therefore a sign of higher component mobility and therefore also the possibility of decreased oxidative stability. After 4–6 months of storage, the radical intensities of the malt kernels had stabilized at lower levels reflecting a steady-state level between formation and decay (Fig. 2A). After 4 months of storage strong linear correlations were found between the stabilized radical intensities and the water activities, where a high water activity resulted in a low radical content (Fig. 2B).
Storage of pilsner and roasted malts
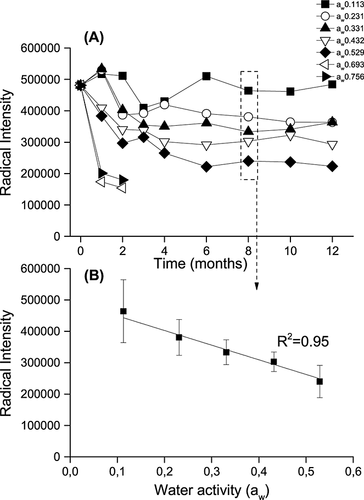
In a separate storage experiment a pilsner malt and a roasted malt were stored for 12 months at 10 or 20 °C and at water activities of 0.231 or 0.432 resulting in four different storage conditions. The roasted malt was produced from the pilsner malt by heating it at 190 °C for 50 min, resulting in a colour of 114 EBC. The two malts were therefore identical except for the roasting process. In this way differences owing to different growth, harvesting and malting conditions were eliminated. The pilsner malt was freshly kilned and the roasting was carried out the day before initiation of the storage experiment. Pilsner malt had initially a radical intensity of 3.9 × 105 and roasted malt a radical intensity of 1.9 × 106. The radical signal from roasted malt was ~7 times larger than the signal from pilsner malt, demonstrating the high level of radicals generated and trapped during the roasting in the roasted malt 18 (Fig. 1). After 1 month of storage, the radical content of the pilsner malt was lower owing to the adjustment of water activity causing increased mobility and radical decay (Fig. 3). The radical contents in both malts were found to be highly influenced by the water activity, and malts stored at the low water activity (0.231) had a significantly larger radical content and a smaller decay compared with malts stored at high water activity (0.432; Fig. 3). When the storage water activity of the malt was low (0.231), the radical intensity was not influenced by the storage temperature (10 and 20 °C). However, when the water activity of the malt was high (0.432), the storage temperature had a significant influence on the radical formation, and pilsner malt and roasted malt stored with a high water activity (0.432) at 20 °C had a significantly larger radical decay compared with storage at 10 °C. The radical content in the roasted malt changed the most during the 12 month storage period, and the high storage temperature and water activity (20 °C, 0.432) conditions resulted in a significant decay in radical content to 62% of the initial level.
Sweet wort made from stored malt
Sweet worts were made from fresh (0 months) pilsner malt and roasted malt as well as malts stored for 6 and 12 months at the different storage conditions. The sweet worts were characterized by colour, pH, filtration time, wort extract yield after 45 min of filtration and sugar content (°Brix) (Table 1). For the roasted malt, the wort colour was significantly darker for wort produced from the fresh malt as compared with the colour of wort produced from malt stored for 6 months, independent of the storage temperature and water activity. The colour of the wort did not significantly change upon further storage of the malt (12 months). The colour of wort made from pilsner malt was not affected by storage time and conditions. The pH of the worts was constant throughout the experiment, and generally the pH of the pilsner wort was approximately one unit higher than that of the wort made from roasted malt, in line with previous findings 6, 19. The sugar content was also constant throughout the 12 months of storage for both types of worts. Sugar content was generally lower for the dark worts than for the pilsner worts, probably explained by increased enzyme inactivation and starch denaturation during roasting. Filtration time of wort made from pilsner malt had a tendency to increase during malt storage, whereas the filtration time of worts made from roasted malt had a tendency to decrease; however, this was not significant. Only wort made from roasted malt stored at a water activity of 0.231 and at 20 °C had a significantly higher filtration time after 12 months of storage than all other worts. All results were based on a double determination; however, the one filtration of the latter worts came to a complete stop during filtration, and its filtration time was therefore not included in the table. The variations of the filtration times were reflected in the extract yields, which slightly decreased for pilsner wort and increased for roasted wort during storage, with the only exception being the roasted malt stored at low water activity (0.231) and 20 °C, where also the extract yield decreased owing to the slow filtration.
Volatiles
The volatile profile was determined on worts produced from pilsner malts and roasted malts stored for 0, 6 and 12 months at all storage conditions. Sixty-nine volatile compounds were identified in the wort volatile profiles through dynamic head-space sampling and GC-MS analysis (Table 2). As expected, the volatile profile of wort made from pilsner malt was very different from the volatile profile of wort made from roasted malt. Furthermore, the volatile profile of roasted malt changed much more during malt storage than that of the pilsner malt (Fig. 4).
| No. | Name | Target Ion | No. | Name | Target Ion | No. | Name | Target ion |
|---|---|---|---|---|---|---|---|---|
| 1 | 2-Methylpropanal | 72 | 24 | 1-Pentanol | 42 | 47 | 1-(2-Furyl)-2-propanone | 81 |
| 2 | 2-Propanone | 58 | 25 | Dihydro-2-methyl-3(2H)-furanone | 43 | 48 | Benzaldehyde | 106 |
| 3 | 2-Methylfuran | 82 | 26 | Methyl-pyrazine, | 94 | 49 | 2-Acetoxymethylfuran | 81 |
| 4 | 2-Butanone | 43 | 27 | 2-Hexanone | 43 | 50 | 5-Methyl-2-furancarboxaldehyde | 53 |
| 5 | 2-Methylbutanal | 57 | 28 | 1-Hydroxy-2-propanone | 43 | 51 | 2-Butylpyridine | 94 |
| 6 | 3-Methylbutanal | 58 | 29 | 2,5-Dimethylpyrazine | 108 | 52 | 1-Ethyl-1H-pyrrole-2-carboxaldehyde | 123 |
| 7 | 2,5-Dimethylfuran | 96 | 30 | 2,6-Dimethylpyrazine | 108 | 53 | 5-Methyl-1H-pyrrole-2-carboxaldehyde | 109 |
| 8 | Pentanal | 44 | 31 | Ethyl-pyrazine | 107 | 54 | 2-Methylbenzaldehyde | 91 |
| 9 | Hexanal | 56 | 32 | 2,3-Dimethylpyrazine, | 108 | 55 | 2-Isoamyl-6-methylpyrazine | 108 |
| 10 | 2-Methyl-2-butenal | 84 | 33 | 2-Ethyl-6-methylpyrazine | 121 | 56 | 2-Isopentyl-3-methylpyrazine | 108 |
| 11 | 2-Methyl-1-propanol | 43 | 34 | Nonanal | 57 | 57 | Phenylacetaldehyde | 91 |
| 12 | 5-Hydroxy-2-hexanone | 43 | 35 | 2-Ethyl-5-methylpyrazine | 121 | 58 | 2-Furanmethanol | 98 |
| 13 | 4,5-Dimethyloxazole | 97 | 36 | 2-Ethyl-3-methylpyrazine | 122 | 59 | 2,5-Dimethyl-3-(3-methylbutyl)pyrazine | 122 |
| 14 | 3-Heptanone | 57 | 37 | Propylpyrazine | 94 | 60 | N-(4-Hydroxyphenyl)-acetamide | 109 |
| 15 | 2-Penten-1-ol | 57 | 38 | 3-Furfural | 95 | 61 | 1-Phenyl-2-propanone | 43 |
| 16 | Cyclopentanone | 55 | 39 | 5-Methyl-2(3H)-furanone | 55 | 62 | 2(5H)-Furanone | 55 |
| 17 | Pyridine | 79 | 40 | Ethenylpyrazine | 106 | 63 | 4-Methyl-2-propylfuran | 95 |
| 18 | Limonene | 68 | 41 | 2,5-Dimethyl-3-ethylpyrazine | 135 | 64 | 3-Phenylfuran | 115 |
| 19 | Trimethyloxazole | 101 | 42 | Furfural | 38 | 65 | 2-Methyl-3-(furyl)propenal | 79 |
| 20 | Pyrazine | 80 | 43 | 2-Acetyl-5-methylfuran | 109 | 66 | Methylphenylpentenal | 174 |
| 21 | 3-Methylpyridine | 93 | 44 | 5-Methylfurfural | 110 | 67 | Phenol | 94 |
| 22 | 2-Pentylfuran | 81 | 45 | 2-Methyl-6-propylpyrazine | 108 | 68 | 5-Methyl-2-phenyl-2-hexenal | 117 |
| 23 | Thiazole | 85 | 46 | 3,5-Diethyl-2-methylpyrazine | 149 | 69 | 1-Furfuryl-2-formylpyrrole | 81 |
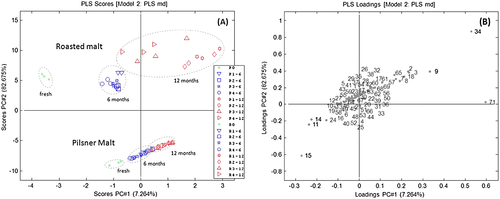
Analysis of the wort volatile profiles with a PCA provided detailed information on how the profiles changed during storage (Fig. 5). The worts made from both pilsner malt and roasted malt were mainly influenced by a decrease in volatile compounds in the first 6 months of malt storage and by formation of compounds from 6 to 12 months (data not shown). Furthermore, the wort analysis showed that the malt remained uninfluenced by storage temperature and water activity within the first 6 months of storage. After 12 months of storage, the volatile profile of wort made from both pilsner malt and roasted malt was highly influenced by the storage temperature. The volatile profile of wort from pilsner malt stored at 20 °C was also significantly influenced by the variation in water activity (Fig. 5-1A, 1B). However, the volatile profile of worts made from roasted malt was significantly influenced by the water activity when the malt had been stored at both 10 and 20 °C (Fig. 5-2A, 2B).
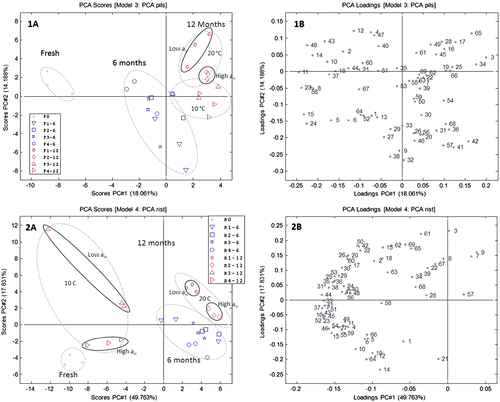
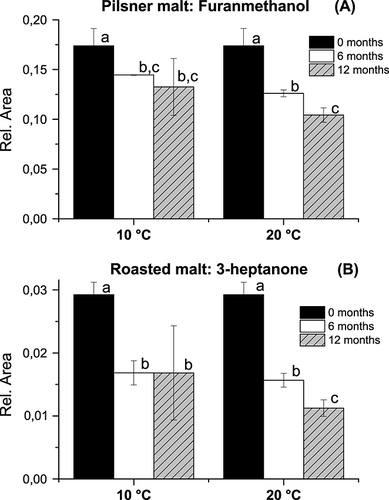
Storage temperature had a larger impact on the volatile profile during malt storage than the water activity. To illustrate how temperature influenced the volatile profile during storage, the impact of water activity was neglected and the results averaged (Figs. 6-8). As the roasted malt had a much more intense volatile profile compared with pilsner malt, the secondary axes were made proportional to the volatile intensity and different axes may appear for the same compound originating from pilsner malt and roasted malt (Figs. 7 and 8). A major part of the change in the volatile profile during storage can be explained by a loss in volatile compounds exemplified for furanmethanol in wort from pilsner malt (Fig. 6A) and 3-heptanone in wort from roasted malt (Fig. 6B). The volatile profile of pilsner malt was furthermore characterized by a loss in alcohols and the volatile profile of roasted malt was characterized by a loss of pyrazines and ketones. Quantitatively more volatiles were lost for roasted malt compared with pilsner malt and generally more compounds were lost during storage at 20 °C compared with storage at 10 °C (Fig. 6). This loss illustrates what is likely to be the case for all volatile compounds in the malt during storage. Therefore, the compounds that are formed during storage are probably also subject to evaporation. The degree of evaporation of each volatile is further complicated by its affinity to the malt matrix, which may also be affected by the water activity.
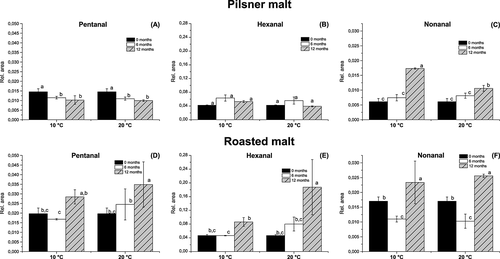
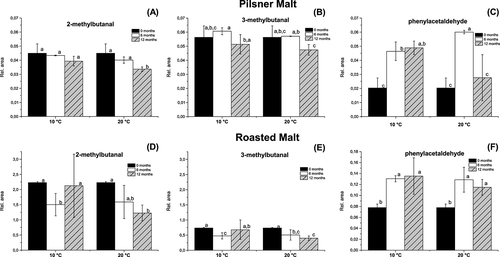
In spite of the relatively large evaporation that occurred, compounds were also found to increase in concentration during storage. Lipid oxidation occurred, particularly in the roasted malt, expressed by the formation of nonanal, hexanal and pentanal (Fig. 7). Hexanal and pentanal levels were found to increase only in roasted malt and not in pilsner malt. High temperature is known to increase lipid oxidation and, despite the increased evaporation, also found in roasted malt, the concentration of hexanal was more than 4 times higher than in pilsner malt after 12 months of storage at 20 °C. Interestingly, a slightly higher quantity of hexanal and pentanal was detected in roasted malt stored at 20 °C and at the low water activity of 0.231 compared with 0.432. The relative concentrations for pentanal were 0.0432 ± 0.0030 at low water activity and 0.0265 ± 0.0003 at high water activity and the relative concentration for hexanal was 0.2444 ± 0.0078 at low water activity and 0.1307 ± 0.0063 at high water activity. The nonanal concentration was found to increase significantly in both the pilsner malt and roasted malt, but was highest in the roasted malt. In addition, methylfuran was formed continuously in both the pilsner and roasted malt during storage. Also in pilsner malt, 2-methyl-3-furylpropenal and pyridine were formed and in roasted malt 2,5-dimethylfuran, propanone and limonene were formed (data not shown).
The Strecker aldehydes 2-methylbutanal, 3-methylbutanal and phenylacetaldehyde are all compounds characteristic for malt flavour 2, 4 and they were present in roasted malt in much larger quantities than in pilsner malt (Fig. 8), which is not surprising owing to the Maillard reactions taking place during roasting. The compounds 2-methylbutanal and 3-methylbutanal tended to decrease in concentration during storage, although this was only significant for storage at 20 °C, where the concentrations in the roasted malt had decreased to approximately half of the original content after 12 months. Phenylacetaldehyde was formed during storage and its presence in pilsner malt was highly influenced by temperature. In roasted malt stored for 12 months at 10 °C, there was a tendency for all three Strecker aldehydes to be present in slightly larger concentrations when stored at a water activity of 0.432 compared with 0.231. It was recently found that water treatment of malt and other dry products resulted in increased formation of phenylacetaldehyde and 2- and 3-methylbutanal 20, which may explain the observations in the current study.
Discussion
The sugar content in the sweet wort was constant throughout malt storage, showing that the major components in the malt and the starch-degrading enzymatic activity were intact. However, the mobility of components in the malt depended on the water activity and temperature during the storage. This resulted in wort samples where the volatile profiles and filtration rates changed during the storage of the malt.
The radical content in pilsner malt was highly correlated with water activity (R2 = 0.95), indicating that water activity is an important factor for radical decay and radical reactivity in malt. The radical content of other dried products has also been found to be influenced by water activity, although with less linear correlation 9, 21. The radical content observed during the storage experiment with both pilsner malt and roasted malt was also influenced by water activity. At the high water activity (0.432), the storage at high temperature (20 °C) further affected the radical decay, whereas at low water activity (0.231), the variation in storage temperature did not influence the radical content significantly. Radical content in roasted malt was approximately 7 times larger than the radical content in pilsner malt, and also the radical decay was larger for the roasted malt. These results were reflected in the volatile analysis, as the volatile profile of wort made from roasted malt changed more during storage than the volatile profile of wort made from pilsner malt. Furthermore, the volatile profiles of both types of wort were highly influenced by temperature, thus correlating with the larger radical decay detected at 20 °C and high water activity (0.432). For the roasted malt, the storage water activity had a significant influence on the volatile profile of the wort at both high (20 °C) and low (10 °C) storage temperatures after 12 months of storage. For pilsner malt, the influence of storage water activity was only significant for storage at high temperature (20 °C). Some of the changes in the volatile profiles of the malts were caused by evaporation, which is independent of oxidative reactions. However, the oxidative changes detected by ESR were large enough to significantly impact the formation of the volatiles detected. These results show that temperature is very important for both the rate of oxidative reactions and for the evaporation of volatiles and that low water activity (0.231) is a limiting factor for oxidative reactions.
Detailed studies of the volatile compounds showed that the radical decay was highly correlated to the oxidative stability of the malt in terms of development of more lipid oxidation-derived compounds such as nonanal, hexanal and pentanal. Lipid oxidation is known to be one of the main contributors to off-flavours in beer 1. In the current study, formation of secondary oxidation products caused by lipid oxidation was not significant until after 12 months of storage (Fig. 7). Hexanal and pentanal are both formed from the oxidation of linoleic acid and nonanal is formed from the oxidation of oleic acid 22. Linoleic acid is the most abundant fatty acid in malt (~60% of the total lipid content) followed by oleic acid (~11%), depending on the barley variety 23. The current study shows that pentanal, nonanal and hexanal in the wort are linked to the roasting of the malt and that they increase in concentration during the storage of the malt (Fig. 7). Lipid oxidation may be caused by a combination of radical induced oxidation and LOX-induced oxidation, and the natural substrate for LOX is linoleic acid 7. The content of LOX has been found to decrease in activity during malt storage 7; however, in the current study lipid oxidation products continued to increase in the wort, indicating that the main part of the lipid oxidation was radical-induced. Lipid oxidation became more pronounced in the worts during storage of the malts at 20 °C compared with 10 °C in accordance with existing knowledge 24. An interesting observation was that a slightly higher quantity of hexanal and pentanal was detected in wort from roasted malt stored at the low water activity of 0.231 compared with 0.432 at 20 °C. As mentioned previously, this could be caused by an increased affinity towards the malt matrix at the lower water activity resulting in decreased evaporation; however, the reaction rate may also be influenced by the water activity. In a recent study, oatmeal, peanuts and pork scratchings were found to have significantly increased hexanal formation at a water activity of 0.231 compared with 0.432 9. A similar tendency was found for oxidation of flaxseed oil where high oxidation rates were found at both low and high humidity conditions 25.
The fact that a large number of compounds, including coloured Maillard reaction compounds, were lost during the first 6 months of storage correlated with the decrease in wort colour (Table 1). Similar to this, Forster et al. 2 also found a correlation between colour and formation of Strecker aldehydes and N-heterocycles, which were found to initially increase, whereafter they decreased to concentrations below the initial content. In a recent study of sweet wort made from malt, with colours varying from 8 to 100 EBC, it was found that malt roasted to a level above 33 EBC had a large volatile loss when heated to 40 °C for 10 h 6. This and the current study underline the fact that malt roasting has a negative effect on the oxidative stability of beer caused by increased radical formation, increased lipid oxidation and a greater loss of volatiles during malt storage, as well as in the initial stages of brewing. This is in agreement with other studies having identified a decrease in oxidative stability with increasing beer colour based on ESR lag phase measurements 10, 26. This suggests the oxidative stability of roasted malts can be correlated to the oxidative stability of the resulting beers.
The changes occurring during malt storage were found to be caused by a combination of (a) compound formation through oxidative processes and (b) loss of volatiles through evaporation. The intensity of volatiles as well as the changes in volatile profiles of worts were relatively small during storage of pilsner malt compared with roasted malt. However, the changes occurring in pilsner malt may be more important, as in general this malt aroma is more precise and delicate. The aroma of roasted malt is stronger and more complex and variations in volatile profile may to some extent be masked by its complexity.
Conclusions
The mobility of components in the malt depended on the water activity and temperature during storage. This resulted in wort samples where the volatile profiles and filtration rates changed during the storage of the malt. However, the malt storage did not affect the sugar content in sweet worts, showing that the major components and starch degrading enzymatic activities were intact in the malts.
Malt roasting had a negative effect on the oxidative stability of beer caused by increased radical formation, increased lipid oxidation and a greater loss of volatiles during malt storage, as well as in the initial stages of brewing. For both roasted malt and pilsner malt, good correlations were found between radical decay and changes in the volatile profile of the sweet wort, where high temperature and high water activity resulted in the largest changes. This study suggests that chemical changes occurring in malts during less than 12 months of storage may potentially affect the aroma of the beer and that water activity and storage temperature both should be kept low in order to maintain a high malt quality.




