Gene expression and immune response kinetics using electroporation-mediated DNA delivery to muscle
Abstract
Background
Injection of DNA encoding exogenic proteins into muscle tissue combined with electroporation often results in a transient increase of the encoded protein concentration in the muscle and the blood. The reduction is normally due to an immune response against the exogenic protein but other factors may also be involved. How various electroporation parameters affect the concentration kinetics of syngenic and exogenic proteins is studied in relation to immune response and muscle damage after electroporation-mediated DNA transfer to muscle.
Methods
Electroporation was applied to mouse quadriceps and rat tibialis anterior muscles after injection of DNA encoding either secreted alkaline phosphatase (SEAP), β-galactosidase (β-gal), luciferase or a mouse IgG molecule. Protein concentrations in blood or muscle and antibody responses were measured for a period up to 3 months. Tissue inflammation and muscle cell damage were studied on muscle cross-sections and assessed by measuring the concentrations of creatine phosphokinase (CPK) in blood.
Results
Mice with the highest SEAP concentration in blood at day 7 also had the highest rate of decrease afterwards, the strongest antibody responses against SEAP and the highest acute levels of CPK in blood. DNA-transfected muscle fibers were significantly reduced in number from days 7 to 14. Mononuclear cells surrounded the reporter gene expressing muscle fibers, thus indicating a cellular immune response. When using DNA encoding a syngenic protein the protein concentration in blood was relatively stabile over a 3-month period, but showed different kinetics for various electroporation parameters.
Conclusions
Our findings suggest that the optimal electroporation parameters for DNA vaccination may be different from the optimal parameters for long-term expression of genes encoding syngenic proteins. Copyright © 2004 John Wiley & Sons, Ltd.
Introduction
Electrical stimulation of skeletal muscles after an intramuscular injection of DNA promotes entry of injected DNA into muscle fibers 1-3. Such stimulation, also called electroporation, induces a transient increase in membrane permeability which allows DNA to enter the cells 4. Compared to DNA injection alone, electroporation substantially increases the production of the DNA-encoded proteins. Electroporation may therefore enable numerous gene therapy and DNA vaccination applications.
Injection of DNA encoding exogenic secreted proteins into muscle tissue with or without electroporation often results in a transient increase of the protein concentration in the blood and the muscle 5-7. Polyclonal antibodies and cellular immune responses specific for the exogenic protein are normally observed within 2 weeks after the DNA transfection. Many factors are believed to influence the induction of an immune response after plasmid DNA injection, such as the properties of the antigen itself, the expression level, location of antigen expression, co-stimulatory factors such as CpG sequences, and the degree of local tissue damage. The choice of pulse pattern and field strength used for in vivo electroporation influence some of these factors. Preliminary experiments on rat tibialis anterior muscle in our laboratory showed that electroporation using trains of high-frequency unipolar pulses resulted in a higher reporter gene activity than bipolar pulses. To establish optimal electroporation parameters for different purposes a better understanding of the factors affecting the production of the DNA-encoded proteins is necessary. This study focuses on how and why various electroporation parameters affect the concentration of the encoded protein in blood over a period for up to 3 months.
First, injection of DNA encoding luciferase into rat tibialis anterior muscle revealed that electroporation with unipolar pulses resulted in a significantly higher luciferase activity than bipolar pulses. This was followed by injection of DNA encoding secreted alkaline phosphatase (SEAP) into mouse quadriceps muscle to compare the efficiency of unipolar or bipolar pulse electroporation at different field strengths in enhancing SEAP concentrations in blood and the DNA transfected muscle. Second, we studied electroporation-induced muscle damage by histological examination of the muscle and creatine phosphokinase (CPK) concentration in blood. Third, we compared the efficiency of the different pulse parameters in inducing antibody responses against SEAP in blood and correlated the antibody responses with the subsequent drop in SEAP concentration in blood. Finally, we studied the kinetics of a syngenic protein in blood after transfection using either unipolar or bipolar pulses with the field strengths shown to result in the highest levels of SEAP in blood.
Materials and methods
Animals and treatment
Male Wistar rats weighing 250–300 g, and female C57BL/6 and Balb/c mice weighing 20–24 g, were obtained from B&K Universal (Sollentuna, Sweden). DNA injection, electroporation, perfusion of muscles, and sacrifice of animals by cervical dislocation were all performed under deep anaesthesia with Equithesin (165 mg chloral hydrate, 84 mg magnesium sulphate, and 40 mg pentobarbital per kg body weight, i.p.). Fluothane (#183335, Zeneca, Oslo, Norway) was used as anaesthesia in the experiments performed to determine CPK concentrations in blood. The experiments were conducted in conformity with the laws and regulations controlling experiments and procedures with live animals in Norway.
Plasmid DNA (50 µl) in 0.9% NaCl was injected either into the exposed rat tibialis anterior muscle or through the skin into the mouse quadriceps muscle. VR1255 plasmid (0.1 µg/µl) DNA encoding luciferase was used in the rat experiments 7. gWiz SEAP (0.2 µg/µl) and gWiz ß-gal (0.2 µg/µl) (Gene Therapy Systems, San Diego, CA, USA) were used in the experiments on C57BL/6 mice. pLNOH2 γ2b VH NP (0.4 µg/µl) and pLNOH λ1 (0.4 µg/µl), together encoding the mouse IgG2b anti-NIP (4-hydroxy-3-iodo-5-nitrophenylacetic acid) antibody, were used in the experiments on Balb/c mice 8. Conductive gel was applied on the mouse skin, and two steel calliper electrodes were placed on the skin on both sides of the quadriceps and used for electroporation. Due to muscle size and high resistance over the rat skin and underlying adipose tissue, the rat tibialis anterior muscles were surgically exposed and electroporated with two steel wire electrodes placed 3 mm apart perpendicularly on each side of the muscle. Electrodes were connected to a pulse generator (Inovio AS, Oslo, Norway) delivering the electrical pulses described in Table 1.
| Column | Pulse duration (µs) | Pulses/train | Trains | V/cm |
|---|---|---|---|---|
| 1 | 400 | 1000 | 10 | 200 |
| 2 | 400 | 1000 | 10 | 150 |
| 3 | 400 | 1000 | 10 | 100 |
| 4 | 200 + 200 | 1000 | 10 | +200/−200 |
| 5 | 200 + 200 | 1000 | 10 | +150/−150 |
| 6 | 200 + 200 | 1000 | 10 | +100/−100 |
| 7 | — | — | — | — |
| 8 | Not treated |
Muscles were removed or blood was collected from the tail vein at the specified days after DNA injection and electroporation.
Cloning of pLNO λ1
The λ1 light chain was PCR-amplified with forward primer 5′-ggt-gtg-cat-tcc-cag-gct-gtt-gtg-act-cag-g-3′ and reverse primer 5′-cgc-gga-tcc-cta-gga-aca-gtc-agc-acg-gg-3′ (as a 660 base-pair product) from a pEE12 vector containing the light chain and cloned into the TOPO-TA cloning vector (#K4500-01, Invitrogen Norge AS, Oslo, Norway). Subsequently, a BamHI-BsmI fragment from TOPO-TA, containing the λ1 light chain, was subcloned into a target vector (pLNOκ 9). The resulting vector is called pLNO λ1, and contains the mouse λ1 light chain.
Luciferase assay
Frozen rat tibialis anterior muscles were homogenized in 1 ml reporter lysis buffer (#E3971, Promega, Madison, WI, USA) and centrifuged for 5 min at 13 000 rpm. Then, 20 µl supernatant was mixed with 100 µl luciferase assay reagent (#E1483, Promega) and measured for 10 s in a luminometer (TD-20/20, Turner Designs, Sunnyvale, CA, USA).
SEAP assay
SEAP concentration in mouse blood and muscle extract was measured according to the manufacturer's protocol using the Phospha-Light system (#T1017, Applied Biosystems, Bedford, MA, USA). Alkaline phosphatase (#P3895, Sigma-Aldrich, St. Louis, MO, USA) was used as standard.
Detection of SEAP in muscles was done on supernatants from muscle extracts. Anaesthetized mice were perfused with 10 ml phosphate-buffered saline (PBS) (#P4417, Sigma-Aldrich) through v. cava to reduce the amount of SEAP located in the blood vessels of the muscle. Quadriceps muscles were homogenized in 1 ml reporter lysis buffer (#E3971, Promega), frozen and thawed once. After centrifugation the supernatant was collected and analyzed for SEAP concentration with a luminometer (#TD-20/20). As a control for perfusion efficiency the untreated contralateral muscles were removed. These muscles had on average only 1.5% of the SEAP concentration in the treated muscles.
CPK assay
CPK concentration was measured in blood collected from v. cava 2 h after DNA transfection and electroporation (#520, Sigma-Aldrich). The procedure was performed according to the manufacturer's instructions, but adapted to smaller volumes. Absorbance was measured at 490 nm with an Emax microplate reader (Molecular Devices, Sunnyvale, CA, USA).
ß-Gal staining and histochemistry
Quadriceps muscles were surrounded with Tissue-TEK O.C.T. (#4583, Bayer Corporation, Pittsburgh, PA, USA) and quickly frozen in a mixture of isopentane and dry ice. Cross-sections (12 µm) were made and dried for 1 h at room temperature. Sections were then fixed with 2% paraformaldehyde and 0.2% glutaraldehyde in PBS (pH 7.4) for 5 min at room temperature, washed twice for 5 min in PBS, and stained for 30 min at 37 °C with X-gal (1 mg/ml, #V394A, Promega), magnesium chloride (2 mM), potassium ferricyanide (4 mM) and potassium ferrocyanide (4 mM) in PBS (pH 7.4). Cross-sections were thereafter washed twice with PBS, and counterstained with haematoxylin, azophloxine and saffron (HAS).
ELISA
Blood was analyzed for SEAP-specific IgG1 and IgG2a subclass antibody responses. Microtiter plates (#3590, Corning Incorporated, NY, USA) were coated with 1 µg alkaline phosphatase (#P3895, Sigma-Aldrich) per well in PBS and incubated overnight at 4 °C. Plates were washed with washing buffer [PBS with 0.1% Tween-20 (#P1379, Sigma-Aldrich)] between all steps. Blood samples were diluted with dilution buffer (PBS with 0.05% Tween-20) in a three-fold series starting at 1 : 50 and incubated in the coated wells for 2 h at 37 °C. Biotinylated goat anti-mouse IgG1 (#553441, Pharmingen, San Diego, CA, USA) and IgG2a (#553388, Pharmingen) were diluted 1 : 1000 with dilution buffer and 100 µl per well was incubated in parallel wells for 1 h at 37 °C. Streptavidin conjugated to horseradish peroxidase (Southern Biotech, Birmingham, AL, USA) was diluted 1 : 1000 in dilution buffer and 100 µl per well was incubated for 1 h at 37 °C. Color development was obtained by incubating 100 µl per well of Sigma FAST OPD (#P9187, Sigma-Aldrich) in the dark at room temperature for 30 min. Absorbance was measured at 450 nm with an Emax microplate reader.
Blood levels of monoclonal mouse anti-NIP IgG2b were detected with ELISA as described by Tjelle et al. 10. Wells were coated with NIP2.6BSA (i.e., 2.6 NIP molecules per BSA molecule) after which diluted sera were added. Biotinylated anti-mouse IgG2b (Pharmingen), streptavidin-alkaline phosphatase (Amersham Pharmacia Biotech UK Limited, UK) and para-nitrophenylphosphate substrate (Sigma-Aldrich) were added sequentially. Standard curves were constructed with anti-NIP31 mAbs (affinity purified from cell transfectants).
Statistical analysis
Spearman correlation and Student t-test analyses were performed using Prism 3.00 (GraphPad Software Inc., San Diego, CA, USA).
Results
Electroporation with unipolar pulses resulted in higher SEAP concentration in blood compared to bipolar pulses
DNA encoding luciferase was injected into rat tibialis anterior muscles and electroporated with either unipolar or bipolar electrical pulses using sets of pulses (2, 3, 5 and 6) shown in Table 1. As shown in Figure 1A, unipolar pulse electroporation at 100 and 150 V/cm resulted in 12- and 21-fold higher luciferase activity compared to bipolar pulse electroporation, respectively. To compare unipolar and bipolar pulse electroporation when using a secreted protein, DNA encoding SEAP was injected into the mouse quadriceps muscle followed by electroporation using the sets of parameters (1–6) shown in Table 1. As shown in Figure 1B, unipolar pulses (1–3) resulted in higher maximal blood concentrations of SEAP than bipolar pulses (4–6) for the same field strengths at day 3 and 7. For both pulse patterns, increasing the field strength from 100 to 200 V/cm resulted in higher SEAP concentration in blood. Regardless of electroporation procedure, the highest SEAP blood concentrations occurred on day 7. Thereafter, the levels dropped rapidly with the highest rate of decrease in the mice with highest SEAP concentration at day 7. Compared to DNA injection alone, the most efficient electroporation parameters enhanced SEAP concentration in blood 120 times. Since higher field strengths are necessary when electroporation is performed through the skin (due to skin resistance) and not directly on the muscle, the relative increase in reporter gene activity in the electroporated muscles versus the non-electroporated muscles was higher for the rat tibialis anterior (direct contact between electrode and muscle) than the mouse quadriceps muscle (electroporation through skin).

Electroporation-enhanced DNA transfection depending on pulse field strength and shape. (A) DNA encoding luciferase was injected into rat tibialis anterior muscle, followed by electroporation with pulse patterns described in Table 1. Muscle extracts were analyzed for luciferase activity 5 days after treatment. Note the higher luciferase activity found in muscles electroporated with unipolar pulses compared to bipolar pulses at the same field strength. n = 10, 5, 10, 2 and 5 for group 2, 3, 5, 6 and 7, respectively. (B) DNA encoding SEAP was injected into mouse quadriceps muscle, followed by electroporation using pulse parameters 1–6 (Table 1). Groups 7 and 8 represent DNA injection alone and untreated muscles, respectively. Note the increase in SEAP concentration in blood on day 7 after transfection, which is greater for unipolar (1–3) than for bipolar (4–6) pulses and for increasing field strengths (1 > 2 > 3, 4 > 5 > 6). Note also that SEAP concentrations in blood at day 7 showed significant negative correlation with the rate of SEAP reduction between days 7 and 14 (r = −0.5864, P < 0.0001). n = 6 for all groups. Values are means ± SEM. Statistical analysis: Spearman correlation
SEAP concentration decreased faster in blood than in muscle
Quadriceps muscles were removed after perfusion for measurement of SEAP concentration in transfected muscle tissue (electroporation parameter set 1, Table 1). As shown in Figure 2, SEAP levels dropped faster in blood from day 7 to day 14 than in muscle. This indicates that, in addition to a reduced SEAP expression in muscle, there is an inactivation or removal of SEAP in the blood.
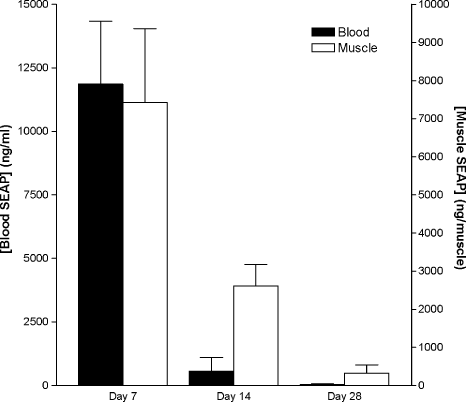
SEAP concentration declined faster in blood than in muscle. DNA encoding SEAP was injected into mouse quadriceps muscles, followed by electroporation (pattern 1 in Table 1). Note larger reduction in SEAP concentration in blood compared to that in muscle extracts from days 7 to 14 after transfection (95 vs. 70%). n = 5 for all groups. Values are means ± SEM
The number of transfected muscle cells was rapidly reduced within 2 weeks after transfection
To investigate if the reduction in SEAP concentration in muscle could be caused by elimination of transfected muscle fibers, DNA encoding SEAP and ß-galactosidase (ß-gal) were electroporated together into the quadriceps muscle (pulse patterns 1 and 4, Table 1). For pulse pattern 1, cross-sections were made from 5, 9 and 6 muscles from days 7, 14 and 56, respectively, while, for pulse pattern 4, cross-sections of 4 muscles at day 14 were made. Cross-sections were stained for ß-gal and counterstained with HAS. Pictures of representative examples of stained cross-sections were taken. Seven days after transfection, dense areas of muscle fibers expressing ß-gal were observed (Figure 3A). In most cases necrotic muscle fibers were found in close proximity of the transfected fibers. These fibers were probably damaged by the electroporation procedure and not destroyed by the immune system. After 14 days the necrotic areas of the muscle were to a large degree replaced by regenerating muscle cells. However, now mononuclear cells were found surrounding ß-gal-positive muscle fibers (Figure 3B). Fifty-six days after transfection, some areas in the muscle tissue were still regenerating. Only one ß-gal-positive fiber was detected in the 6 muscles analyzed (Figure 3C). The surrounding mononuclear cells and the significantly reduced circumference of the transfected fiber compared to normal muscle fibers suggest that this apparently last stronghold of transfected muscle fibers is about to fall. As shown in Figure 3D, there was a clear reduction in ß-gal-positive muscle fibers at day 14 compared to day 7. Also, when applying electroporation (bipolar pulses at 200 V/cm) resulting in a low level of inflammation, we observed mononuclear cells surrounding DNA-transfected cells at day 14 (Figure 3E), but to a lower extent than with unipolar pulses. Both SEAP and ß-gal are antigenic proteins, and the cellular immune responses are therefore likely to be directed at epitopes on both proteins. This may explain why the reduction of ß-gal-positive muscle fibers is more pronounced than the reduction of SEAP in the muscle. Even so, this shows that immune reactions cause transfected muscle fibers to die, thereby contributing to the loss of SEAP both in the muscle and in the blood (Figures 1 and 2).
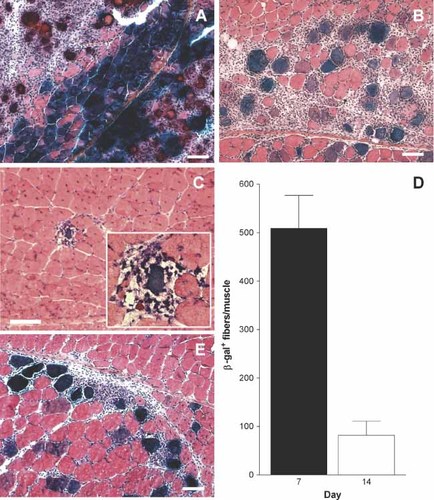
Transfected muscle fibers became surrounded by mononuclear cells. DNA encoding ß-gal and SEAP was injected into mouse quadriceps muscles, followed by electroporation (Table 1, pattern 1: A–D, pattern 4: E). Cross-sections made from muscles 7 (n = 5), 14 (n = 13), and 56 (n = 6) days after transfection were stained for ß-gal and counterstained with HAS. Representative examples of stained cross-sections are shown in A–C and E. (A) Areas of ß-gal-positive fibers with areas of nearby necrotic fibers at day 7. (B) Less-pronounced areas of transfected muscle fibers surrounded by mononuclear cells and necrotic areas at day 14. (C) The only ß-gal-positive muscle fiber found at day 56 in muscles surrounded by mononuclear cells. (D) Number of ß-gal-positive muscle fibers in sections from 5 muscles on days 7 and 14. (E) Also when applying electroporation with pulses resulting in a low degree of tissue inflammation (pulse pattern 4) did mononuclear cells surround DNA transfected cells, but to a lower extent than with pulse pattern 1. Values are means ± SEM. Scalebar: 100 µm
Muscle cell damage after electroporation
To determine the degree of electroporation-induced acute muscle damage, blood was examined for CPK activity 2 h after electroporation with parameter sets 1–6 in Table 1. As shown in Figure 4A, unipolar pulses caused more leakage of CPK than bipolar pulses applied with the same field strength, while for both groups the CPK concentration increased with increasing field strengths. HAS-stained cross-sections of quadriceps muscles removed 3 days after DNA transfection were analyzed to evaluate if the measured CPK concentrations were related to the degree of muscle damage. Figure 4B shows that electroporation with unipolar pulses at 200 V/cm resulted in a 10-fold larger area of damaged muscle tissue compared to bipolar pulses.
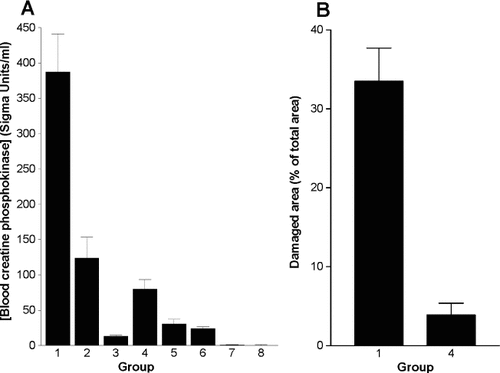
Muscle cell damage after electroporation. DNA encoding ß-gal was injected into mouse quadriceps muscles, followed by electroporation (patterns 1–6 in Table 1). (A) Blood levels of CPK were measured 2 h later. Note the greater increase in CPK levels after unipolar (patterns 1–3) compared to bipolar (patterns 4–6) pulses. n = 5 (except groups 5 and 6 where n = 4). (B) Cross-sections were made from muscles removed 3 days after electroporation, stained with HAS and evaluated for muscle damage as damaged area in percent of total cross-sectional area. Groups 1 (n = 10) and 4 (n = 8) correspond to patterns 1 and 4 in Table 1. Values are means ± SEM
Increasing the field strength for electroporation resulted in a stronger antibody response
Following injection of DNA encoding SEAP, electroporation was applied using the sets of parameters (1–6) shown in Table 1. As shown in Figure 5, increasing field strength (100–200 V/cm) resulted in higher antibody titers for both pulse configurations. The IgG1 antibody response was faster than the IgG2a response, since the IgG1 response most often was equally strong on days 28 and 56, whereas antigen-specific IgG2a antibodies reached the highest endpoint titer on day 56. Even though the antibody responses obtained with unipolar and bipolar electroporation at the same field strength were not statistically significantly different, the electroporation with unipolar pulses generally resulted in higher IgG1 antibody titers than bipolar pulses at the same field strength.
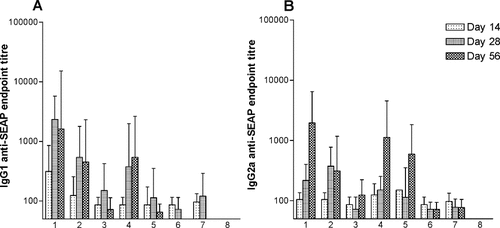
SEAP DNA transfection-induced appearance of antibodies against SEAP in blood. DNA encoding SEAP was injected into mouse quadriceps muscles, followed by electroporation (patterns 1–6 in Table 1). Blood samples taken at days 14, 28 and 56 after DNA transfection were analyzed for IgG1 (A) and IgG2a (B) anti-SEAP antibody concentration. Note that on day 14 the strongest IgG1 (r = 0.3597, P = 0.0103) response against SEAP was found in animals with the highest blood SEAP concentration on day 7, whereas on day 56 both IgG1 (r = 0.6191, P < 0.0001) and IgG2a (r = 0.6463, P < 0.0001) levels were highest in mice with the highest blood SEAP concentration on day 7. n = 6 for all groups. Values are means ± SEM. Statistical analysis: Spearman correlation
As shown in Figure 1 the most marked reduction of SEAP in blood from days 7 to 14 was found in the mice with the highest SEAP concentration in blood at day 7. Consequently, we wanted to find out if this reduction was correlated with a specific antibody response against SEAP and if the strongest antibody responses were found in the mice with the highest SEAP concentration at day 7. We pooled all the data from Figures 1 and 5 and found that there was a significant correlation between the relative reduction of SEAP concentration in blood from days 7 to 14 and the SEAP-specific IgG1 antibody response at day 14 (Figure 6A). A similar correlation was not seen for IgG2a for these early times. In addition, the strongest IgG1, but not IgG2a, responses at day 14 were found in the mice with the highest SEAP concentrations at day 7. This early reduction of SEAP from the blood may therefore be more dependent on the IgG1 response than the IgG2a response. As shown in Figure 6B, at day 56 both IgG1 and IgG2a show positive correlation with the relative reduction of SEAP concentration in blood from days 7 to 56. Also at day 56 both the strongest IgG1 and IgG2a antibody responses against SEAP were found in the mice which had the highest SEAP concentration in blood 1 week after DNA transfection. The IgG2a response can hence also be important in eliminating SEAP, but at a later stage than IgG1.
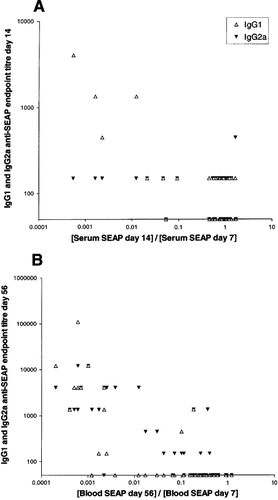
Early elimination of SEAP showed IgG antibody subclass specificity. Data from all groups in Figures 1 and 5 were pooled. The strongest IgG1 (open triangles) (r = −0.3304, P = 0.0204) response on day 14 was found in mice with the highest relative decrease in blood SEAP concentration from days 7 to 14 (A), whereas on day 56 both IgG1 (r = −0.7056, P < 0.0001) and IgG2a (r = −0.7451, P < 0.0001, closed triangles) responses were strongest in mice with the highest relative decrease in SEAP concentration from days 7 to 56 (B). Statistical analysis: Spearman correlation
Different electroporation parameters resulted in different long-term kinetics for syngenic protein concentration in blood
We wanted to apply the most effective unipolar and bipolar electroporation patterns (1 and 4 in Table 1) to compare long-term protein concentration in blood when using DNA encoding a syngenic protein (mouse IgG2b anti-NIP). As seen in Figure 7, IgG2b anti-NIP concentration in blood was higher in the group electroporated with unipolar pulses at days 7 and 14. After day 22 the IgG2b anti-NIP concentration was highest in the bipolar group. The protein levels showed only a slight decrease in the animals receiving bipolar pulses whereas the unipolar pulses resulted in a continuous reduction of expression levels. After 77 days the group receiving bipolar pulses retained expression levels that appeared more stable and were at least twice the levels in the group receiving unipolar pulses.
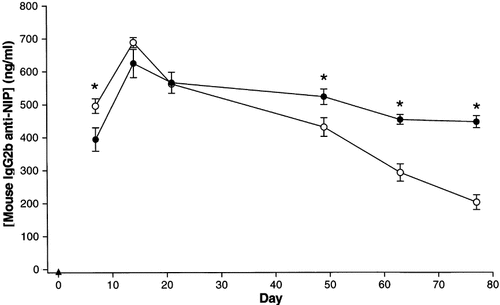
Expression of DNA encoding a syngenic antibody molecule was relatively stable after transfection and electroporation. DNA encoding mouse monoclonal IgG2b anti-NIP was injected into mouse quadriceps muscles, followed by electroporation with pattern 1 (open circles, n = 8) or pattern 4 (filled circles, n = 7), see Table 1. At different times thereafter, the amount of antibodies in the blood against NIP (IgG2b anti-NIP) was measured. Untreated animals (filled triangles, n = 5). *Statistically significant difference between the unipolar and the bipolar groups at the specific time points (P < 0.05, Student's t-test)
Discussion
In the present work, we have observed that when DNA encoding an exogenic protein (SEAP) is injected and electroporated into skeletal muscle, the concentration of SEAP in blood increases to a certain level and then rapidly decreases during the first 2 weeks. The highest rate of decrease and the strongest antibody response against SEAP were found in the animals with the highest SEAP concentration in blood after 1 week. Interestingly, SEAP was more rapidly cleared from the blood than from the transfected muscle, indicating that a humoral immune response plays an important role in neutralizing the secreted protein. Transfection of DNA encoding a syngenic mouse IgG2b molecule with electroporation led to a more persistent protein concentration in the blood than when using DNA encoding the exogenous SEAP protein.
Comparison of unipolar and bipolar pulses for transfection of SEAP revealed that the unipolar pulse patterns much more efficiently caused production of SEAP at early stages (7 days), perhaps because unipolar pulses cause more extensive movements of DNA. While electroporation using bipolar pulses may make DNA oscillate back and forth, unipolar pulses will cause the DNA to move unidirectionally. The probability of the injected DNA being transferred into the cytoplasm or nucleus of the cells may therefore be higher using unipolar than bipolar pulses, and could at least partly explain the observed difference in reporter gene expression between the two different pulse patterns. Extensive movement of other charged molecules during unipolar electroporation may, however, induce toxic conditions for the cells, and thus cause muscle cell damage. This is suggested by our results from histological analyses of muscle and with acute CPK concentrations in blood.
CPK concentration in blood has been used as an enzymatic marker for acute muscle cell injury after electroporation 11, 12. We found a clear positive correlation between field strength and CPK in blood, and unipolar pulse electroporation at 200 V/cm caused a 600-fold greater increase in CPK concentration than DNA injection alone. CPK probably leaks out from the cytoplasm and into the extracellular space from cells through both transient and permanent defects in the plasma membrane of injured cells. CPK levels thus give an indication not only of fatally damaged cells, but also of the temporary permeabilization needed for successful DNA transfection. However, permanent cell ruptures are likely to be the dominant cause of the CPK in blood, because the CPK levels correspond closely to the degree of muscle tissue damage as seen on muscle cross-sections.
Transient expression of exogenous gene products has been reported for many different gene products, including SEAP, luciferase, ß-gal, chloramphenicol, acetyltransferase and clotting factor IX 5-7. Consistent with these results SEAP concentration in blood decreased rapidly in all cases of electroporation after day 7. Although the reduction could be caused by DNA degradation or inactivation, persistent expression of reporter genes in immunodeficient mice 5, as well as syngenic protein (present results and 13), argues against a general mechanism affecting DNA processing to explain the rapid reduction in expression levels.
The most plausible reason for a protein clearance in blood would be an induced immune response against the exogenic proteins. Specific antibody responses are generally detected 3–4 weeks after an immunization, which is much later than the observed rapid clearance of SEAP. Both in this work and other reports, the IgG responses have been detected as early as 14 days after DNA injection and electroporation 14, 15, and can therefore partially explain the decrease in SEAP concentration in blood. We describe in this work that mice with the highest relative reduction of SEAP in blood from days 7 to 14 also had the strongest IgG1, but not IgG2a, responses against SEAP at day 14. However, at day 56, both IgG1 and IgG2a antibody responses correlated with the relative reduction in SEAP from days 7 to 56. This suggests that IgG1 is more involved than IgG2a in the early reduction of SEAP from the blood, while IgG2a becomes more important at a later stage. Mouse IgG2a is known as an opsonizing antibody triggering cellular immune responses and complement activation, and a late IgG2a activation may partly explain the relatively late clearance of SEAP from the muscle. Taken together, this suggests that a high number of antigens in circulation trigger a strong immune response against the antigen which in turn eliminates the antigen from the blood. In addition to a high antigen concentration it is likely that mechanisms involved in tissue inflammation, such as cytokine release and the recruitment of immune cells, act together to establish the immune response. Also, we cannot rule out the possibility that different electroporation parameters affect different cell populations within the muscle, which may influence the immune response 16.
In this study SEAP was more rapidly eliminated from blood than from the transfected muscle. Different mechanisms removing SEAP must therefore be functioning in the blood and the DNA-transfected muscle. Inactivation of SEAP in muscle cells may be caused by a cellular immune response against SEAP. Paster et al. recently showed a prolonged and significantly higher level of antigen-specific CD8+ cells when DNA injection was followed by electroporation than without 17. For specific CD8+ cells to recognize transfected muscle fibers expressing the antigen, the muscle fibers have to display antigenic epitopes on major histocompatibility complex class I (MHC I). Muscle fibers do not normally express MHC I, but up-regulate MHC I during inflammation 18. We have in this study observed reporter gene expressing muscle fibers surrounded by mononuclear cells 14 days after DNA transfection. These mononuclear cells are probably active in the process of attacking the transfected muscle fibers, but could also participate in the removal of cell debris. At day 56 only a low percentage of the treated animals had detectable levels of SEAP and only one ß-gal-positive muscle fiber in 6 muscles was observed. This shows that there is a dramatic reduction in DNA-transfected muscle fibers, but on the other hand it also shows that, even after 2 months with antigenic stimulation, the immune system has not totally eliminated the antigen.
Long-lasting expression of a therapeutic protein is of crucial importance for treatment of some diseases. Using a syngenic protein, EPO, Rizzuto et al. found only a limited reduction in blood EPO concentration during a period of 84 days when combining DNA injection with electroporation 13. In the present study we applied DNA encoding a syngenic immunoglobulin molecule (mouse IgG2b anti-NIP) to mimic a self-protein. Seven days after transfection the IgG2b anti-NIP concentration in blood was slightly higher in the group electroporated with unipolar pulses compared to bipolar pulses. However, the difference was rather small compared to the SEAP experiments. With SEAP the unipolar pulses resulted in a 2–3-fold higher concentration in blood compared to bipolar pulses, while the mouse IgG2b concentration was only 0.25-fold higher when using unipolar pulses. This illustrates the inter-gene variability when comparing two electroporation schemes. After day 14 the mice electroporated with unipolar pulses had a more rapid decline in IgG2b anti-NIP concentration than the bipolar group. This may be caused by muscle damage which is then followed by a decrease in muscle cells producing the protein. The damage caused by electroporation with the unipolar pulses might be sufficient to work as an efficient adjuvant for induction of immune responses against the variable regions of the antibody despite the report that these regions are known not to be very immunogenic 19. Another possibility is that electroporation with unipolar pulses results in muscle fibers with a higher number of DNA plasmid copies than when using bipolar pulses. This may be toxic in itself or may prevent synthesis of proteins essential for cell survival.
In conclusion, this study has shown that electroporation parameters such as pulse pattern and field strength affect muscle cell damage, immune responses and gene expression of both syngenic and exogenic proteins, and that one set of parameters is not optimal for all tasks. Electroporation with high-frequency unipolar pulses generally results in higher antibody responses than bipolar pulses at the same field strength, while bipolar pulses result in more stable and, on average, higher concentrations of syngenic proteins than unipolar pulses. The electroporation parameters should therefore be chosen in each case according to the particular application for gene transfer.
Acknowledgements
We thank Terje Lømo, University of Oslo, for very constructive help with the manuscript. The Norwegian Cancer Society (grant: C 02057/004) and EU project QLRT-2000-00530 supported these studies.




