Serum Ferritin in Women With HFE p.C282Y Homozygosity: Positive Associations With Age, Live Births, Menopause, and Transferrin Saturation
Funding: National Heart, Lung, and Blood Institute in conjunction with the National Human Genome Research Institute. The study was supported by The University of Alabama at Birmingham (N01-HC05188); University of Minnesota (N01-HC05185); Howard University (N01-HC05186, N01-CM-07003-74) and the Minority Community Clinical Oncology Program; Kaiser Permanente Center for Health Research (N01-C05189); University of California Irvine (N01-HC05190); London Health Sciences Centre (N01-HC05191); Wake Forest University (N01-C05192). Additional support was provided by The University of Alabama at Birmingham General Clinical Research Center, Grant M01-RR00032; and Southern Iron Disorders Center.
Trial Registration: The authors have confirmed clinical trial registration is not needed for this submission.
ABSTRACT
Background
We sought to determine associations of serum ferritin (SF) with live birth numbers and other iron-related variables in women with HFE p.C282Y (rs1800562) homozygosity.
Methods
We studied non-pregnant, non-Hispanic white women in post-screening evaluations to determine associations of SF with age, pregnancy and live birth numbers, dichotomous menopause and therapeutic phlebotomy reports, daily food and supplemental iron intakes, and transferrin saturation (TS).
Results
There were 136 women with mean age 51 ± 13 (SD) years and median SF 238 µg/L (range: 8, 2960). There were 376 pregnancies (median: 3/woman (1, 8)) and 296 live births (median: 2/woman (0, 6)). A total of 70 women (51.5%) reported menopause and 31 women (22.8%) reported phlebotomy. Median heme + non-heme food iron intake was 13.4 mg/d (3.1, 57.3). Mean TS was 62 ± 25%. Pearson's coefficient of ln SF versus age was 0.1955 (p = 0.0226). Median SF of women with and without menopause was 386 µg/L (8, 2960) and 165 µg/L (8, 1894), respectively (p < 0.0002). SF associations with phlebotomy and iron intakes were not significant. Spearman's coefficient of SF versus TS was 0.5079 (p < 0.0001). Four mean ln SF values of 66 women without menopause subgrouped by live birth numbers were similar (one-way ANOVA p = 0.4460). Multiple regressions on SF using pregnancy numbers revealed positive associations with menopause (p = 0.0157) and TS (p < 0.0001) and using live birth numbers revealed positive associations with live births (p = 0.0389), menopause (p = 0.0305), and TS (p < 0.0001).
Conclusions
SF levels in 136 women with p.C282Y homozygosity are positively associated with age, numbers of live births, menopause reports and TS.
1 Introduction
Hemochromatosis in persons of European descent is usually associated with homozygosity for p.C282Y (rs1800562), a common missense mutation of the homeostatic iron regulator (HFE, chromosome 6p22.2) [1, 2]. The estimated prevalence of p.C282Y homozygotes in non-Hispanic white persons in North America is 1 in 227 [3] and in persons of European descent in the United Kingdom, it is 1 in 156 [4]. HFE, a non-classical Class I major histocompatibility complex protein, is an upstream regulator of the peptide hormone hepcidin (HAMP, chromosome 19q13.12) and thus of iron homeostasis [5]. Lower serum hepcidin levels [6] and lower hepatic expression of HAMP mRNA [7] in HFE p.C282Y homozygotes than in control subjects account for increased iron export from storage cells [8] and enhanced intestinal iron absorption [9, 10].
Elevated transferrin saturation (TS), a surrogate marker of increased plasma iron transport, and elevated serum ferritin (SF), a surrogate marker of increased iron stores, occur in 40%–60% of untreated female HFE p.C282Y homozygotes and in 75%–100% of untreated male p.C282Y homozygotes [3, 11-13]. Iron overload-related disease is also less prevalent in women than in men with p.C282Y homozygosity [14, 15].
The net iron cost of a normal singleton neonate, including maternal blood loss at delivery, is ∼740 mg in women with normal iron reserves who are not selected for hemochromatosis phenotypes [16]. Increasing numbers of pregnancies in healthy women who are not selected for hemochromatosis phenotypes or HFE genotypes are associated with decreasing SF levels [17, 18]. Accordingly, early investigators postulated that iron losses due to pregnancy also contribute to the lower SF levels and the lower prevalence of iron overload in women than in men with hemochromatosis [19]. It was the unexpected conclusions of three studies that SF levels or quantities of iron removed by phlebotomy to achieve iron depletion in women with hemochromatosis were not significantly associated with their numbers of pregnancies [20-22].
Maternal daily requirements for absorbed iron during pregnancy increase from < 0.8 mg in the first trimester to 4–5 mg in the second trimester, and to > 6 mg in the third trimester [23]. The iron content of human fetuses increases in proportion to fetal weight [24]. Accordingly, we reasoned that the maternal iron costs of pregnancies are lower than those of live births on average, although we found no report of the association of SF levels or iron stores with numbers of live births in women with HFE p.C282Y homozygosity.
The aim of this study was to determine the associations of SF with the following iron-related independent variables in 136 non-pregnant women with HFE p.C282Y homozygosity at post-screening evaluations: age, reports of numbers of pregnancies and of live births, dichotomous reports of menopause and therapeutic phlebotomy, estimated daily intakes of heme, non-heme, and supplemental iron, and TS. We compare our observations with the results of other studies of women with and without hemochromatosis and discuss the effects of age, the numbers of pregnancies, numbers of live births, menopause, and TS on iron balance.
2 Methods
2.1 Primary Care-Based Screening
The HEIRS Study was designed as a cross-sectional primary care-based screening study only. [3, 25]. All participants reported race/ancestry categories approved by the National Heart, Blood, and Lung Institute and the National Human Genome Research Institute [3, 25]. A total of 98% of self-reported non-Hispanic white participants were recruited at Field Centers in Alabama, California, Ontario, and Oregon/Hawaii [3, 25]. Laboratory testing performed at screening included only TS and SF phenotyping and HFE p.C282Y (rs1800562) and p.H63D (rs1799945) allele-specific genotyping [3, 25]. Of 299 p.C282Y homozygotes detected by screening (138 men, 161 women), 94.0% reported non-Hispanic white ancestry [3].
2.2 Post-Screening Evaluations
Invitations to participate in post-screening evaluations were extended to all HEIRS Study participants with HFE p.C282Y homozygosity [25, 26]. Evaluations included the following: 1) questionnaires completed by participants that addressed iron-related medical histories (Supporting Information); 2) University of Hawaii Multi-Ethnic Dietary Questionnaires [27]; 3) focused physical examinations performed by HEIRS Study physicians [25, 26]; and 4) laboratory testing of blood specimens [25, 26]. The median interval between primary care-based screening and post-screening evaluations was eight months [26].
Analyses of the Dietary Questionnaires at the University of Hawaii provided estimates of the average daily intake of heme, non-heme, and supplemental iron for the previous year [27, 28]. Dietary iron attributed to the consumption of meat, fish, and poultry was classified as heme iron. Other dietary iron was classified as non-heme iron [27]. Iron intakes were expressed as mg/d.
2.3 Post-Screening Evaluation Participants
The present cohort includes 136 non-Hispanic white women with HFE p.C282Y homozygosity who reported that they were not pregnant, who fasted overnight before attending evaluations, who answered medical history questions pertinent to the present study, and who had complete TS and SF data. The mean TS and median SF of the 136 women measured in screening and in post-screening evaluations did not differ significantly (Table S1).
Age, TS, and SF data in the present 136 women did not differ significantly from those of 25 other non-Hispanic white women with HFE p.C282Y homozygosity who attended post-screening evaluations but who were excluded from this study because they reported pregnancy or possible pregnancy or some of their pertinent data were missing (Table S2).
2.4 Laboratory Testing
A morning blood sample was obtained from each woman after an overnight fast. Testing included TS and SF measurements and HFE genotype confirmation performed at the HEIRS Study Central Laboratory (Fairview-University Medical Center Clinical Laboratory, University of Minnesota, Fairview, MN, USA) [3]. Reference ranges for TS and SF in women were 10%–45% and 20–200 µg/L, respectively [3]. The HFE genotype p.C282Y homozygosity was confirmed in each of the present women.
2.5 Statistics
The dataset for the present analyses consisted of observations on 136 women. Data for age, numbers of pregnancies, numbers of live births, TS, and SF are displayed to the nearest integer. Other continuous data are displayed to the nearest single decimal place. We assigned dichotomous variables to questionnaire responses about menopause and therapeutic phlebotomy.
Kolmogorov–Smirnov testing demonstrated that age and TS data did not differ significantly from those that are normally distributed. We displayed these data as means ± 1 standard deviation (SD) and compared them with Student's t-test for unpaired data (two-tailed). We displayed other continuous data as medians (ranges) and compared them using Mann–Whitney U tests (two-tailed). We normalized SF data as ln SF for some analyses. Categorical data were compared using Fisher's exact test (two-tailed). We computed Pearson's correlation coefficient (r) and Spearman's rank correlation coefficient (rho, ρ), as appropriate. We subgrouped 66 women without menopause reports by increasing numbers of their live birth reports and compared the mean ln SF of the four subgroups using a one-way ANOVA test.
We evaluated these independent variables for suitability in multiple regressions on SF: age, numbers of pregnancies and live births, dichotomous reports of menopause and therapeutic phlebotomy, estimated daily intakes of heme, non-heme, and supplemental iron, and TS. The Spearman's rank correlation of numbers of pregnancies and numbers of live births was significant (ρ136 = 0.7886; p < 0.0001). Thus, we used only one or the other of these two variables in a corresponding regression on SF, as appropriate. The Spearman's correlation of heme and non-heme iron intakes was also significant (ρ136 = 0.5062; p < 0.0001) and so we added these two variables to create a single food iron intake variable. In a correlation matrix, age was significantly associated with numbers of pregnancies, numbers of live births, and reports of menopause, and so we deleted age as an independent variable. Preliminary regression models revealed that standardized beta coefficients (β) were low and values of p were high for both food iron and supplemental iron intakes, and thus we excluded these variables from final regression models. We report the contribution of each remaining independent variable to the final regressions as β. We report the proportion of variance in SF explained by the variable(s) as R2 (adjusted R2).
We defined p < 0.05 to be significant. We used Excel 2000 (Microsoft Corp., Redmond, WA, USA) and GraphPad Prism 8 (2018; GraphPad Software, San Diego, CA, USA).
3 Results
3.1 Characteristics of 136 Women With HFE p.C282Y Homozygosity
3.2 Age, Transferrin Saturation, and Serum Ferritin
The mean age of 136 women was 51 ± 13 years. The mean TS and median SF were 62 ± 25% and 238 µg/L (8, 2960), respectively. The combination of TS > 45% and SF > 200 µg/L occurred in 65 women (47.8%). SF < 200 µg/L occurred in 61 women (66.9%). The combination of TS ≤ 45% and SF ≤ 200 µg/L occurred in 24 women (17.6%). Seven women (5.1%) had SF <10 µg/L.
3.3 Pregnancies, Live Births, and Menopause
The total number of pregnancies was 376 (median: 3 pregnancies/woman (1, 8)). One pregnancy was reported by each of 25 women, two pregnancies by 39 women, three pregnancies by 38 women, and four or more pregnancies by 34 women. The ratio of the total number of pregnancies to the total number of women was 2.8:1.
The total number of live births was 296 (median: 2 live births/woman (0, 6)). No live births were reported by each of 13 women, one live birth by 22 women, two live births by 51 women, three live births by 33 women, and four or more live births by 17 women. The ratio of the total number of live births to the total number of women was 2.2:1.
The ratio of the total number of pregnancies to the total number of live births was 1.3:1. In this cohort, 78.9% of reported pregnancies resulted in live birth reports.
Menopause was reported by 70 women (51.5%). Their median age at menopause was 49 years (26, 59).
3.4 Phlebotomy Treatment
A total of 31 women (22.8%) reported that they had been treated with phlebotomy for iron overload or hemochromatosis.
3.5 Iron Intake
The median heme and median non-heme iron intakes were 2.0 mg/d (0.4, 23.9) and 11.1 mg/d (2.5, 41.7), respectively. The median intake of food (heme + non-heme) iron was 13.4 mg/d (3.1, 57.3). The median supplemental iron intake was 0.0 mg/d (0.0, 453.0).
3.6 Serum Ferritin Comparisons in 136 Women With HFE p.C282Y Homozygosity
3.7 Serum Ferritin and Age
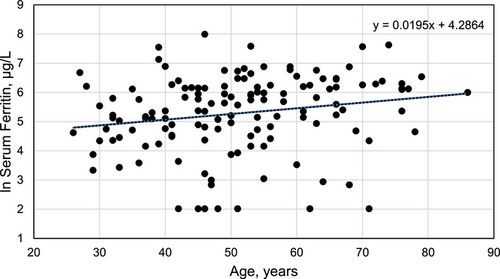
Pearson's correlation of ln SF with age was significant (r136 = 0.1955; p = 0.0226) (Figure 1).
3.8 Serum Ferritin and Menopause Reports
Median SF in women who reported menopause was 2.3-fold higher than that of women who did not report menopause (Table 1).
| Characteristic | Menopause reports (n = 70) | No menopause reports (n = 66) | p value |
|---|---|---|---|
| Mean age at post-screening evaluation, years (SD) | 61 ± 10 | 41 ± 8 | < 0.0001 |
| Mean transferrin saturation, % (SD) | 64 ± 24 | 59 ± 26 | 0.2263 |
| Median serum ferritin, µg/L (range) | 386 (8, 2960) | 165 (8, 1894) | 0.0002 |
| Median number of pregnancies (range) | 3 (1, 7) | 2 (1, 8) | 0.2363 |
| Median number of live births (range) | 2 (0, 6) | 2 (0, 4) | 0.1303 |
- Abbreviation: SD, standard deviation
3.9 Serum Ferritin and Therapeutic Phlebotomy
The median SF of women with and without therapeutic phlebotomy reports did not differ significantly (178 µg/L (8, 2960) vs. 277 µg/L (8, 1960), respectively; p = 0.3877).
3.10 Serum Ferritin and Iron Intakes
Spearman's rank correlations of SF with heme, non-heme, food, and supplemental iron intakes were not significant (Table S3).
3.11 Serum Ferritin and Transferrin Saturation
Spearman's rank correlation of SF with TS was significant (ρ136 = 0.5079; p < 0.0001).
3.12 Serum Ferritin and Live Births in Women Without Menopause Reports
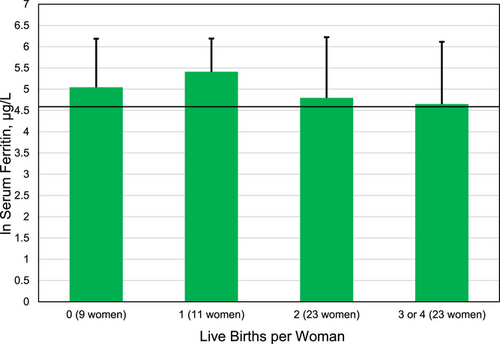
There were 66 women without menopause reports whose mean age was 41 ± 8 years. We computed the mean ln SF of these women subgrouped by numbers of live birth reports (Figure 2). The four mean ln SF values did not differ significantly (one-way ANOVA p = 0.4460). Each of the four mean ln SF values represented SF > 200 µg/L (Figure 2).
3.13 Regression on Serum Ferritin Using Numbers of Pregnancies
We used these four independent variables: numbers of pregnancies, reports of menopause and therapeutic phlebotomy, and TS. The regression revealed two positive associations with SF: menopause reports (p = 0.0157; β = 0.1835); and TS (p < 0.0001; β = 0.5179). The association of SF with numbers of pregnancies was not significant (p = 0.3011; β = 0.0783). The association of SF with therapeutic phlebotomy reports (dichotomous) was not significant (p = 0.2529; β = 0.0861). R2 (adjusted R2) of this regression was 0.3103 (0.2822). The ANOVA p of this regression was < 0.0001.
3.14 Regression on Serum Ferritin Using Numbers of Live Births
We used these four independent variables: numbers of live births, reports of menopause and therapeutic phlebotomy, and TS. The final regression revealed three positive associations with SF: numbers of live births (p = 0.0467; β = 0.1511); menopause reports (p = 0.0305; β = 0.1639); and TS (p < 0.0001; β = 0.5310). The association of SF with therapeutic phlebotomy reports was not significant (p = 0.3090; β = 0.0759). R2 (adjusted R2) of this regression was 0.3253 (0.3048). The ANOVA p of this regression was < 0.0001.
4 Discussion
Two novel findings of this post-screening evaluation of non-Hispanic white women in North America with HFE p.C282Y homozygosity are that: 1) the four mean ln SF values of 66 women without menopause reports who were subgrouped by live birth numbers did not differ significantly and each of the four mean ln SF values represented SF > 200 µg/L; and 2) the SF levels of 136 women not selected for menopause reports were positively associated with their numbers of live births after adjustment for other variables. Together, these observations demonstrate that SF levels, surrogate measures of iron stores, do not decrease on average as a consequence of increasing numbers of live births in women with p.C282Y homozygosity. Consistent with these observations, total iron per organ (liver, spleen, heart, and pancreas) was higher in pluriparous than nulliparous hfe−/− mice [29]. Corresponding hepatic hepcidin mRNA expression (both Hepc1 and Hepc2) was lower in pluriparous than nulliparous hfe−/− mice [29]. These observations in mice further suggest that mechanisms other than those responsive to body iron stores contribute to the regulation of iron absorption and metabolism during pregnancy in women with p.C282Y homozygosity.
There was no significant association of SF with the numbers of pregnancies in the present cohort, in agreement with the conclusions of three studies of women of European descent with hemochromatosis [20] or HFE p.C282Y homozygosity [21, 22]. In French women with p.C282Y homozygosity, the mean amount of iron removed by phlebotomies to achieve iron depletion was significantly higher in women who reported having had one or more pregnancies than in women who reported having had no pregnancies [22]. Contrary to a previous postulate [19], these observations demonstrate that iron losses due to pregnancies do not contribute significantly to the lower prevalence of iron overload phenotypes and iron overload-related disease in women than in men with p.C282Y homozygosity [21, 22]. These observations also suggest that another factor(s), especially in pre-menopausal women with p.C282Y homozygosity, accounts for their lower SF levels and iron overload prevalence than those of men with p.C282Y homozygosity. Menstrual blood loss may be such a factor [30].
SF levels of the present women were positively correlated with age. In 27,099 non-Hispanic white women in the United States aged ≥ 25 years who were not selected for iron phenotypes or HFE genotypes, SF rose with age until the approximate age of 62 years and increased less rapidly with age thereafter [31]. The prevalence of non-hemochromatosis disorders that increase SF also increases with age [32].
The median age at menopause reported by the present 70 women was 49 years. The median age at natural menopause or hysterectomy reported by French and Canadian women with homozygous hemochromatosis was 50 years [20]. In 22,282 community-dwelling women in the United States who were not selected for iron phenotypes or HFE genotypes, the median age at natural menopause was 50 years [33].
SF levels of the present women were positively associated with menopause reports after adjustment for other variables, in agreement with previous studies [20, 22, 30]. Likewise, SF increased significantly after the fifth decade of life in 1179 Danish women who were not blood donors [34] and in 27,099 non-Hispanic white women in the United States who were not selected for iron phenotypes or HFE genotypes [31].
Age-related increases of SF after the fifth decade of life in women are caused in part by net iron accumulation from dietary sources after cessation of both menstruation [30, 31] and childbearing [31]. Serum hepcidin concentrations are lower in pre-menopausal than in post-menopausal women [35]. This suggests that the amount of dietary iron absorbed daily before menopause is greater than the amount absorbed daily after menopause. Inflammation or tissue injury also contributes to aggregate age-related increases of SF [32].
SF was not significantly associated with the estimated total daily intakes of food or supplemental iron in this study. In a study of post-menopausal women with HFE p.C282Y homozygosity in the United Kingdom, there was a positive correlation between SF and heme iron intake but not non-heme iron intake [36].
Mean TS was positively associated with SF in the present study, consistent with mixture modeling of TS and SF values in the HEIRS Study non-Hispanic white participants at risk for HFE mutations or self-reported liver disease [37]. In untreated p.C282Y homozygotes, there are inverse correlations of hepatic hepcidin mRNA expression with both TS and SF [38].
A strength of the present study is the availability for analysis of diverse iron-related variables of 136 non-Hispanic white women with HFE p.C282Y homozygosity who attended post-primary care screening evaluations. The mean age and iron phenotypes of the 136 women we included and the 25 women we excluded did not differ significantly, indicating that there was little, if any, bias in selecting subjects for the present study.
Limitations of this study include that HEIRS Study physicians did not interview participants about their past medical histories or review their questionnaire responses or medical records, including inquiries about iron supplements, menses, blood donation, other blood loss, iron deficiency, therapeutic phlebotomy, or iron overload, by study design. Another limitation is the lack of observations of women aged < 25 years [3]. Unavailable data pertinent to iron balance in the present women include the following: estimated menstrual blood/iron losses, iron supplementation and SF measurements during pregnancy, complications of pregnancy, prevalence of singleton live births, blood losses at delivery, and reports of lactation. Performing mutation analyses to detect potential genetic modifiers of SF or iron overload phenotypes [39, 40] in all post-primary care screening evaluation participants with HFE p.C282Y homozygosity, measuring hepcidin levels, recommending additional diagnostic evaluations, and providing treatment were also beyond the scope of the HEIRS Study.
SF is a surrogate marker of both iron stores and inflammation or tissue injury [15, 41]. It is unknown whether each of the 75 present women with SF > 200 µg/L had iron overload, inflammatory or tissue injury disorders, or both because evaluating participants for these conditions was beyond the scope of the HEIRS Study. Nonetheless, the correlation coefficient of log10 SF versus iron removed by phlebotomy to achieve iron depletion in 54 adults with HFE p.C282Y homozygosity in a US health maintenance clinic screening study was moderately strong (r54 = 0.5488; p = 0.0002) [42]. Our regression analyses also demonstrate that variables we did not analyze account for more than two-thirds of the variance in SF values in the present cohort.
5 Conclusions
We conclude that SF levels in 136 women with HFE p.C282Y homozygosity are positively associated with age, numbers of live births, menopause reports, and TS.
Author Contributions
Each author contributed equally to this study. James C. Barton conceived this study and its methodology, evaluated participants in post-screening evaluations, performed analyses, and drafted the manuscript. J. Clayborn Barton conceived study methodology, curated data, performed analyses, and drafted the manuscript. Ronald T. Acton conceived this study and its methodology, performed analyses, curated data, and drafted the manuscript. Each author approved the manuscript in its final form.
Acknowledgments
The Hemochromatosis and Iron Overload Screening (HEIRS) Study (January 2000-January 2006) was initiated and funded by the National Heart, Lung, and Blood Institute in conjunction with the National Human Genome Research Institute. The study was supported by contracts N01-HC05188 (The University of Alabama at Birmingham); N01-HC05185 (University of Minnesota); N01-HC05186, N01-CM-07003-74, and the Minority Community Clinical Oncology Program (Howard University); N01-C05189 (Kaiser Permanente Center for Health Research); N01-HC05190 (University of California Irvine); N01-HC05191 (London Health Sciences Centre); and N01-C05192 (Wake Forest University). Additional support was provided by The University of Alabama at Birmingham General Clinical Research Center grant M01-RR00032; and Southern Iron Disorders Center. The funders had a role in HEIRS Study design, data collection, and analysis. The funders had no role in the decision to publish the present work or in preparation of the manuscript. The following individuals also performed data collection (2001-2003) and received compensation for their work: Paul C. Adams, MD (Department of Medicine, London Health Sciences Centre, London, ON, Canada); John H. Eckfeldt, MD, PhD (Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN); Victor R. Gordeuk, MD (Division of Hematology and Oncology, Department of Medicine, University of Illinois at Chicago, Chicago, IL); Emily Harris, PhD (Epidemiology and Genomics Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Bethesda, MD); Helen Harrison, RN (The Western-Fanshawe Collaborative BScN Program, Fanshawe College, London, ON, Canada); Christine E. McLaren, PhD (Department of Epidemiology, University of California, Irvine, CA); and Gordon D. McLaren, MD (Division of Hematology/Oncology, Department of Medicine, University of California, Irvine, CA and Department of Veterans Affairs Long Beach Healthcare System, Long Beach, CA).
Ethics Statement
The Hemochromatosis and Iron Overload Screening (HEIRS) Study, conducted by the National Heart, Lung, and Blood Institute and the National Human Genome Research Institute, in accordance with principles of the Declaration of Helsinki, evaluated diverse aspects of hemochromatosis, iron overload, and iron-related disorders in a primary care-based sample of 101, 168 adults enrolled during the interval 2001–2003 at four Field Centers in the United States and one in Canada [3]. Local Institutional Review Boards of the HEIRS Study Coordinating Center, Central Laboratory, and Field Centers approved the HEIRS Study protocol that is described in detail elsewhere [3, 25].
Consent
HEIRS Study participants ≥ 25 years of age were recruited from outpatient facilities affiliated with the five Field Centers and gave written informed consent for screening and post-screening evaluation [3, 25].
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Summary data supporting the conclusions of this study are displayed in the article (and its supplemental file). The National Heart, Lung, and Blood Institute does not permit investigators to submit data directly to journals, related repositories, or other sources. Parties interested in obtaining HEIRS Study screening and post-screening evaluation data from which the present data were derived are referred to the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) (https://biolincc.nhlbi.nih.gov/studies/heirs/).




