Short hairpin RNAs against eotaxin or interleukin-5 decrease airway eosinophilia and hyper-responsiveness in a murine model of asthma
Abstract
Background
Eosinophilia plays the major role in the pathogenesis of asthma and correlates with the up-regulation of eotaxin, which, together with interleukin (IL)-5, is important for differentiation, chemo-attraction, degranulation, and survival of eosinophils in local tissue. In a previous study, we found that administration of lentivirus-delivered short hairpin RNA (shRNA) to suppress the expression of IL-5 inhibited airway inflammation. The present study aimed to investigate the role of eotaxin shRNA and the synergistic effect of eotaxin and IL-5 shRNAs on airway inflammation in an ovalbumin (OVA)-induced murine model of asthma.
Methods
Lentivirus-delivered shRNAs were used to suppress the expression of eotaxin and/or IL-5 in local tissue in an OVA-induced murine asthma model.
Results
Intra-tracheal administration of lentivirus containing eotaxin shRNA expressing cassette (eoSEC3.3) efficiently moderated the characteristics of asthma, including airway hyper-responsiveness, cellular infiltration of lung tissues, and eotaxin and IL-5 levels in bronchio-alveolar lavage fluid. Administration of lentiviruses expressing IL-5 or eotaxin shRNAs (IL5SEC4 + eoSEC3.3) also moderated the symptoms of asthma in a mouse model.
Conclusions
Local delivery of lentiviruses expressing IL-5 and eotaxin shRNAs provides a potential tool in moderating airway inflammation and also has the potential for developing clinical therapy based on the application of shRNAs of chemokines and cytokines involved in T helper 2 cell inflammation and eosinophilia. Copyright © 2008 John Wiley & Sons, Ltd.
Introduction
Asthma is a chronic airway inflammatory disease characterized by eosinophil infiltration and airway hyper-responsiveness (AHR). Both clinical and experimental studies show that the presence of eosinophils and their products in the airways correlates with AHR 1. Mediators released by eosinophils induce smooth muscle contraction, mucous secretion, and structural tissue damage resulting in bronchial hyper-responsiveness and epithelial cell injury. Eosinophils may also play a role in airway remodelling by releasing the potent pro-fibrotic cytokines, transforming growth factor (TGF)-β, and other fibrogenic factors 2.
Eotaxin is the key chemo-attractant of eosinophils. It selectively binds to CCR3 that is highly expressed on eosinophils, basophils, and mast cells, all of which are important in the pathogenesis of asthma. In asthmatic airways, there is increased eotaxin level in the bronchial airway lumen and mucosa. Fujisawa et al. 3 reported that chemokine-induced eosinophil degranulation is mediated through only CCR3. Eotaxin is more potent than other chemokines that bind CCR3, such as RANTES, MIP3 and MIP4, to induce eosinophil degranulation and leukotriene C4 release 3.
Interleukin (IL)-5 is another key cytokine in the growth and differentiation of eosinophils in bone marrow 4-6 and the release of eosinophils into the peripheral circulation 7, 8. In addition, IL-5 enhances eosinophil recruitment, activation, and survival in the inflammation site 3, 9. It is mainly produced by activated T cells, CD8 T cells, mast cells, eosinophils, and airway epithelial cells 10-12 and, in asthmatic patients, its levels are correlated with clinical features 13. IL-5 mRNA is up-regulated in the bronchial mucosa after allergen-provocation 14 and IL-5 deficient mice do not develop AHR, lung damage, and eosinophilia in a mouse asthma model and eosinophilia in a parasite model 15, 16. Therefore, inhibiting or reducing IL-5 and eotaxin expression comprises a promising therapeutic strategy in eosinophil-mediated diseases.
Manipulation of gene expression in mRNA levels is more efficient than in protein levels because multiple copies of a protein (approximately 5000 copies) are produced by each mRNA 17. Therefore, mRNA targeting strategies, such as hybridization-based anti-sense oligonucleotides (AS-ONs) and RNA interference (RNAi), have been investigated for their therapeutic effect on asthma and other allergic diseases. To date, T helper (Th) 2 cytokines (IL-4, IL-5, and IL-13) and their receptors (IL-5Rα and common beta chain of granulocyte-macrophage colony-stimulating factor (GM-CSF)/IL-3/IL-5 receptor), adhesion molecules (interstitial cell adhesion molecule-1 and very late activation antigen-4), intra-cellular signal transduction molecules (Syk, Btk, Fyn, and p38α mitogen activated protein kinase), transcription factors (GATA-3, STAT6, and nuclear factor-κB), and other factors involved in allergic inflammation (stem cell factor, TGF-β1, and tumour necrosis factor-α) have been used as the targets of AS-ON or RNAi therapy in allergic diseases 17.
In a previous study, IL-5 targeting shRNA efficiently moderated symptoms of asthma 18. Therefore, the present study investigated the role of eotaxin shRNA in reducing airway inflammation, as well as its synergistic effect with IL-5 shRNA in an ovalbumin (OVA)-induced murine model of asthma.
Materials and methods
Construction of SEC
The shRNA expression cassettes (SECs) targeting mouse eotaxin gene were synthesized by a shRNA expression cassette kit (Ambion, Houston, TX, USA). The eoSEC3.1 and eoSEC3.3 targeting eotaxin cDNA sequence were 5′-CTTCCTGCTGCTTTATCAT-3′ and 5′-CACAATGGGACGAGTTAGG-3′. The IL5SEC4 targeting cDNA sequence was 5′-AAGAAATTCCTGTAGCGCAGG-3′. The control shRNA sequence of the mock virus (SECneg) was 5′-GTCAGACTGTGCCATGACTG-3′. The control shRNA was provided by the shRNA expression cassette kit, which lacked homology to any mouse gene. The SECs driven by U6 promoter were digested with EcoRI and HindIII and ligated into the pSEC™ hygro vector, and then changed into pTY-linker vector 19-21 for the lentivirus-delivery system.
Preparation of SEC-expressing lentiviruses
Plasmids (pTY-linker, pHP-dl.Nde/Ase, pCEP4-tat, and pHEF-VSV-G) for producing lentivirus were from the Graduate Institute of Microbiology of the National Taiwan University. The lentivirus vectors, containing the SEC or enhanced green fluorescent protein (eGFP) with three other plasmids for virus generation, pHP-dl.Nde/Ase, pCEP4-tat, and pHEF-VSV-G, were mixed at ratios of 3, 3, 2, and 1, respectively, and co-transfected into 293T cells. After 18–24 h, fresh complete medium were changed. Lentivirus particles were produced and secreted into the supernatant 48 h after transfection. Filtrated lentivirus-containing supernatant was concentrated with a centricon 100 kDa centrifuge tube and stored at − 80 °C.
Determination of cytokine expression
IL-5, eotaxin, and interferon (INF)-γ levels were assayed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's recommended protocols (R&D, Minneapolis, MN, USA).
Determination of suppression efficiency of SECs in vitro
Female BALB/c mice aged 4–6 weeks were sacrificed by cervical dislocation to avoid the influence of ether or pentobarbital. The lungs were removed and washed with 1 × phosphate-buffered saline (PBS) buffer until the blood was removed. The connective tissue and blood vessels were removed while the lung tissues were cut into small pieces and centrifuged. The cell precipitate was collected while the supernatant was discarded. The lung cells were cultured with minimal essential medium (Life Technologies, Grand Island, NY, USA) complete medium including 10% fetal bovine serum, 4 mM L-glutamine, 25 mM HEPES (pH 7.2), 100 U/ml penicillin, 100 µg/ml streptomycin and 0.25 mg/ml amphotericin. After 10–14 days, the primary cell population were 80% confluent.
The cells were re-seeded with 1 × 105 cells per well in 48-well plates and transfected with two SEC-containing lentiviruses or eGFP-expressing lentiviruses for 24 h. The transfected lung cells (1 × 105/ml) were then treated with rmIL-4 (3000 U/ml) for 48 h. Eotaxin level in the supernatants was measured by ELISA.
OVA-induced allergic airway inflammation
Six to 8-week-old BALB/c mice were obtained from the Animal Center of the College of Medicine, National Taiwan University and sensitized by intra-peritoneal injection of 20 µg of OVA emulsified in 2 mg of aluminium hydroxide in a total volume of 200 µl PBS on day 0, and boosted with 50 µg of OVA emulsified in 2 mg of aluminium hydroxide on days 14 and 28. On day 37, different kinds of lentiviruses (1.5 × 106 IFU) were administered intra-tracheally into the anesthetized animals, whereas the positive control mice were administered PBS. The naïve group was not sensitized with OVA or administered with any treatment.
For challenge, all of the mice were treated intra-nasal with OVA (100 µg in a total volume of 40 µl in PBS) on days 40, 41, and 42. AHR was assessed 24 h after the last OVA-challenge. On day 44, sera and bronchio-alveolar lavage (BAL) fluid were collected. The lungs were partially cut and fixed with 10% neutralized buffered formalin. Sections, 5 µm thick, were prepared, subjected to hematoxylin and eosin staining, and examined by light microscopy.
Airway function determination
Airway function was measured by detecting changes in lung resistance (RL) in response to increasing doses of aerosolized methacholine (MCh) in anesthetized mice using a modification of the techniques described by Glaab et al. 22. Mice were anesthetized with 70–90 mg/kg pentobarbital sodium (Sigma, St Louis, MO, USA), tracheostomized, and mechanically ventilated at a rate of 150 breaths/min, a tidal volume of 0.3 ml, and a positive end-expiratory pressure of 3–4 cmH2O with a computer-controlled small animal ventilator (Harvard Rodent Ventilator, model 683, Southnatick, MA, USA). PE-50 tubing was inserted into the oesophagus to the level of the thorax, coupled with a pressure transducer (LDS Gould, Valley View, OH, USA).
Flow was measured by electronic differentiation of volume signal and changes, in pressure, flow, and volume were recorded. Pulmonary resistance was calculated using the software (Model PNM-PCT100 W, LDS PONEMAH Physiology Platform, LDS Gould). MCh aerosol was generated with an in-line nebulizer and administrated directly through the ventilator. The resistance of the oro-tracheal tube (0.45 cmH2O · s · ml−1) was subtracted from all airway resistance measurements. Data were expressed as RL in the ratio of RL after PBS nebulization.
OVA-specific antibody assay
ELISA determined sera anti-OVA immunglobulin (Ig)E, IgG1 and IgG2a antibody titers. Briefly, 96-well microtiter plates were coated with 1 µg/well OVA in NaHCO3 buffer, pH 9.6. After overnight incubation at 4 °C, the plates were washed and blocked with 3% bovine serum albumin (BSA) in PBS for 2 h at room temperature. Serum samples were diluted and added to each well overnight at 4 °C. Plates were then washed. Either biotin-conjugated anti-mouse IgE, IgG1, or IgG2a (0.5 mg/ml, Pharmingen, San Diego, CA, USA) diluted in 3% BSA-PBS buffer (1 : 500) was added for 45 min at room temperature. Avidin-conjugated horseradish peroxidase (1 : 5000, Pierce Biotechnology, Rockford, IL, USA) was then added for another 30 min at room temperature.
The reaction was developed by peroxidase substrate, 2,2′-azino-bis(3-ethylzothiazoline-6-sulfonic acid) diammonium salt (0.5 mg/ml). Absorbance was determined at 420 nm in a microplate reader. Antibody levels were compared to standard serum and IgG1, IgE and IgG2a concentrations in standard serum were arbitrarily assigned one ELISA unit (1 EU).
BAL fluid
BAL fluid was obtained by cannulating the trachea of each mouse and washing the airways with 1 ml of HBSS. The BAL fluid was centrifuged at 1500 r.p.m. for 10 min at 4 °C. The supernatant was stored at − 20 °C to determine eotaxin and IFN-γ production by ELISA. The cell pellets were re-suspended with secondary collected BAL fluid, washed with HBSS, and re-suspended in 1 ml of HBSS. Appropriate cells of BAL fluid (approximately 2 × 104) were cytospined and stained with Liu's stain. Based on morphology, a minimum of 200 cells were counted and classified as macrophages, lymphocytes, neutrophils, or eosinophils to analyse the inflammatory cell population in BAL fluid.
Statistical analysis
Differences between experimental groups were assessed by one-way analysis of variance followed by the Newman–Keuls multiple comparison test. p < 0.05 was considered statistically significant. Values for all measurements were expressed as the mean ± SEM.
Results
Intra-tracheal delivered eotaxin-shRNA decreased airway hyper-responsiveness
Two shRNA fragments were designed against eotaxin. Prior to the SEC-eotaxin administration to mice, the present study examined whether shRNA could suppress eotaxin expression efficiently in vitro. Primary lung cells were transfected with eoSEC3.1, eoSEC3.3 or eGFP for 24 h to screen which had the best inhibitory effect. Transfected cells were then treated with IL-4 (3000 U/ml) for 48 h and ELISA assayed eotaxin levels in the supernatant. eoSEC3.1 and eoSEC3.3 decreased the IL-4-stimulated eotaxin protein expression to 50% and 80% of FuGENE6/IL4-treated lung cells, respectively (see Supporting information, Figure S1).
The effect of eoSEC3.3 and the combination of eoSEC3.3 and IL-5 shRNAs (IL5SEC4) on airway hyper-responsiveness and eosinophilia in the murine model of asthma was also investigated by intra-tracheal administration of eoSEC3.3 and/or IL5SEC4 to BALB/c mice on day 37 after the first OVA sensitization. To determine the effect of shRNAs on airway function, the mice were exposed to MCh aerosols. Airway hyper-responsiveness was measured by an invasive technique in anesthetized mice.
As shown in Figure 1, intra-tracheal administration of PBS and SECneg led to a significant increase in RL to inhaled MCh compared to that of naïve mice, which were not sensitized but challenged with OVA on days 41–42. Administration of eoSEC3.3-containing lentiviruses resulted in significant decreases in RL to inhaled MCh compared to those of control mice, which were administered PBS or SECneg. Simultaneous administration of IL5SEC4- and eoSEC3.3-containing lentiviruses (IL5SEC4 + eoSEC3.3) resulted in a similar decrease in Penh and RL levels with single SEC-treated mice (Figure 1).
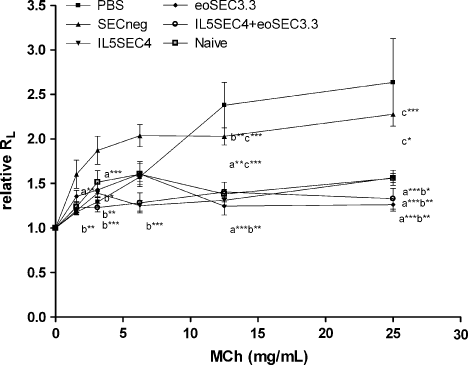
Local SEC-containing lentiviruses delivery reduced AHR as measured by invasive body plethysmography. Results, expressed as the mean ± SEM, are expressed for each methacholine concentration of lung resistance (RL) in the ratio of RL after PBS nebulization of three independent experiments (n ≥ 5). a*, p < 0.05; a**, p < 0.01; and a***, p < 0.001 compared to the PBS group. b*, p < 0.05; b**, p < 0.01; and b***, p < 0.001 compared to the SECneg group. c*, p < 0.05; c**, p < 0.01; and c***p < 0.001 compared to the naive group
Inhibition of eosinophilia by eotaxin shRNA-expressing lentiviruses
The influx of inflammatory cells into lungs 48 h after the last OVA challenge was examined. In BAL fluid of PBS- or SECneg-treated mice, eosinophil and neutrophil infiltration were significantly increased compared to naïve mice (Figure 2). Administration of eoSEC3.3-containing lentiviruses efficiently inhibited eosinophil infiltration into the lungs (Figure 2). Histopathologically, inflammatory cell infiltration around the airways was noted in the PBS and SECneg groups. However, inflammatory cell infiltrates were less in the eoSEC3.3-treated mice (Figure 3). In the IL5SEC4 + eoSEC3.3 group, inflammatory cell infiltration was similar to individual delivery of IL5SEC4 and eoSEC3.3. (Figures 2 and 3)
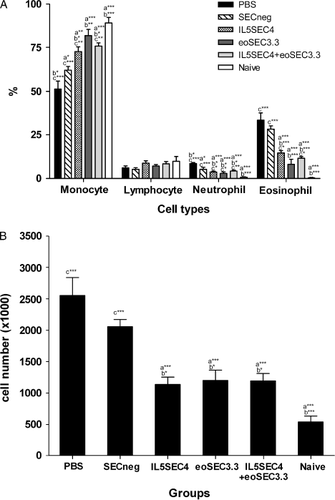
Delivery of local SEC-containing lentiviruses moderated airway eosinophilia. BAL fluid was obtained and the cell population was assayed as previously described (n = 5–7 per group). (A) Cell composition of BAL fluid. (B) Total cell numbers of BAL fluid. Results are expressed as the mean ± SEM. a*, p < 0.05; a**, p < 0.01; and a***, p < 0.001 compared to the PBS group. b*, p < 0.05; b**, p < 0.01; and b***, p < 0.001 compared to the SECneg group. c*, p < 0.05; c**, p < 0.01; and c***, p < 0.001 compared to the naive group
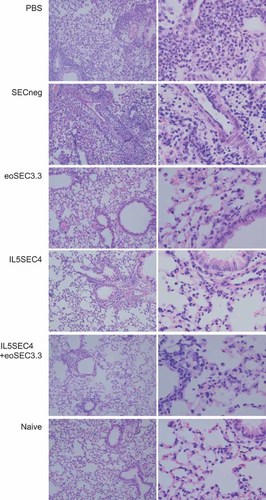
Both IL5SEC4 and eoSEC3.3 decreased the infiltration of lymphocytes in the lungs. On day 44, lungs of different mice groups were excised after collecting BAL fluid and fixed with 10% buffered formalin. Sections, 5 µm thick, were prepared and subjected to hemotoxylin and eosin staining (magnification: left, × 100; right, × 400)
eoSEC3.3 suppressed IL-5 and eotaxin in BAL fluid
Eotaxin is an important chemokine in the recruitment of eosinophils, basophils, and Th 2 lymphocytes in the lungs, and therefore we evaluated the eotaxin levels in the BAL fluid. The results showed that eoSEC3.3 treatment significantly suppressed eotaxin and IL-5 production. However, IL5SEC4 + eoSEC3.3 did not result in a more effective reduction of eotaxin or IL-5 (Figure 4).
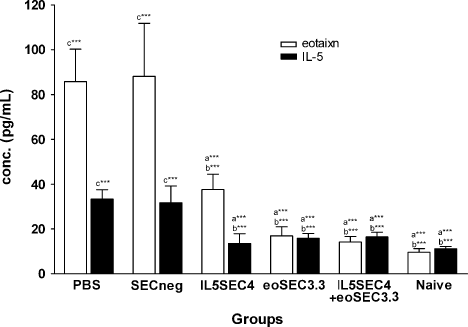
Eotaxin and IL-5 levels in BAL fluid decreased in SEC-treated mice. The levels were measured by ELISA (n = 5–7 per group) and the results are expressed as the mean ± SEM. a***, p < 0.001 compared to the PBS group. b***, p < 0.001 and b**, p < 0.01 compared to the SECneg group. c***, p < 0.001 compared to the naive groups
Delivery of SEC-containing lentiviruses did not affect sera allergen-specific immunoglobulin levels
On day 44, the mice were bled to examine serum immunoglobulin levels. Compared to the naïve group, OVA-specific IgE in OVA sensitized groups, including PBS-, SECneg-, IL5SEC4-, eoSEC3.3, and IL5SEC4 + eoSEC3.3-treated groups, were significantly increased (Figure 5). However, OVA-specific IgE levels were not significantly different among the SEC, SECneg, and PBS-treated mice (Figure 5). There was also no difference among the OVA-specific IgG2a and IgG1, Th2 and Th1 response-related immunoglobulins.
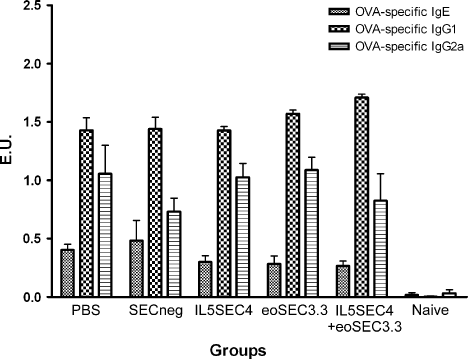
There was no difference in serum OVA-specific IgE, IgG1, and IgG2a levels among the different SEC-treated mice. On day 44, blood samples were obtained and OVA-specific IgE, IgG1, and IgG2a concentrations were measured by ELISA (n = 7–8 per group)
Discussion
Th2 related cytokines, such as IL-4, IL-5, and IL-13, are important in the development of allergic asthma. Among them, IL-5 plays a key role in the development, recruitment, activation, and survival of eosinophils 4, 5, 23. By contrast, eotaxin is the major chemokine for the recruitment and degranulation of eosinophils 3. Decreased eosinophils in the airway lumen of IL5SEC4- or eoSEC3.3-treated mice may be caused by the suppression of the differentiation and recruitment of eosinophils from the bone marrow. Nonetheless, both IL-5 and eotaxin are important for the survival of eosinophils.
Recent studies have demonstrated that apoptotic eosinophils in sputum from asthmatic patients correlate negatively with IL-5 and eotaxin levels 9. In the present study, decreased eosinophil infiltration might be due to the shorter lifespan of emigrated eosinophils in IL-5- or eotaxin-shRNA-treated mice. In addition, the number of airway eosinophils in SEC-treated mice was reduced by only approximately 28–45% compared to that of SECneg-treated mice. This implies that other cytokines, such as IL-3 24 and GM-CSF 25, may also be important in eosinophil survival and persistence in tissues. However, the mechanism of less eosinophilic infiltration of shRNA-treated mice warrants further study.
The IL-5 and eotaxin levels in the BAL fluid decreased with respect to IL5SEC4- or eoSEC3.3-treated mice. The reduced eotaxin levels in IL5SEC4-treated mice may be due to the influence exerted on the recruitment and survival of eosinophils, which are the sources of eotaxin. Eosinophils are also the source of IL-5 and less eosinophil infiltration and activation in the lungs of eoSEC3.3-treated mice results in reduced IL-5 levels in BAL fluid.
Allergic asthmatic responses can be divided into the early and late phases. Cross-linking of allergic specific IgE bound to the surface of mast cells by allergen, degranulation of these cells, and mediator release, are all responsible for the early allergic response. In the present study, circulating IgE levels are not different between IL5SEC4-treated and control mice (Figure 5), but other late allergic responses significantly improved. shRNA was applied as a modulatory tool to suppress the IL-5 and eotaxin expression locally, which successfully alleviated inflammation, eosinophilic infiltration, and airway hyper-responsiveness, but not serum levels of OVA-specific antibodies. Local effects of IL-5 and eotaxin may explain why levels of OVA-specific IgE, IgG1, and IgG2a were not significantly different among the study groups.
In a recent study in mice, intra-airpouch administration of CAT-213, which is an antibody that neutralizes human eotaxin-1, attenuated dermal eosinophilia induced by human eotaxin-1 26. In another recent clinical study, CAT-213 reduced sub-mucosal eosinophil infiltration induced by allergen in patients with rhinitis 27, 28. However, there was no evidence of the therapeutic effect of CAT-213 in asthma patients or in an asthmatic murine model.
Anti-IL-5 therapy results in an improvement of symptoms in patients with eosinophilic esophagitis 29 and chronic rhinitis with nasal polyposis 22, 30, who have lymphocytic variants of hyper-eosinophilic syndromes. By contrast, anti-IL-5 therapy shows only moderate or no clinical effects in patients with bronchial asthma or atopic eczema. Studies on asthmatic patients have shown a dramatic suppression of circulating eosinophils and reduced sputum eosinophilia after allergen challenge following a single intravenous administration of mepolizumab, an anti-IL-5 antibody. However, there has been no significant effect on late asthmatic response or airway hyper-responsiveness induced by histamine 31-33. These results suggest that anti-IL-5 and anti-eotaxin treatment has local effects but does not affect the Th1/Th2 lymphocyte function and cytokine secretion in peripheral blood 32.
In the present study, the simultaneous inhibition of eotaxin and IL-5 does not have a better modulatory effect in the OVA-induced murine model of asthma compared to the suppression of eotaxin or IL-5 alone. In a previous study, GATA-3 shRNA alone efficiently inhibited OVA-induced airway inflammation 34. A combination of IL-4 and IL-13 shRNAs can inhibit AHR and eosinophilia more efficiently than a single delivery of IL-4- or IL-13-shRNA (unpublished data). Other Th2 cytokines or transcriptional factors may also play important roles in allergen-induced airway inflammation.
One potential problem of RNAi-based drugs is the induction of interferon response, which causes nonspecific inhibition of gene expression. Previous studies have also shown that lentiviral mediated-delivery of shRNA can induce a subset of interferon-stimulated genes 35. The results obtained in the present study show that SECneg-treated mice have some reduction in both AHR and IL-5 and eotaxin levels in BAL fluid (Figures 1 and 4) compared to PBS-treated mice. In a previous study, lentiviruses used to deliver shRNA did not increase the expression of IFN response genes, including 2′,5′-oligoadenylate synthetase and myxovirus-resistance protein A, in lungs of lentiviruses-treated mice compared with naïve mice 34. Therefore, the present study excluded the possibility that the IFN response induced by lentiviruses reduced AHR. Previous studies have also demonstrated that shRNA causes nonspecific gene regulation by partial homology 36, 37. However, it still remains unclear whether unanticipated off-target effects occurred in the present study. More studies are needed to understand the mechanisms of nonspecific off-target effects.
In summary, a single treatment of SEC-lentivirus against eotaxin or IL-5 in an OVA-induced murine asthma model can efficiently reduce levels of IL-5 and eotaxin in BAL fluid, AHR, and eosinophil infiltration. Concomitant delivery of IL-5 and eotaxin shRNAs can likewise modulate asthma symptoms, although the modulatory effects are not better than those with single shRNA treatment.
Supporting information
Supporting information may be found in the online version of this article.
Acknowledgements
The authors express their thanks to Dr Lih-Hwa Hwang of the Graduate Institute of Microbiology, National Taiwan University for providing the lentivirus-producing plasmids.




