Functional transfer of eukaryotic expression plasmids to mammalian cells by Listeria monocytogenes: a mechanistic approach
Abstract
Background
Cystic fibrosis (CF) is one of the most common monogenic disorders in the caucasian population. Gene therapy for CF is principally feasible and bacterial transfer systems might provide novel possibilities for therapy. However, transfection efficiencies are low and need to be improved. Thus, more detailed understanding of the DNA transfer mechanism is necessary to systematically eliminate these restrictions.
Methods
Functional transfer of GFP-CFTR (cystic fibrosis transmembrane conductance regulator) to eukaryotic cells using attenuated Listeria monocytogenes mediated gene transfer (bacteriofection) was shown by fluorescent microscopy, flow cytometry, immunoblotting and whole cell patch clamping. The characteristics of plasmid transfer were studied by use of electron and fluorescence microscopy, flow cytometry and Southern blotting. Polymerase chain reaction (PCR) was used to screen the genome of bacteriofected cells for cotransfer of chromosomal bacterial DNA.
Results
Correct intracellular localization and functionality of the GFP-CFTR fusion protein after bacteriofection was shown. Efficient bacterial lysis and release of bacterial content was demonstrated using antibiotics to kill intracellular bacteria. Although only low transfection rates were observed, high numbers of transferred plasmids were detected in host cells under these conditions. However, they were associated with high molecular weight entities and not available to cytosolic transcription. Cotransfer of bacterial genomic DNA was observed in transfectants but occurred at low frequencies.
Conclusions
In this work we demonstrate that low rates of bacteria-mediated transfection are not due to poor invasion of bacteria, insufficient bacterial lysis, or plasmid DNA degradation. Our data suggest that the transferred plasmid DNA is associated with higher macromolecular structures inhibiting nuclear transport and transgene transcription. Copyright © 2005 John Wiley & Sons, Ltd.
Introduction
A large number of diseases are caused by alterations of the genetic information in germ line. For such monogenically caused dysfunctions, only treatment of symptoms is available at present. Gene therapy, which aims at the somatic repair of the genetic defect, thus addressing the cause of the disorder, has been carried out successfully only in a few special cases so far 1.
In the last decade, bacteria (in particular intracellular pathogens) have arisen as a new class of vehicles for gene transfer. Transfer of eukaryotic expression plasmids to mammalian cells has now been reported for several Gram-negative species as well as for the Gram-positive species Listeria monocytogenes 2-6. Thus, bacterial carriers represent a viable alternative to currently used viral and non-viral gene therapy vectors.
L. monocytogenes is an ubiquitous, facultative intracellular Gram-positive bacterium, that can infect humans and a variety of other vertebrates 7. Its ability to spread to neighboring cells 8 renders L. monocytogenes especially attractive for gene therapy, since it might be able to deliver the therapeutic DNA to cells in deeper tissue layers that are not accessible for other vectors, like viral vectors or naked DNA.
One disease that could be subject to somatic gene therapy is cystic fibrosis (CF). CF is the most common recessively inherited lethal disorder in the caucasian population, affecting approximately 1 in 2500 newborns 9. CF is caused by loss-of-function mutations within the cystic fibrosis transmembrane conductance regulator (CFTR) gene 10-12. Life expectancy of such patients is roughly 30 years in countries with advanced health care systems, but over 90% of affected individuals finally succumb to persistent infections and sustained severe neutrophil-mediated inflammation of the lung 13. Current therapy, including regular chest physiotherapy and the aggressive use of antibiotics, is aimed at slowing the inevitable progression of lung disease rather than halting it or even preventing its onset. This, however, could be achieved by the novel approach to complement the defective gene by gene therapy.
Clinical studies have demonstrated that gene transfer and expression of CFTR in the airway epithelium of CF patients is in principle feasible, and that partial correction of the chloride conductance is possible (for review, see 14). However, the efficiency of gene transfer as well as the level and duration of expression were too low for clinical benefits, indicating the necessity for further improvement of the vectors and gene transfer systems used 15-17.
A L. monocytogenes-based in vitro gene transfer system that involves antibiotic-mediated killing of intracellular bacteria has been established and optimized by our group. In previous studies, this system allowed successful transfer of green fluorescent protein (GFP) as well as functional CFTR to mammalian cells 18, 19. For the in vivo application of this transfer system, however, several obstacles have to be overcome and safety issues need to be addressed. For instance, an efficient reporter system has to be established allowing the detection of even low numbers of bacteriofected cells in vivo. High transfer rates are observed only in selected cell lines, hence low transfer rates in vivo might be expected. In order to allow a rational improvement of plasmid transfer, the mechanism of bacteriofection needs to be elucidated. By identifying and eliminating the restrictions of this approach the transfer efficiencies should be significantly enhanced. Finally, the fate of potentially cotransferred bacterial chromosomal DNA needs to be clarified.
In this work, we address several of these issues by using the eukaryotic expression plasmids pERL3-CMVGFP and pERL3-CMVGFPCFTR-neo encoding GFP or a fusion protein between GFP and CFTR, respectively, as reporters. First, the newly constructed plasmid pERL3-CMVGFPCFTR-neo was tested for functionality. We show here that bacteria-mediated transfer of GFP-CFTR results in a functional chloride channel, thus providing a simple detection system for further studies on CFTR gene therapy. Furthermore, we identify steps limiting the process of L. monocytogenes-mediated DNA transfer using electron microscopy, fluorescence microscopy, Southern blot analysis and flow cytometric methods. Finally, we show that fragments of bacterial chromosomal DNA also integrate into the host cell genome which, however, in all probability is a rare event.
Materials and methods
Bacterial strains and plasmids
The Escherichia coli (E. coli) strain TOP 10 (Invitrogen, Groningen, The Netherlands) was used for general cloning. E. coli were grown in Luria Bertani (LB) broth or on LB-agar plates 20 at 37 °C. Ampicillin (Sigma, Taufkirchen, Germany) was added at 100 µg/ml or erythromycin (Sigma) at 400 µg/ml when required. L. monocytogenes strains EGDe 21 and hlyW491A 19 were grown in brain heart infusion (BHI) broth or on BHI-agar plates (Difco, Detroit, MI, USA) at 37 °C. Medium was supplemented with 5 µg/ml erythromycin when indicated. Transformation of bacteria has been described elsewhere 20, 22.
Construction of expression plasmids
pERL3-CMVGFP-neo
The neomycin phosphotransferase gene was derived from the plasmid pVETKNE 18 by digestion with XhoI and SalI. The excised fragment was inserted in the SalI site of pERL3-CMVGFP 18. The resulting plasmid was named pERL3-CMVGFP-neo.
pERL3-CMVGFPCFTR-neo
A PstI fragment containing a human CFTR cDNA was prepared from plasmid pBSSK−/CFTR (kind gift from Dr. B. Tümmler, Hannover, Germany) and cloned into the corresponding restriction site of plasmid pEGFP-C2 (Clontech, Palo Alto, CA, USA). The resulting plasmid pEGFP-CFTR was then digested with MluI and NsiI to isolate the eukaryotic expression cassette encoding N-terminally GFP-tagged CFTR. This was subsequently blunt-ended with T4 DNA polymerase and ligated with the SalI-digested, T4 DNA polymerase-treated plasmid pERL3-neo, which was generated by cloning a eukaryotic expression cassette for neo as a SalI-XhoI fragment derived from pPVETKNE 18 into SalI-opened pERL-3 23. The resulting plasmid was named pERL3-CMVGFPCFTR-neo.
pERL3-T7-IRES-GFP
The IRES-containing fragment from plasmid pMC-1 24 was isolated by NotI digestion followed by Klenow treatment to generate blunt ends, and subsequent digestion with EcoRI. The vector pBluescript II KS(+) (Stratagene, La Jolla, CA, USA) was treated in the same way, and the two fragments were ligated, resulting in the plasmid pBS-IRES. As the T7 promoter an adaptor fragment was used consisting of the oligonucleotides AAT TTC CCT ATA GTG AGT CGT ATT AG (EcoRI-pT7-SalI-V) and TCG ACT AAT ACG ACT CAC TAT AGG GA (EcoRI-pT7-SalI-R) that contained ends compatible with SalI and EcoRI. The sequence was designed such that the EcoRI site was destroyed upon ligation. This fragment was ligated into the corresponding restriction sites of pBS-IRES, giving rise to the plasmid pBS-T7-IRES. The coding sequence of EGFP was obtained from pEGFP-1 (Clontech) by digestion with the enzymes SalI and Bsp120I and was inserted into the SalI and NotI sites of pBS-T7-IRES resulting in the plasmid pT7-IRES-GFP. The NotI restriction site was then destroyed by treatment with Klenow and a XhoI site was introduced using the linker CCCTCGAGGG. Then the T7 promoter controlled expression cassette was isolated by digestion with XhoI and PstI and ligated into the XhoI and PstI sites of the plasmid pERL-3 19. By this step, the kanamycin resistance gene was destroyed. The final plasmid was named pERL3-T7-IRES-GFP.
pERL3-CMVDsRed2
The coding sequence of DsRed2 was obtained by restriction digestion of the plasmid pDsRed2-N1 (Clontech) with XmaI and NotI. The coding sequence of EGFP was removed from the plasmid pERL3-CMVGFP 18 by digestion with the same enzymes and the DsRed2-containing fragment was ligated into these sites.
Cell lines
CHO-K1 cells (ATCC CCL-61) and HEp-2 cells (ATCC CCL-23) were cultured in IMDM (Gibco BRL, Eggenstein, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS; Integro, Zaandam, The Netherlands) and 0.25 mM β-mercaptoethanol (Serva, Heidelberg, Germany). The cell line ST31-7 was grown in the same medium but containing additional G418 (1 mg/ml; Gibco BRL) 19. Cells were cultured at 37 °C and 5% CO2 in a humidified atmosphere.
The clones CHO-GFPCFTR+.1 and .2 stably expressing GFP-CFTR were generated by transfecting the plasmid pERL3-CMVGFPCFTR-neo into CHO-K1 cells using the method of calcium phosphate precipitation 25. CHO-K1 transfectants were selected by growth in medium supplemented with G418 (1 mg/ml; Gibco BRL). Transfectants were cloned by sorting of single, GFP-positive cells using a FACSVantage (Becton Dickinson, Heidelberg, Germany) and screened by fluorescence microscopy.
L. monocytogenes-mediated transfer of eukaryotic expression plasmids to CHO-K1 and HEp-2 cells
Cells were seeded 24 h before infection in 24-well plates at a density of 5 × 104 cells/well, resulting in approximately 1 × 105 cells/well at the time of infection. Overnight cultures of L. monocytogenes strains were diluted 1 : 50 and grown for 3–4 h. After washing once with phosphate-buffered saline (PBS), bacteria were added to cell cultures at the indicated multiplicity of infection (MOI) and in a total volume of 200 µl medium. Subsequently, cultures were centrifuged to enhance infection (5 min at 800 rpm in a Beckman GS-6KR centrifuge) and incubated for 1.5 h at 37 °C. Cells were then washed twice with PBS and cultured in medium supplemented with 50 µg/ml gentamicin to kill the remaining extracellular bacteria. After an additional 4 h of incubation at 37 °C, intracellular bacteria were lysed by exchanging the gentamicin-containing medium for fresh medium supplemented with penicillin G (100 U/ml) and streptomycin (100 mg/ml) (Cytogen, Ober-Mörlen, Germany). Cells were incubated for the indicated time periods at 37 °C and finally transient transgene expression was analyzed or cells were processed for electron microscopy or DNA isolation.
To detect GFP or GFP-CFTR expression by fluorescence microscopy, cells grown on coverslips were fixed with 3.7% formaldehyde in PBS for 20 min at room temperature and counterstained with DAPI (Roche, Mannheim, Germany). Coverslips were mounted in Fluoprep (bioMérieux, Marcy l'Etoile, France) and preparations examined by epifluorescence microscopy using an Axiovert 135 TV microscope (Zeiss, Oberkochen, Germany) equipped with a Plan-Apochromat 100 × /1.40 NA oil immersion objective. Images were recorded with a cooled, back-illuminated CCD camera (TE/CCD-1000 TKB; Princeton Instruments, Trenton, NJ, USA). Flow cytometry for quantitation of GFP- or GFP-CFTR-expressing cells was performed with trypsinized, unfixed cells using a FACSCalibur (Becton Dickinson) and CellQuest software (Becton Dickinson).
Stably transfected clones expressing GFP-CFTR were generated by selection for G418 resistance (1 mg/ml; Gibco BRL), sorting of single, GFP-positive cells using a FACSVantage (Becton Dickinson), and screening by fluorescence microscopy. Three CFTR-expressing clones named ST37-2M.12, ST37-2M.19 and ST37-2H.43 were used for further experiments. As a control, a transfectant clone ST33-20.8 stably expressing β-galactosidase of E. coli was established by Listeria-mediated gene transfer using a plasmid in which GFP was replaced by the lacZ gene. Gene expression was detected after fixation as described 19.
Immunoblotting
Confluent cells of a 25-cm2 culture flask were harvested in HEPES buffer (20 mM HEPES, pH 7.4, 1 mM EGTA, 0.4 mM EDTA, 5 mM DTT) using a cell scraper and homogenized by ultrasound in the presence of protease inhibitors (complete protease inhibitor cocktail tablets; Roche, Mannheim, Germany). Subsequently, microsomes were prepared by differential centrifugation. Nuclei, mitochondria and large debris were removed by centrifugation at 4000 g for 10 min at 4 °C. The resulting supernatant was centrifuged at 40 000 g for 1 h at 4 °C to yield the microsomal membrane fraction. Microsomes were solubilized in SDS gel-loading buffer and aliquots denatured by incubation for 10 min at 37 °C. Samples were then separated by 6% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes (Millipore, Bedford, MA, USA) by semi-dry blotting. Detection of CFTR was performed with the mouse monoclonal antibody MATG1104 followed by a polyclonal horseradish peroxidase-conjugated goat anti-mouse IgG (Dianova, Hamburg, Germany). Finally, ECL reagent (Amersham Pharmacia Biotech, Freiburg, Germany) was added and chemiluminescence detected by exposure to ECL Hyperfilms (Amersham Pharmacia Biotech). Peptide competition for MATG1104 binding was carried out as described 26. Isotype control was performed with the mouse monoclonal antibody S23 as primary antibody.
Whole-cell patch-clamp recordings of GFP-CFTR-expressing cells established by Listeria-mediated DNA transfer
CHO-K1 cells transfected with GFP-CFTR or a control vector were plated in 35-mm dishes 48 h prior to the experiments. Cells (about 4 × 105 per 35-mm dish) were washed with extracellular solution (see below), and the recordings were started 10–30 min after the washing procedure using a bath volume of 2 ml. Activators of CFTR channels were applied with a pipette directly into the bath solution 2–5 min after a stable recording configuration had been obtained. Conditions of whole-cell patch-clamp recordings have been described in detail 27. Data are expressed as means ± SEM. All measurements were performed at 20–22 °C. Solutions and drugs used: Cells were bathed in extracellular solution composed of 140 mM NaCl, 3 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 15 mM glucose, 10 mM HEPES (4-[2-hydroxyethyl]-1-piperazinethanesulfonic acid), pH 7.35, adjusted with NaOH. Extracellular solution with low Cl− contained 140 mM Na+ gluconate instead of NaCl. The recording pipette contained an intracellular solution composed of 140 mM K+ glutamate, 20 mM NaCl, 2 mM MgCl2, 10 mM HEPES, pH 7.3, adjusted with KOH. Intracellular solution with high Cl− contained 140 mM KCl instead of K+ glutamate. The intracellular solution contained ATP (5 mM) to avoid ATP depletion of the patch-clamped cell and to retain full CFTR response over time. A free intracellular Ca2+ concentration ([Ca2+]i) of 100 nM was obtained using 100 µM of the Ca2+-chelator BAPTA (1,2-bis[2-aminophenoxy]ethane-N,N,N′,N′-tetraacetic acid) and a total Ca2+ concentration of 30 µM, assuming an apparent dissociation constant KD of 0.24 µM (pH 7.3) for the Ca2+-BAPTA complex. Differences in osmolarity between extra- and intracellular solutions were compensated for as described 28. Bath and pipette solutions were filtered through 0.2-µM pore filters (Renner, Dannstadt, Germany). Forskolin and IBMX (3-isobutyl-1-methylxanthine) were obtained from Sigma (Taufkirchen, Germany).
Transmission electron microscopy
For morphological analysis, the infected monolayers were fixed with 2% glutaraldehyde and 5% formaldehyde in cacodylate buffer (0.1 M cacodylate, 0.9 M sucrose, 0.01 M MgCl2, 0.01 M CaCl2, pH 6.9) overnight on ice, washed three times with cacodylate buffer, treated with 1% aqueous osmium tetroxide for 1 h at room temperature, and washed with cacodylate buffer. Samples were then dehydrated with a graded series of acetone (10, 30, 50%) on ice for 30 min for each step. Then, samples were left in 70% acetone with 0.5% uranyl acetate overnight, and further dehydrated with 90% and 100% acetone. Infiltration of the samples was done with the low viscosity epoxy resin according to the described procedure by Spurr 29. Finally, samples were placed into gelatine capsules, filled with pure resin and polymerized for 8 h at 70 °C. Ultrathin sections were cut with a diamond knife and sections picked up with copper grids (300 mesh). Counter-staining of the sections was performed with lead citrate for 2 min. After air-drying, samples were examined in a Zeiss EM 910 transmission electron microscope at an acceleration voltage of 80 kV.
Plating and determination of viable bacteria by fluorescence microscopy
The number of viable intracellular bacteria was estimated by plating lysates of infected cells at different time points after infection. Cells were lysed by addition of 0.1% Triton X-100 (Serva) in water and incubation for 15 min at room temperature. Serial dilutions of the lysates were prepared and aliquots were plated on BHI agar plates. The number of colony-forming units (CFU) was determined and the number of viable bacteria per well was calculated. In addition, viable and non-viable bacteria were stained with the LIVE/DEAD BacLight bacterial viability kit as recommended by the manufacturer (Molecular Probes, Leiden, The Netherlands).
Isolation of DNA and Southern blotting
For the separation of nuclei and cytosol, infected cells were harvested by trypsin treatment 48 h after infection and single cell suspension in cold 5% citric acid were prepared at a density of 108 cells/ml or less. To lyse the cells, cold 10% Igepal solution (Sigma) was then added to a final concentration of 0.5% while mixing the cells thoroughly. The cell suspension was underlayed with a cold solution of 30% sucrose/0.5% Igepal/5% citric acid. The gradient was centrifuged for 10 min at 2500 rpm in a Beckman GS-6KR centrifuge at 4 °C. To obtain DNA present in the upper phase of the gradient, 0.3 M sodium acetate, 1 µg/ml glycogen (Boehringer, Mannheim, Germany) and 0.7 volumes isopropanol were added and incubated at—20 °C overnight. The precipitate was spun down, washed once with 70% ethanol, air-dried and resuspended in 10 mM Tris-HCl (pH 7.6)/1 mM EDTA (pH 8.0). The sediment containing the nuclei was resuspended in 100 mM Tris-HCl (pH 8.5)/5 mM EDTA (pH 8.0)/200 mM NaCl/0.2% SDS. After addition of proteinase K (Sigma) at a final concentration of 0.4 mg/ml, the samples were incubated for 14–20 h at 54 °C, DNA was precipitated by addition of 0.7 volumes of isopropanol, and sedimentation at 13 000 rpm in a table-top centrifuge. The pellet was washed in 70% ethanol and after air drying resuspended in 10 mM Tris-HCl (pH 7.6)/1 mM EDTA (pH 8.0).
For Southern blot analysis, indicated amounts of DNA from the nuclear fractions and the cytosolic fractions were digested with EcoRI. As a control, different amounts of plasmid DNA (as indicated) were mixed with 5 µg of genomic DNA from uninfected HEp-2 cells and digested with EcoRI. Subsequently, the samples were separated by agarose gel electrophoresis. DNA was then transferred to a nylon membrane (Gene Screen plus, Perkin Elmer, Belgium) by capillary force under alkaline conditions. After the transfer, the membrane was washed for 1–2 min in 2 × SSC 20. DNA was fixed to the membrane by UV crosslinking with a Stratalinker 2400 (Stratagene), applying 120 mJ. The GFP-specific probe was obtained by PCR using pERL3-CMVGFP as template and oligonucleotides GCGTGTCCGGCGAGGGCGAGGGCG as forward primer and GCCGAGAGTGATCCCGGCGGCGG as reverse primer. Radioactive labeling was carried out with the Ladderman labeling kit (Takara, Japan) according to the manufacturer's recommendations. The membrane was then prehybridized with QuickHyb hybridization solution (Stratagene) for 45 min. Then, the radioactive probe was added and the membrane was hybridized for 4 h at 65 °C. After two short washings with 2 × SSC/0.1% SDS and three washings with 0.1 × SSC/0.1% SDS for 10 min at 65 °C, the membrane was exposed to a BioMax MS film (Kodak, Rochester, NY, USA) for 1–2 h. Alternatively, a phosphoimager was used.
Isolation of genomic DNA and PCR analysis of bacterial DNA from bacteriofected CHO-K1 cells
Bacterial chromosomal DNA was isolated as previously described 20. For isolation of DNA from CHO-K1 cells stably transfected by recombinant L. monocytogenes, or untreated controls, 107 cells were digested with proteinase K (200 µg/ml) for 48 h at 55 °C in 1 ml proteinase K buffer (10 mM Tris HCl, 5 mM EDTA, 1% SDS, 300 mM NaAc, pH 8.0). The solution was transferred to 7 ml Vacutainer Brand SST tubes (Becton Dickinson), where the gel matrix completely separates the organic and aqueous phases. One volume of a phenol/chloroform (1 : 1) mixture was added and the sample was mixed and centrifuged for 10 min at 1400 g. To remove the remaining traces of phenol, 1 volume of chloroform was added and the sample was again mixed and centrifuged. The solution was transferred to 2-ml reaction tubes, DNA was precipitated with 1 volume of absolute ethanol, followed by centrifugation for 15 min at >20 000 g. After washing once with 70% ethanol, DNA was resuspended in H2O.
-
L.m.RS1-V: 5′-GGTAATCAAACGCCAGTAGGGACT-3′;
-
L.m.RS1-R: 5′-ATATGGCGTAGAAGGTTTC GCACC-3′;
-
L.m.RS2-V: 5′-AATCCGGTTCACCTGCACCAGTAA-3′;
-
L.m.RS2-R: 5′-AA TCAAGCGGCCGCTGATATTGCA-3′;
-
L.m.RS3-V: 5′-GGATTTTAAAGCAGTTGCAGC ACG-3′;
-
L.m.RS3-R: 5′-TGTCAAAGTCGCTAGAGTAGCGAA-3′;
-
L.m.RS4-V: 5′-ATGA ACACGATGGTATCGCCGTTC-3′;
-
L.m.RS4-R: 5′-AGGATGGATGCTGCTCGAACG TTT-3′;
-
L.m.RS5-V: 5′-GGTCACATTCTCTTCGGCTGCAAT-3′;
-
L.m.RS5-R: 5′-CTGGTTTTGAATGGCGTGAACCTG-3′;
-
L.m.RS6-V: 5′-CGTTTTATGAACGATTTGGA TGGC-3′;
-
L.m.RS6-R: 5′-CTCAATGCAATAGTACTCGACTCC-3′;
-
L.m.RS7-V: 5′-ATGCCTGATGCTAGGGTTGGTACA-3′;
-
L.m.RS7-R: 5′-TCCTATAAAACAAACGGCTCGCTG-3′.
-
GAPDH FOR: 5′-ATCTTCTTGTGCAGTGCCAGC-3′
-
GAPDH REV: 5′-ACTCCACGACATACTCAGCACC-3′
A standard PCR reaction was performed with 40 cycles of amplification using 60 °C (L. monocytogenes-specific primers) or 58 °C (GAP-DH-specific primers) as annealing temperature in a Hybaid Touchdown thermocycler. To determine the sensitivity of the PCR assay, the reaction was carried out with serial dilutions of purified genomic DNA of L. monocytogenes and DNA from 3000 CHO-K1 cells as a background. Under these conditions, specific bands could be detected with all primer pairs used, when an equivalent of 10 copies of the L. monocytogenes genome was present in the reaction (data not shown).
Results
Expression of GFP-CFTR in CHO-K1 and HEp-2 cells after CaPO4 and L. monocytogenes-mediated transfection
The newly constructed reporter plasmid encoding a fusion protein between GFP and CFTR under control of the CMV promoter was first tested for expression. Conventional calcium phosphate transfection using pERL3-CMVGFPCFTR-neo was carried out to establish two stable CHO-K1 clones, designated CHO-GFPCFTR+.1 and .2. Expression of the GFP-CFTR fusion protein could be readily detected by immunoblotting, flow cytometry and fluorescence microscopy, as shown in Figures 1A and 1B. Immunoblotting also demonstrated that the glycosylation of the fusion protein is similar to the native CFTR, since the two expected mature and precursor forms were observed (black and red arrowheads, respectively, in Figure 1A), albeit shifted to a higher molecular weight due to the GFP fusion partner (Figure 1A).
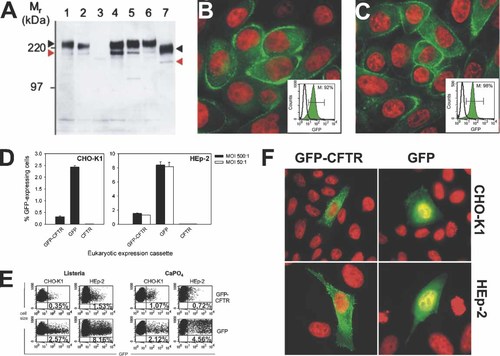
GFP and GFP-CFTR expression after CaPO4 or L. monocytogenes hlyW491A-mediated transfer of pERL3-CMVGFP and pERL3-CMVGFPCFTR-neo. (A) Detection of CFTR in microsome preparations of stably transfected clones using the monoclonal antibody MATG1104 and a horseradish peroxidase-conjugated secondary antibody. Lanes 1 and 2: CHO-GFPCFTR+.1 and .2 transfected via CaPO4 transfection; lane 3: parental CHO-K1 cells; lane 4: ST37-2M.12; lane 5: ST37-2M.19; lane 6: ST37-2H.43, these three clones were derived from bacteriofection with the above plasmid; lane 7: ST31-7 (CHO-K1-derived cell line stably expressing CFTR after L. monocytogenes-mediated transfer of pERL3-CMVCFTR-neo 19). As expected, a mature (black arrowhead) and a precursor (red arrowhead) form of CFTR were observed. (B, C) Corresponding fluorescence microscopy of CHO-GFPCFTR+.1 and ST37-2M.19, respectively (green: GFP; red: DAPI, false color). Inset: Flow cytometric analysis of the control clone CHO-GFPCFTR+.1 established by CaPO4 transfection (B) and the clone ST37-2M.19 bacteriofected by L. monocytogenes (C) using the expression plasmid pERL3-CMVGFPCFTR-neo. Green: GFP expression; solid line, parental CHO-K1 cells; M: indicates percentage of living GFP-expressing cells. (D) Cells were transfected with different derivatives of L. monocytogenes hlyW491A at a MOI of 50 : 1 or 500 : 1 carrying either the eukaryotic expression plasmid pERL3-CMVGFPCFTR-neo (GFP-CFTR), pERL3-CMVGFP (GFP) or pERL3-CMVCFTR-neo (CFTR, negative control). GFP or GFP-CFTR expression of host cells was evaluated by flow cytometry (mean ± SEM, n = 3). (E) Flow cytometric analysis of GFP or GFP-CFTR expression in transiently calcium phosphate-transfected or bacteriofected CHO-K1 and HEp-2 cells. Dots in the upper right quadrant represent viable, GFP-expressing cells. Equimolar amounts (1 pmol) of each plasmid DNA were used for calcium phosphate precipitations. (F) Fluorescent micrographs of transiently transfected CHO-K1 cells and HEp-2 cells after bacteriofection with the plasmids pERL3-CMVGFP and pERL3-CMVGFPCFTR-neo, respectively (green: GFP; red: DAPI, false color)
For Listeria-mediated bacteriofection we used our previously optimized in vitro gene transfer system 19. The variant strain of L. monocytogenes hlyW491A, secreting an attenuated listeriolysin and carrying the eukaryotic expression plasmid pERL3-CMVGFPCFTR-neo, was used for gene transfer into CHO-K1 cells. At a multiplicity of infection (MOI) of 500 around 0.3% GFP-CFTR-expressing cells were found, whereas about 2.6% were found to express GFP when bacteriofected with the GFP-encoding plasmid 18 (Figures 1D and 1E). This discrepancy between transient GFP and GFP-CFTR expression is consistent with data found previously for GFP and CFTR 19.
To examine whether the difference in GFP and GFP-CFTR expression is restricted to CHO-K1 cells, HEp-2, a CFTR-negative human epithelial cell line 30 highly susceptible to L. monocytogenes-mediated gene transfer, was bacteriofected with either the GFP-CFTR- or the GFP-encoding vector. The frequency of GFP-CFTR-expressing cells was increased in HEp-2 as compared to CHO-K1 cells and comparable at both MOIs (Figure 1D). However, it was significantly lower (1.5%) when compared with the frequency of GFP-expressing cells. Around 8.4% GFP-expressing cells were found at a MOI of 500 (Figure 1D) and a comparable percentage was transfected at the 10-fold lower MOI of 50. In addition, a lower mean fluorescence was observed for GFP-CFTR-expressing cells compared to GFP-expressing CHO-K1 and HEp-2 cells, regardless of whether L. monocytogenes-mediated gene transfer or calcium phosphate transfection was used (Figure 1E).
Transient expression of GFP-CFTR in CHO-K1 and HEp-2 cells was confirmed by fluorescence microscopy. In both cell lines, transfection of plasmid pERL3-CMVGFPCFTR-neo led to a distinct, apparently vesicular GFP signal (Figure 1F). This is in clear contrast to the GFP expression after transfer of pERL3-CMVGFP, which appears to be homogenous and is also found in the nucleus (Figure 1F). Thus, the GFP-CFTR fusion protein appears to be targeted to cellular membranes via its CFTR portion.
Stable transfectants of CHO-K1 cells were generated for further analysis of GFP-CFTR expression. Three CHO-K1 clones, denoted ST37-2M.12, ST37-2M.19 and ST37-2H.43, were established by L. monocytogenes-mediated transfer of pERL3-CMVGFPCFTR-neo and sorting for single GFP-expressing cells. Individual transfectant clones were screened by fluorescence microscopy. As shown for ST37-2M.19 in Figure 1C, clones with more than 90% GFP-expressing cells could be generated, exhibiting fluorescence in the area of the plasma membrane similar to the control CHO-GFPCFTR+.1 (Figure 1B).
These findings were confirmed by immunoblot analysis. GFP-CFTR was detected in microsomal membrane fractions of the stable transfectants. Immunoreactive bands representing the mature and immature GFP-CFTR fusion protein were found for ST37-2M.12, ST37-2M.19 and ST37-2H.43, but not for untransfected CHO-K1 cells (Figure 1A).
Electrophysiological characterization of GFP-CFTR fusion protein after Listeria-mediated cDNA transfer into CHO-K1 cells
Using patch-clamp analysis of whole cells, we also examined whether the GFP-CFTR fusion proteins expressed in bacteriofected CHO-K1 cells show the typical functional activity of native CFTR channels. This was important as the GFP fusion partner could influence the physiological activity of the CFTR, and also the procedure of the bacteriofection might alter the properties of the recombinant cells. Normal CHO-K1 cells do not exhibit endogenous CFTR channels 19, and, as expected, application of the CFTR channel activators forskolin and IBMX had no effect on the membrane current of control vector-transfected CHO-K1 cells ST33-20.8 (Figure 2A). In contrast, in ST37-2H.43 cells expressing the GFP-CFTR fusion protein, forskolin and IBMX led to a marked increase in membrane current within 1–2 min (Figure 2B). The maximal current amplitude was 1283 ± 460 pA (n = 3) at +30 mV membrane holding potential. Typical current-voltage relationships of the membrane currents before and after activation by forskolin and IBMX are shown in Figure 2C. The reversal potential of the activated membrane current was—38.7 ± 1.9 mV (n = 5), which is close to the equilibrium potential for Cl− of −46.3 mV using physiological intra- and extracellular solutions. To demonstrate that this activated membrane current is predominantly carried by Cl−, we used combinations of intra- and extracellular solutions with different Cl− concentrations and determined the reversal potentials of the activated membrane currents. According to the predictions of the Nernst equation for chloride ions, the reversal potentials depended mainly on the intra- and extracellular Cl− concentrations (Figure 2D). The more the Cl− conductance determines the reversal potential of the membrane current, the more the measured curve matches the theoretical curve. The small divergence between the CHO-CFTR curve and the theoretical curve is due to the contribution of other ions (e.g. K+ and Na+) to the activated membrane current. Thus, analysis of GFP-CFTR expression of stably transfected CHO-K1 cells clearly demonstrates successful L. monocytogenes W491A-mediated gene transfer of a functional GFP-CFTR to this cell line.
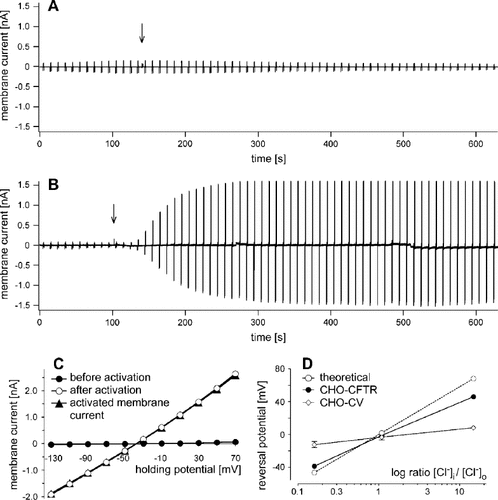
Electrophysiology of GFP-CFTR transfectants. Typical whole-cell patch-clamp registrations of CHO-K1 cells transfected either with (A) a control vector (ST33-20.8) or (B) GFP-CFTR (ST37-2H.43). GFP-CFTR Cl− channels were activated by application of forskolin (10 µM) and IBMX (100 µM), as indicated by arrows. Physiological solutions were used. The sequence of the holding potential was −30 mV for 8.5 s followed by −90, −30, and +30 mV each for 0.5 s. The membrane current had a negative amplitude at −90 mV and a positive amplitude at +30 mV. (C) Current-voltage relationships of the membrane currents before and after maximal activation by forskolin and IBMX of a CHO-K1 cell expressing GFP-CFTR (ST37-2H.43). Current amplitudes were measured at the end of voltage pulses lasting 400 ms in 20-mV increments from −130 to +70 mV. (D) Dependence of the reversal potentials of the membrane currents on the ratio of the chloride concentrations in different intracellular ([Cl−]i) and extracellular ([Cl−]o) solutions. Data represent mean values from 5–9 cells expressing GFP-CFTR (ST37-2H.43 indicated as CHO-CFTR) during maximal current activation by forskolin and IBMX and from 4–5 cells transfected with a control vector (ST33-20.8 indicated as CHO-CV). Error bars indicate SEM unless smaller than symbols. The calculated reversal potentials for the different Cl− concentrations according to the Nernst equation (theoretical) are also presented
A minor fraction of the intracellular carrier bacteria transfer plasmids to the nucleus of the host cell
Functional transfer of GFP-CFTR could be shown, thus providing a simple detection system for future studies. However, transfection efficiencies were too low to be of potential clinical benefit. To be able to rationally improve the efficiency, it is necessary to understand the mechanism of DNA transfer in order to find the limiting step.
First, we wanted to investigate whether all bacteria that have entered the host cell are able to transfer their plasmid load to the nucleus. To this end, we used two different recombinant strains of L. monocytogenes harboring the expression plasmids pERL3-CMVGFP, encoding the green fluorescent protein, and pERL3-CMVDsRed2 that encoded a red fluorescent protein. The hypothesis was that when all intracellularly lysed bacteria are able to transfer expression plasmids, a coinfection of HEp-2 cells with both carrier bacteria at high MOIs (740 for pERL3-CMVGFP and 630 for pERL3-CMV-DsRed2) should result in expression of both fluorescent proteins in all cells that could be transfected. This was clearly not the case, as shown by fluorescence microscopy 48 h after initiation of DNA transfer (Figure 3A). Similarly, when bacteriofected cells were analyzed by flow cytometry only 6.3% of the cells were expressing both proteins (Figures 3B and 3C). Despite the extremely high MOIs used for both carrier bacteria, 18.1% of the bacteriofectants expressed GFP alone and 7.1% were single positive for DsRed2 (Figures 3B and 3C). Thus, only very few of the many carrier bacteria that infect a host cell are able to transfer their plasmid load to the nucleus of the host cell.
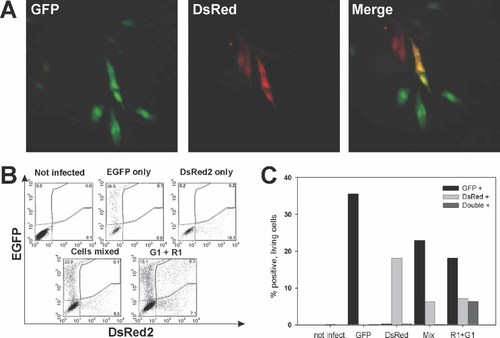
Cotransfection of HEp-2 cells with two different eukaryotic expression plasmids mediated by L. monocytogenes. (A) Fluorescent micrographs of cotransfected cells. Cells were incubated with a mixture of L. monocytogenes W491A carrying the plasmid pERL3-CMVGFP and L. monocytogenes W491A carrying the plasmid pERL3-CMVDsRed2. Cells were analyzed 48 h post-infection. (B) Flow cytometric analysis of cotransfected cells. HEp-2 cells were transfected by L. monocytogenes W491A carrying the plasmid pERL3-CMVGFP (EGFP only), the plasmid pERL3-CMVDsRed2 (DsRed2 only), or a mixture of both strains (G1 + R1). Uninfected cells were used as a negative control. For gating, cells from the single transfections were mixed (Cells mixed). The quantitation of this experiment is shown in (C). This experiment was repeated twice with similar results
Plasmids liberated from carrier Listeria are not accessible in the cytosol of host cells
A possible explanation for this low transfection rate could be that the plasmids remain in the cytosol and are inefficiently transported to the nucleus. We, therefore, argued that strong expression should be possible when the complete expression process takes place exclusively in the cytosol. To address this point, we equipped the carrier bacteria with the plasmid pERL3-T7-IRES-GFP that consists of an EGFP encoding cDNA under the control of the T7 promoter and an IRES from poliovirus type I. As host cell we used the BHK-21 derivative BSRT7/5, that constitutively expresses the T7 RNA polymerase. Under these circumstances, RNA should be transcribed from the plasmid by the T7 polymerase directly in the host cell cytosol and the IRES should render the transgene translation Cap-independent.
We expected a quick onset of transgene expression and, if expression plasmids remained freely in the cytosol, a strongly elevated number of cells expressing the transgene. To verify this idea, BSRT7/5 cells and BHK-21 cells were transfected with pERL3-T7-IRES-GFP or pERL3-CMVGFP by the calcium phosphate precipitation method 20. Flow cytometry revealed the first GFP-positive BSRT7/5 cells transfected with pERL3-T7-IRES-GFP already after 4 h. At this time point, no GFP expression was observed in BSRT7/5 cells transfected with pERL3-CMVGFP or BHK-21 cells transfected with pERL3-T7-IRES-GFP. Six hours after transfection a few GFP-expressing cells were found in cells transfected with pERL3-CMVGFP (Figures 4A and 4C). At 24 h, BHK-21 cells transfected with pERL3-CMVGFP and BSRT7/5 cells transfected with pERL3-T7-IRES-GFP showed comparable numbers of GFP-expressing cells. In addition, expression from the T7-driven plasmids decreased already 48 h after transfection while the number of GFP-positive BSRT7/5 cells transfected with pERL3-CMVGFP was low after 24 h but increased at 48 h after transfection.
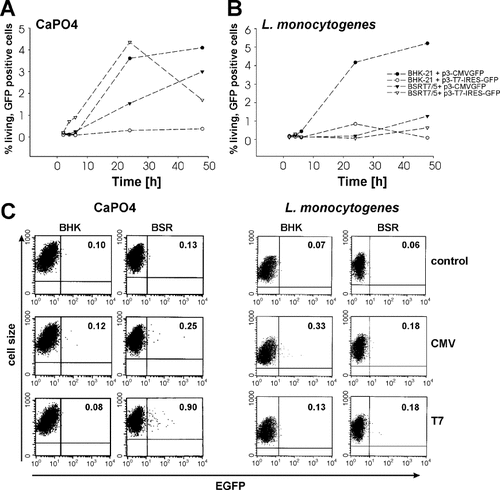
Bacteriofection of BHK-21 and BSRT7/5 cells with a plasmid potentially expressed in the cytosol. BHK-21 and BSRT7/5 cells were transfected with the plasmids pERL3-CMVGFP and pERL3-T7-IRES-GFP, respectively, by either calcium phosphate precipitation (A) or by L. monocytogenes-mediated gene transfer (B). The number of living, GFP-expressing cells was measured by flow cytometry at 2, 4, 6, 24 and 48 h after initiation of DNA transfer. (C) Flow cytometric analysis at 6 h post-transfection. Dots in the upper right quadrant represent viable, GFP-positive cells. BHK: BHK-21 cells, BSR: BSRT7/5 cells, control CaPO4: mock transfected cells, control L. monocytogenes: uninfected cells, CMV: transfection with plasmid pERL3-CMVGFP, T7: transfection with plasmid pERL3-T7-IRES-GFP. Significant expression could be detected only when the plasmid was conventionally transfected
When similar transfections were performed as bacteriofections using L. monocytogenes W491A carrying the corresponding plasmids, early onset of transgene expression in BSRT7/5 cells bacteriofected with pERL3-T7-IRES-GFP was no longer observed (Figures 4B and 4C). GFP-positive cells were only found after 24 and 48 h in BHK-21 cells bacteriofected with pERL3-CMVGFP. Remarkably, transfection of BSRT7/5 cells with Listeria using pERL3-CMVGFP as expression plasmid led to a low transgene expression only after 48 h. The cause of this effect cannot be explained at this time.
Bacteria are efficiently lysed and their content is released after treatment with antibiotics
The above results suggest that most plasmids introduced into the host cells by L. monocytogenes do not become accessible to the host cells' expression mechanisms. They might be either associated with proteins, possibly of bacterial origin, or they might be rapidly degraded. To distinguish between these possibilities we first wanted to analyze the fate of the bacteria after treatment with antibiotics since the plasmids might remain associated with lysed bacteria. To this end, we first established the number of viable bacteria during the course of the experiment by plating, as shown in Figure 5C. Already 24 h after the addition of the antibiotics the number of colony-forming bacteria dramatically decreased and, by 48 or 72 h, hardly any platable bacteria were found.
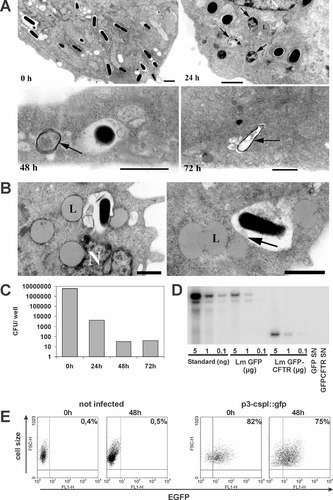
Efficient release of bacterial content into the cytosol of host cells and association of plasmids with high molecular weight components after Listeria-mediated DNA transfer. (A) Transmission electron micrographs of L. monocytogenes W491A-infected HEp-2 cells at different time points after addition of antibiotics. Arrows indicate disrupted bacterial cell walls. Interestingly, despite the long time after invasion, many bacteria are found in vacuoles. In (B) the aggregation of lysosomes at sites of bacterial degradation is shown. The arrow indicates the fusion of a lysosome and a bacteria-containing vesicle. Black bars correspond to 1 µM. L: Lysosome; N: Nucleus. (C) Number of viable intracellular bacteria as determined by plating of cell lysates at different time points after addition of antibiotics. CFU, colony-forming units. (D) Southern blot analysis of nuclear and cytosolic fractions of bacteriofected HEp-2 cells. DNA was isolated from the cells 48 h post-infection. Standard: pERL3-CMVGFP plasmid DNA mixed with HEp-2 genomic DNA as carrier DNA. Lm GFP: nuclear fractions from HEp-2 cells transfected by L. monocytogenes W491A carrying the plasmid pERL3-CMVGFP. Lm GFP-CFTR: nuclear fractions from HEp-2 cells transfected by L. monocytogenes W491A carrying the plasmid pERL3-CMVGFPCFTR-neo. GFP SN/GFPCFTR SN: corresponding cytosolic fractions. (E) Flow cytometric analysis of HEp-2 cells after infection with L. monocytogenes W491A constitutively expressing GFP (pERL3-cspl::gfp). 0 h: cells were analyzed just before addition of antibiotics. 48 h: cells were analyzed 48 h after the addition of antibiotics. Uninfected HEp-2 cells were used as negative control (not infected)
This was confirmed using fluorescent live/dead staining for bacteria. Only a few live bacteria were detected at time points later than 24 h following initiation of antibiotic treatment (data not shown). Interestingly, also the number of detectable dead bacteria decreased dramatically under these circumstances (data not shown). Since the determination of dead bacteria is based on the staining of bacterial DNA with propidium iodide that can be discerned as red fluorescent entities within the cytosol of the host cells, these data suggest that the content of the bacteria is no longer concentrated within the bacteria. Therefore, we employed transmission electron microscopy to directly examine the fate of the bacteria. Figure 5A shows that, at the time of adding antibiotics, bacteria have an intact structure and are found either in typical phagocytic vacuoles or already in the cytosol. At 24 h, bacteria that are only partially filled with electron-dense material can be found. Some structures which obviously represent lysed bacteria or bacteria that are at the verge of releasing their content into the cytosol were found (Figure 5A, arrows). Similar structures were observed at later time points, although they become rather rare at 48 and 72 h.
Interestingly, at 24 h and later, several bacteria are still found in vesicles. At this time all bacteria would have been expected to have escaped into the cytosol. Apparently, such vesicles fuse with lysosomes (Figure 5B, arrows).
The electron micrographs indicated that the bacterial content is released quickly into the cytosol of the host cells after adding the antibiotics. This suggests that bacterial proteins and other macromolecules should be found in the cytosol of the host cells shortly after bacterial lysis. We therefore employed Listeria that heterologously expressed GFP under the control of a bacterial promoter (pERL3::cspL+GFP 19). As shown in Figure 5E, already at the initiation of the antibiotics treatment, 80% of infected cells contain significant amounts of GFP. However, GFP was exclusively associated with bacteria, as observed by fluorescence microscopy. Roughly the same number of cells still contained GFP 48 h later, but at this time point hardly any bacteria could be observed within the host cells. In addition, GFP was now evenly distributed over the entire host cell (data not shown). The fluorescence intensity observed after 48 h was similar to that detected at the time when antibiotic treatment was started, but was significantly lower than seen after Listeria-mediated DNA transfer. Apparently, GFP expressed by the bacteria is released upon lysis of the bacteria into the cytosol of the host cells.
Transferred plasmids are associated with fast sedimenting entities
Following efficient release of the bacterial content the plasmid DNA should remain detectable in the cytosol of the host cells unless it is rapidly degraded.
Therefore, HEp-2 cells were infected with L. monocytogenes W491A carrying the plasmids pERL3-CMVGFP and pERL3-CMVGFPCFTR-neo, respectively. The infected cells were lysed 48 h after transfection and the lysate was centrifuged over a 30% sucrose cushion to separate the cell nuclei from soluble cytosolic compounds. DNA was isolated from both fractions and analyzed by Southern blotting. To estimate the number of plasmids in the nuclear and cytosolic fractions, three different amounts of pERL3-CMVGFP plasmid DNA mixed with HEp-2 genomic DNA were loaded on the gel for comparison. Results are shown in Figure 5D. Signals specific for EGFP were found exclusively in the fraction sedimenting through the sucrose cushion when cells bacteriofected with pERL3-CMVGFP and pERL3-CMVGFPCFTR-neo were tested, respectively. No signals were detectable in the cytosolic fractions of these cells, while soluble plasmid DNA added to the cell lysate just before centrifugation was to a large extent found in the cytosolic fraction (data not shown). By comparing the intensity of signals from nuclear fractions with the plasmid standard, we estimated that the number of plasmids recovered was in the range of the number of plasmids carried by the intracellular bacteria that were added at the time of infection. Keeping in mind the low transfection efficiency, it is highly unlikely that all plasmids are transferred into the nucleus. We, therefore, interpret the above result as evidence that the plasmid DNA is associated with macromolecules that are of sufficient mass to sediment through the sucrose cushion during the separation of cell nuclei from cytosolic compounds. Furthermore, it clearly shows that the plasmid DNA is not degraded rapidly, but remains within the host cells for a considerable amount of time.
Integration of bacterial DNA into the host cell genome after L. monocytogenes-mediated gene transfer
The rapid release of the complete bacterial content upon lysis of L. monocytogenes within eukaryotic cells also results in the release of large quantities of bacterial chromosomal DNA. This could in turn result in transfer and subsequent integration into the host cell genome. To test this possibility, genomic DNA was isolated from different CHO-K1-derived cell lines and clones that had been bacteriofected with L. monocytogenes. A PCR was performed using seven primer pairs to scan and detect fragments of the listerial chromosome. The primer pairs that were selected did not show any amplification product with DNA from the parental CHO-K1 cells (data not shown). A DNA amount equivalent to 3000 CHO-K1 genomes was tested. The sensitivity of this assay allowed reproducibly the detection of DNA amounts equivalent to 10 L. monocytogenes genomes.
First, the cell line ST37, from which the above-mentioned GFP-CFTR-expressing clones were derived, was tested. A specific signal was obtained for all seven primer pairs (Figure 6). When this cell line was further sorted for GFP-expressing cells (ST37-S1), only three of the seven bands remained detectable. Repeated sorting of medium and high fluorescent cells from the ST37-S1 population resulted in the two subpopulations, ST37-S2H and ST37-S2M, but neither of them gave rise to specific amplification products. The same was true for the clone ST37-2H.43 derived from ST37-S2H and for two clones, ST37-2M.12 and ST37-2M.19, derived from the ST37-S2M line.
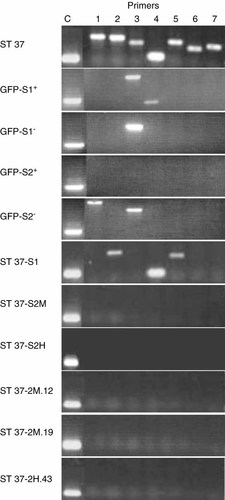
Presence of DNA fragments derived from the bacterial chromosome in the genome of CHO-K1 cells after L. monocytogenes-mediated transfection. Primer pairs (1–7) were specific for L. monocytogenes genomic DNA or GAP-DH (C). All cell lines and clones were CHO-K1 derived and stably transfected by L. monocytogenes-mediated gene transfer. ST37, bulk culture expressing GFP-CFTR; ST37-S1, GFP-positive subpopulation of ST37; ST 37-S2M, medium fluorescent subpopulation of ST37-S1; ST37-S2H, high fluorescent subpopulation of ST37-S1; ST37-2M.12 and ST37-2M.19, clones derived from ST37-S2M; ST37-2H.43, clone derived from ST37-S2H; GFP-S1+ and GFP-S1−, GFP-positive and -negative subpopulations of a bacteriofection employing the GFP-encoding plasmid pERL3-CMVGFP-neo; GFP-S2+ and GFP-S2−, positive and negative subpopulations of a bacteriofection employing the GFP-encoding plasmid pERL3-EPI1, a supposedly episomally replicating eukaryotic expression plasmid 37. Results shown are representative for at least three independent experiments
To confirm these results, cells bacteriofected with other plasmids were also examined. Again CHO-K1 cells stably transfected by L. monocytogenes with the GFP-expressing plasmids pERL3-CMVGFP-neo or pERL3-EPI1 showed specific bands for all seven primer pairs (data not shown). In contrast, the neomycin-resistant but GFP-negative subpopulation of the pERL3-CMVGFP-neo transfection (Figure 6, GFP-S1−) resulted in a single amplification product, whereas the GFP-positive fraction exhibited two such products (Figure 6, GFP-S1+). Cells bacteriofected with the plasmid pERL3-EPI1 were also sorted for GFP-positive (GFP-S2+) and negative (GFP-S2−) populations and showed either no or two specific amplification products, respectively. PCR from five clones from a transfection using the β-galactosidase-encoding plasmid pERL3-CMVβ-neo did not result in any primer-specific bands (data not shown). Thus, the more homogenous a bacteriofected cell population becomes, the less bacterial DNA can be detected. These results indicate that integration of L. monocytogenes chromosomal DNA into the host cell genome occurs, but appears to be a rare event and probably involves only small fragments of bacterial DNA.
Discussion
An in vivo application of the Listeria-mediated DNA transfer system, for instance to treat cystic fibrosis, has to satisfy several prerequisites, the most important one being a sufficient transfection rate of the airway epithelium to allow complementation of the defect in CFTR function. The transfer of a functional CFTR encoding DNA by L. monocytogenes to eukaryotic cells appears to be feasible, and an efficient detection system for gene transfer is introduced here by fusing GFP with the N-terminus of CFTR. Using our improved transfer protocol 19, CHO-K1 and HEp-2 cells could be transfected with this construct by L. monocytogenes in vitro. The resulting fusion protein exhibited the correct subcellular distribution as well as the expected biophysical behavior suggesting expression of a functional recombinant CFTR protein. This was demonstrated by both fluorescence-localization and electrophysiological studies. After Listeria-mediated transfer of the GFP-CFTR-encoding vector, the cell line CHO-K1 devoid of endogenous CFTR was clearly shown to express a typical CFTR channel that transported chloride ions and could be activated by forskolin and IBMX.
For CFTR channels, single-channel conductances in the range of 3–11 pS have been reported 31, 32. Taking into account the amplitude of the membrane current that is activated by forskolin and IBMX in GFP-CFTR-transfected CHO-K1 cells, and assuming a single-channel conductance of 10 pS, we estimate a minimum number of 1700 simultaneously active GFP-CFTR Cl− channels per cell. Since the open-state probability of expressed GFP-CFTR Cl− channels is not known, the real number may be substantially higher. Thus, a number of CFTR molecules within a physiological range is expressed upon L. monocytogenes-mediated DNA transfer.
Although bacteriofection resulted in appropriate numbers of recombinant CFTR channels, the lower frequency of transient transfectants expressing GFP-CFTR compared to GFP is still poorly understood. The same discrepancy was found in CHO-K1 as well as in HEp-2 cells, and also observed when these cell lines were transfected using standard calcium phosphate transfection. Hence, the effect is not specific for CHO-K1 cells nor for L. monocytogenes-mediated gene transfer. Flow cytometric analysis revealed that the mean fluorescence intensity of GFP-CFTR-expressing cells is lower than that of GFP-expressing cells, suggesting a lower average number of GFP-CFTR molecules per cell. This implies that the signal of some of the GFP-CFTR-expressing cells might not exceed the background fluorescence thus explaining the lower transfection rates. The lower expression could be a result of a shorter half-life of GFP-CFTR compared to GFP. Other explanations include the less efficient transcription and/or translation due to sequence peculiarities, as well as the known inefficiency of CFTR processing 33.
Although the level of CFTR channels per cell resembles the natural situation, the number of cells transfected by either GFP or GFP-CFTR is still very low. It is therefore of utmost importance to understand the mechanism of DNA transfer in molecular terms, in order to understand its limitations and then, subsequently, achieve the elimination thereof. There are three obvious steps that might limit the process of bacteria-mediated gene transfer: (1) invasion of the host cell; (2) bacterial lysis and plasmid release; and (3) stability and transport of the expression plasmid to the nucleus.
It is clear from our former data 18, 19, 34 and data presented here that invasion of the host cell by the bacteria is not a critical step since most cells are infected and contain several bacteria. Furthermore, when L. monocytogenes were grown in minimal medium which leads to the upregulation of virulence factors, we observed a 5- to 6-fold increase in the numbers of intracellular bacteria during infection. However, only a 2- to 3-fold increase in transfected cells could be detected (unpublished observation). Thus, transfer efficiency is not directly correlated with invasiveness of the bacteria.
Bacteria are efficiently lysed within the cytosol of the host cell upon antibiotic treatment. Their cell walls were disrupted quickly and the bacterial content was released. This was confirmed by showing that the GFP protein expressed by the bacteria is quickly distributed within the cytosol of the eukaryotic host cells upon bacterial lysis. Virtually all cells that were initially infected contained evenly distributed GFP 48 h after antibiotic treatment. Consequently, the efficiency of bacterial lysis and release of the bacterial content into the cytosol of host cells should be high enough to allow effective gene transfer.
Nevertheless, under these circumstances, transfer of the released plasmids is comparatively low. In fact, introduction of two types of Listeria carrying different plasmids suggests that normally only a few, if not a single, plasmids are transferred to the nucleus to be expressed. Alternatively, bundled plasmids derived from a single bacterium might be transferred under these conditions. In any case, low transfer rates are not due to rapid degradation of the plasmids since large amounts of intact plasmids could be recovered even 48 h after initiation of antibiotics treatment. Rather, the plasmids appear to be associated with macromolecules or organelles as indicated by their sedimentation properties. Spiking the lysates with control plasmids showed that the high sedimentation rate of the bacterially introduced plasmids is most likely not an artefact of the lysis procedure. Most of the control plasmids could be recovered in the slowly sedimenting fraction during these experiments. Strong association with cellular or bacterial components would also explain why these plasmids, unlike those introduced by calcium phosphate transfection, cannot be transcribed in the cytosol by the T7 polymerase.
Thus, the most important limitation for an efficient gene transfer using L. monocytogenes as carrier, and its application in vivo, is the association of the plasmid DNA with macromolecules or organelles after the lysis of the bacteria. The next step should be now to further characterize these complexes. It might be possible to modify the plasmids or the macromolecules associated in a way to facilitate transfer of DNA to the nucleus.
Since high bacterial MOIs were used under our bacteriofection conditions, it is plausible that fragments of the bacterial chromosome can also reach the nucleus of the host cell. It was therefore important to clarify the fate of the bacterial genomic DNA released from lysed L. monocytogenes in the host cell cytosol. To avoid listerial DNA that was only transiently present after infection, we employed stable bacteriofectants that had been in culture for a considerable time. Under these circumstances, bacterial DNA is diluted out due to cell division unless it is integrated into the genome of the host cell. Only then can it be replicated and stably distributed to the daughter cells.
Chromosomal DNA of L. monocytogenes was detected in the genomic DNA of the transfected host cell. However, the more homogenous a cell population was, the less bacterial DNA was detected. In sorted subpopulations only a few specific signals were observed, while in all of the clones analyzed so far, no bacterial DNA was detected. The sequences that are covered by the primer pairs represent only about 0.15% of the L. monocytogenes chromosome. Thus, undetected bacterial DNA could be present. Nevertheless, we conclude that integration of bacterial chromosomal fragments, although clearly taking place, is a rare event under our conditions. Whether the mechanisms leading to plasmid and bacterial DNA transfer are identical remains to be investigated.
Taken together, we have addressed several aspects of Listeria-mediated DNA transfer that have to be understood prior to application in vivo. Most importantly, we have for the first time attempted to understand the mechanisms that are involved in plasmid transfer. Contrary to our original idea that the plasmids are simply released upon bacterial lysis 35, 36, we now find the plasmids to be associated with high molecular weight components. These findings suggest further studies towards the investigation of the plasmid transfer pathway and its rational improvement. This should drastically lower the dose of bacteria required to achieve bacteriofection and thus render integration of bacterial DNA negligible. In concert with further improvements, e.g the introduction of highly attenuated strains, we are confident that L. monocytogenes-mediated gene transfer will find its place as a vector system in gene therapy. Upon availability of an optimized system its potential will surely be considered for other therapeutic complementation of somatic gene defects and therapy of cancer.
Acknowledgements
The expert technical assistance of S. zur Lage, R. Lesch and S. Krämer is gratefully acknowledged. We wish to thank K. E. J. Dittmar for the confocal microscope images as well as Transgene, Strasbourg, and the Association Française de Lutte contre la Mucoviscidose (AFLM), Paris, for supplying the monoclonal antibody MATG1104. We thank Christofer Samuelsson for critically reading the manuscript. This work was supported in part by grants from BMBF, DFG and the Deutsche Krebshilfe.




