Cutaneous Reactions to Iron Infusions: A Case Report and Clinical Review
Funding: The authors received no specific funding for this work.
ABSTRACT
Anemia is prevalent globally and often treated with intravenous (IV) iron formulations, including iron sucrose, iron dextran, ferric carboxymaltose, ferric derisomaltose, and ferumoxytol. IV iron has a high efficacy and safety profile, quickly improving hemoglobin levels with low rates of adverse events. In this work, we report an uncommon case of a cutaneous hypersensitivity reaction following IV iron. A review of the literature shows that acute and delayed cutaneous reactions are possible but rare and respond well to management. Patients should be educated, and staff should be vigilant as to these cutaneous reactions.
Clinical Trial Registration:The authors have confirmed that clinical trial registration is not needed for this submission.
1 Introduction
Anemia is prevalent in patients with cancer, affecting 30–90% of patients undergoing chemotherapy [1]. The etiology is often multifactorial, including components of iron deficiency from gastrointestinal bleeding, malnutrition, and poor enteral absorption [2]. The National Comprehensive Cancer Network (NCCN) recommends investigating anemia with a complete blood count, serum ferritin, and transferrin saturation [3]. Where indicated, iron repletion is preferred using intravenous (IV) infusions, especially in those with functional iron deficiency (serum ferritin >30 ng/mL and TSAT <30%) [2]. Guidelines from the American Society of Clinical Oncology (ASCO) provide similar recommendations to the NCCN [4]. IV iron infusion formulations include iron sucrose [5], iron dextran [6], ferric carboxymaltose [7], ferric derisomaltose [8], and ferumoxytol [9]. Differences in formulation use relate to cost, institution formulary, and the timing to receive the dose [10]. IV iron has a high efficacy and safety profile as proven by numerous studies, quickly improving hemoglobin levels with low rates of adverse events [11]. In this work, we report a rare case of a cutaneous hypersensitivity reaction and review the existing literature on the topic for clinicians.
2 Case
A 56-year-old Caucasian male was diagnosed with iron-deficiency anemia. With a hemoglobin of 6.2 g/dL, he underwent endoscopy, showing an ‘apple core’ lesion in the right colon. Pathology confirmed adenocarcinoma, and body imaging did not show any metastases. He proceeded with resection, leading to a diagnosis of pT3N1, Stage III colon cancer. He was recommended oral iron supplementation and to consider adjuvant chemotherapy when recovered.
At ten weeks post-surgery, the patient remained with symptomatic anemia; laboratory testing confirmed poor enteral iron absorption with serum iron level 30 µg/dL, transferrin saturation 7% and ferritin 16 ng/mL. IV iron dextran infusion was recommended. Halfway into the infusion, the patient had a minor reaction consisting of flushing of the face, without associated tachypnea, tachycardia, hypotension, wheezing, stridor, or oedema. He was treated with diphenhydramine 25 mg and famotidine 20 mg, and the reaction resolved; he went on to complete the infusion.
One day after infusion, the patient reported developing a diffuse serpiginous rash with target-like lesions on the extremities (Figure 1). The rash did not improve with topical steroids, and he was presented to the hospital on Day 7 post-infusion. A punch biopsy was performed, showing a neutrophilic infiltrate with leukocytoclastic vasculitis (Figure 2). Extensive evaluation for autoimmune disease and infection was negative, including testing for hepatitis, antiphospholipid antibody syndrome, rheumatoid arthritis, lupus, and cryoglobulinemia. He was started on oral prednisone 1 mg/kg dosing daily with improvement. After 5 days, he was discharged to complete a prednisone taper over 3 weeks. He followed up in clinic at 2 weeks post-discharge and had a good response and was able to begin adjuvant chemotherapy while finishing the steroid taper. He has done well since with no recurrence of symptoms.
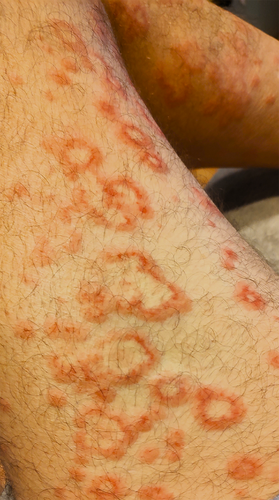
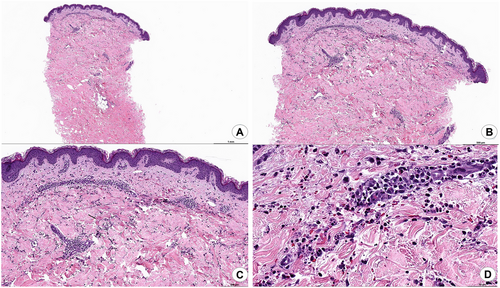
3 Discussion
Intravenous iron infusions are influential in the cancer patients' treatment course, especially those undergoing chemotherapy. Several studies have proven intravenous iron infusions superior in treating iron deficiency anemia in cancer patients undergoing chemotherapy. Many studies in the last two decades have found that IV iron infusions replenish iron levels and hemoglobin quickly than oral treatments, essential biomarkers in a patient's quality of life and tolerance to chemotherapy. IV iron has a high efficacy rate, with Auerbach et al. calculating it to be about 97% [12].
A recent meta-analysis by Litton et al. found a correlation between IV iron and a reduction in the need for blood transfusion, proving that IV iron is highly effective in increasing hemoglobin levels and reducing the risk of transfusions [11]. There was a nonlinear, positive, and statistically significant correlation between hemoglobin levels and quality of life questionnaire scores, showing a correlation between both [13]. IV iron was found to lead to increased hemoglobin recovery compared to oral iron treatments. This is an essential factor affecting a cancer patient's treatment plan and tolerance of chemotherapy [14]. This also improves the quality of life in those dealing with multifaceted anemia while having cancer treatment [15].
Table 1 summarizes reported data on cutaneous reactions in large studies on IV Iron. Although delayed cutaneous reactions can occur from Intravenous iron infusions, overall, their occurrence is extremely rare. Cutaneous reactions range in severity from a mild acute reaction with transient flushing of the skin and face to the more severe delayed reaction reported in this case with persistent rash due to leukocytoclastic vasculitis. When comparing each formulation of IV iron, iron dextran has been reported to have a slightly higher rate of cutaneous reactions compared to the newer iron formulations, showing 3.3% compared to 1.9 and 2.1%, respectively [16].
| Study | N | IV iron formulation | Underlying disease |
Cutaneous reaction reported (% infusions) |
Management |
|---|---|---|---|---|---|
| Arastu et al. [16] | 12,237 | Iron sucrose, ferric carboxymaltose, ferumoxytol, and iron dextran | Multiple cancer types | 3.9% urticarial type | Diphenhydramine, famotidine, and/or corticosteroid |
| Stojanovic et al. [17] | 11,130 | Iron polymaltose, ferric carboxymaltose, and ferric derisomaltose | Iron-deficiency anemia | 0.6% urticarial type; <0.1% nonlaryngeal angioedema | Diphenhydramine, famotidine, and/or corticosteroid; rechallenge with the same or different formulation for mild or moderate reactions |
| Henry et al. [18] | 187 | Iron dextran, iron sucrose, and ferric gluconate | Genitourinary cancer | 1.5% urticarial type | Supportive care and observation |
The underlying mechanisms of reactions to IV iron and not well understood and are mostly thought not to be IgE-mediated, but rather complement-mediated, through activation of mast cells and basophils in the blood [17]. A self-limiting reaction is known as the “Fishbane reaction.” This reaction, characterized by transient flushing and chest/back pain (truncal myalgia), resolves quickly with supportive care and occurs in approximately one in 100 patients given intravenous iron [17]. There are no severe symptoms, such as hypotension, wheezing, stridor, or laryngeal oedema. The pathogenesis of Fishbane reactions is also not exactly understood. The transient flushing experienced in this case during the infusion had the typical features of a mild hypersensitivity or Fishbane reaction and was not felt to be related to the later delayed cutaneous reaction.
To further investigate delayed reactions, a review of case reports/series reported in PubMed through October 31, 2024, is presented in Table 2. The most common delayed reaction was found to be a cutaneous exanthema. In the present case, leukocytoclastic vasculitis occurred after the patient was given an iron dextran infusion. This type of reaction is scarce, with little data on the relationship between iron-dextran infusions and vasculitis. However, there are cases with similarity reported, also managed with systemic corticosteroid. Our patient responded well to the prednisone taper, with a complete response in a short amount of time. Chemotherapy was resumed within the month post-treatment with the continuation of a steroid taper.
| Study | IV iron formulation (n) | Comorbidity | Cutaneous reaction type | Management |
|---|---|---|---|---|
| Stojanovic et al. [17] | Iron polymaltose (8) and ferric carboxymaltose (3) | Unknown | Delayed exanthematous reaction, facial flushing, and urticaria 2 days after infusion | Specific treatment not reported; two cases rechallenged with alternative formulation |
| Shi et al. [19] | Ferric derisomaltose (1) | Stage 4 CKD and lupus nephritis | Skin staining due to extravasation | Dermatology offered laser therapy |
| Mantri et al. [20] | Ferumoxytol (1) | History of iron deficiency and anemia, and hypothyroidism | Exanthematous drug reaction | Methylprednisolone 40 mg intravenously bid; total 5 days of corticosteroid treatment |
| Crowley et al. [21] | Ferric carboxymaltose (1) | Iron deficiency anemia, currently in gestation | Skin stain reaction | Specific treatment was not reported |
4 Conclusion
Cutaneous reactions following IV iron infusion can occur in both acute and delayed settings. A review of the literature shows that delayed reactions are uncommon and treatable. Patients and staff should be educated and monitored for this adverse effect.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information.




