Exploring gene-deleted adenoviral vectors for delivery of short hairpin RNAs and reduction of hepatitis B virus infection in mice
Abstract
Background
RNA interference based therapeutic approaches hold promise for the treatment of patients chronically infected with hepatitis B virus (HBV). To conquer HBV infection, long-term suppression of target transcripts in all hepatocytes without toxic effects may be required. The present study explored gene-deleted adenoviral vectors (GD-AdV) lacking all viral coding sequences for delivery of the previously described short hairpin RNA (shRNA) HBVU6no.2, which was demonstrated to result in post-transcriptional knock-down of HBV transcripts.
Methods
We established conditions for shRNA delivery expressed from GD-AdV in vitro and in vivo and observed up to 96% shRNA-mediated knockdown of luciferase expressed in mouse liver. To investigate in vivo efficacy of HBVU6no.2 expressed from a GD-AdV, we explored a transient and a transgenic mouse model for HBV infection.
Results
We observed an up to 68% drop in serum HBV surface antigen (HBsAg) levels in the transient and the transgenic mouse model for HBV infection, respectively. Interestingly, we detected an up to 86% drop in HBsAg levels in both animal models after administration of a control GD-AdV encoding β-galactosidase. In concordance with reduced serum HBsAg levels, we observed reduced HBV replication as demonstrated by Southern blot analysis of HBV genomes.
Conclusions
The present study demonstrates that GD-AdV can be used against HBV infection but the design of DNA sequences including shRNAs contained in the vector and virus–host interactions during superinfection needs to be carefully considered. Copyright © 2008 John Wiley & Sons, Ltd.
Introduction
The enveloped hepatitis B virus (HBV), a member of the hepadnavirus family, can cause lifelong infection, cirrhosis of the liver, acute and chronic hepatitis, and liver cancer 1. Worldwide, two billion people have been infected with HBV, 350 million have chronic infection 2. Current therapeutic strategies for HBV infection reduce symptoms of liver disease but complete eradication of the infection is one of the major challenges of our time. Therapeutic options include treatment with lamivudine and/or interferon-α, adefovir and entecavir 3, but resistance to these drugs due to the relatively high mutation rate of HBV genomes remains a problem 4. Gene therapy approaches for the treatment of HBV infection based on the introduction of gene drugs into hepatocytes by various viral and nonviral gene transfer systems may represent an attractive alternative to conventional treatment strategies. In previous studies, gene drugs included antisense RNA and DNA approaches 5, 6, ribozymes 7, 8, dominant negative HBV antigen mutants 9, single chain antibodies 10, gene products for enhancement of anti-HBV immunity, and small hairpin RNAs (shRNA). It was demonstrated that expression of shRNAs expressed from a nonviral 11 or a viral vector 11-16 leads to RNA interference-mediated post-transcriptional reduction of HBV target transcripts. The present study focused on recombinant adenoviral vectors for shRNA delivery against HBV, but prototype foamy virus 13 and adeno-associated viruses (AAV) 12, 14 were also evaluated previously.
Recombinant adenoviral vectors remain an attractive tool for gene therapy approaches. Adenovirus has several advantages over other viral based gene therapy approaches, which include the ability to produce high titers, an efficient infection of a broad range of cell types, and the ability to infect dividing and nondividing cells. First generation (fg) adenoviral vectors, which are deleted for the immediate early genes E1 and E3, show direct cytotoxic effects due to the production of immunogenic viral proteins or toxicity resulting from a cell-mediated immune response 17. After performing further improvements, a system for production of gutless gene-deleted adenoviral vectors (GD-AdV) lacking all viral coding sequences was generated 18. This allows the transfer of up to 35 kb of foreign DNA with long-term expression in rodents and nonhuman primates. Various studies showed that GD-AdV results in a significantly reduced cytotoxic response and long-life phenotypic correction in mouse models 19-21. Recent studies also explored recombinant adenoviral vectors with deletions of adenoviral early genes E1 and E3 for delivery of shRNA expression cassettes, including shRNA approaches against HBV infection 15, 16. However, as mentioned above, these early generation adenoviruses may result in cytotoxic side-effects. Furthermore, there is accumulating evidence that adenovirus-associated RNA (VA RNA) still expressed from these vectors interferes with the shRNA/micro RNA pathways 22. By contrast, no VA RNA transcription units are present in the genome of GD-AdV, which may increase shRNA potency.
The present study aimed to evaluate GD-AdV for the delivery of shRNAs. To test the efficacy of GD-AdV in a therapeutic setting, we aimed to suppress HBV transcripts using a previously published shRNA coding sequence against HBV (HBVU6no.2). HBVU6no.2 was demonstrated to be highly efficient for RNA interference-mediated knockdown of HBV transcription products using a nonviral approach 11 and AAV vectors 12. However, after systemic administration of an AAV2/8 vector encoding HBVU6no.2 at high dose, lethality was observed in mice 12.
Materials and methods
DNA constructs and adenoviral vector production
The nucleotide shRNA expression cassettes against luciferase and HBV (HBVU6no.2) were described previously 11, 12, 23. Throughout the study, the plasmid, encoding shRNA against HBV, is referred to as pHBVU6no.2. All shRNAs were transcribed under the control of the U6 promoter. Gene-deleted adenoviral vectors (GD-AdV) with shRNA expression cassettes (FTC/lucRNAi and FTC/HBVU6no.2) and the vectors FTC-hFIX-attB-(FRT)2 and AdFTC/cFIX/ChMAR without shRNA expression cassette were based on the adenoviral backbone AdFTC 19. As additional stuffer DNA FTC/lucRNAi and FTC/HBVU6no.2 contained a transgene expression cassette encoding the human coagulation factor IX (hFIX) 19. For construction of the vector pFTC/lucRNAi, the bacteriophage integrase phiC31 recognition site attB and the inverted repeats for Sleeping Beauty transposase-mediated somatic integration were flanked by the Flp recognition sites FRT. FTC-hFIX-attB-(FRT)2, a GD-AdV with a split hFIX expression cassette, and AdFTC/cFIX/ChMAR containing a canine FIX expression cassette, were used as control vectors and are described elsewhere 24, 25. Further details regarding the cloning strategy and DNA sequences contained in all GD-AdV can be obtained upon request from the authors. The GD-AdV HD28E4LacZ, which contained a transgene expression cassette encoding beta-galactosidase (β-gal) under the control of the cytomegalovirus promoter, is described elsewhere 26. The fg adenoviral vector fgAdluc was based on the adenoviral plasmid pAdHM4 27. The luciferase expression cassette contained in fgAdluc encodes luciferase under the control of Simian Virus 40 (SV40) promoter and was based on the pGL3-control vector (Promega, Madison, WI, USA). The plasmid pTHBV2 with the complete HBV genome is described elsewhere 28 and the plasmid pRSV.hAAT.bpA (phAAT) expressed the human α1-antitrypsin (hAAT) coding sequence transcribed under the control of the rous sarcoma virus (RSV) promoter 29.
GD-AdV production was based on a previously published system 26. In brief, plasmids pFTC/lucRNAi, pFTC/HBVU6no.2 and pFTC-hFIX-attB-(FRT)2 were linearized by NotI digest and transfected into 116 cells 26. For amplification of HD28E4LacZ, the respective plasmid was linearized with PmeI. Eighteen hours post-transfection, 116 cells were infected with the helper virus AdNG163R-2 26 and three serial passaging steps were performed. Three litres of 116 cells were grown in suspension and co-infected with the lysate from serial passaging step 3 and the helper virus AdNG163R-2. Forty-eight hours later, cells were harvested. To purify recombinant adenoviral vectors, we performed CsCl gradients. The amplification and purification of the fg adenoviral vectors (fgAdluc and fgAdhFIX) are described elsewhere 19.
To determine transducing units of FTC/lucRNAi, pFTC/HBVU6no.2 and FTC-hFIX-attB-(FRT)2 in final vector preparations (all vectors contain hFIX coding sequences), we performed a Southern blot analysis as described previously 19. In brief, HeLa cells were infected with different volumes of the GD-AdV preparations and defined multiplicities of infection (MOI) of the fg adenoviral vector fgAdhFIX 19. Three hours post-infection, cells were harvested and genomic DNA isolated. This step was followed by a Southern blot probed with a hFIX complementary DNA probe (HindIII/EcoRI fragment from pAAVCM2). The intensity of bands obtained with the GD-AdV was compared with the standard curve, which displayed bands of defined MOI of the fg vector fgAdhFIX. Infectious units of the GD-AdV HD28E4LacZ were determined by quantification of blue forming units. In brief, HeLa cells were infected and blue forming units were counted. For quantification of transducing units contained in the final vector preparation of the fg vectors fgAdluc and fgAdhFIX, we performed a plaque forming assay.
Furthermore, for all viral vector preparations, viral DNA was released from virions obtained from CsCl gradients by incubation in TE buffer with 0.1% sodium dodecyl sulfate (SDS). The A260 was measured to determine the viral titre. An A260 of 0.01 indicates 107 adenoviral transducing units per µl.
Cell culture and in vitro studies
The cell line 2.2.15 (stably transduced with the HBV genome) and the human hepatoma cell line Huh7 were maintained in Dulbecco's modified Eagle's medium (DMEM) media supplemented with L-glutamine, penicillin/streptomycin, non-essential amino acids (Gibco BRL, Gaithersberg, MD, USA), and fetal bovine serum (10%). HeLa cells were grown in DMEM media supplemented with L-glutamine, penicillin/streptomycin, and fetal bovine serum (10%).
For in vitro assays using adenovirus in HeLa cells, cells were co-infected with the fg adenoviral vector fgAdluc using a MOI of 10 and the GD-AdV FTC/lucRNAi expressing shRNA against luciferase at a MOI of 10, 50, 100 and 500. To infect all cells with the identical total amount of virus, groups were spiked with an irrelevant GD-AdV (AdFTC/cFIX/ChMAR) 25. Luciferase levels were measured 3 days post-infection using a luciferase reporter assay system (Promega).
For plasmid based assays in Huh-7 cells, either 0.67 µg of the plasmid pFTC/HBVU6no.2 (used for production of the GD-AdV FTC/HBVU6no.2) or 0.67 µg of pHBVRNAno.2 were co-transfected with 0.67 µg of pTHBV2 into a well of a six-well plate. Control groups received stuffer DNA, respectively. As an internal control and for normalization, all groups received the plasmid pRSV.hAAT.bpA. Hepatitis B virus antigen (HBsAg) levels in the supernatant were measured 3 days post-transfection by enzyme immunoassay (Abbott Laboratories, Abbott Park, IL, USA).
For studies in Huh7 cells using adenoviral vectors, cells were transfected with the plasmid pTHBV2. Twenty-four hours post-transfection, cells were transduced with the GD-AdV FTC/HBVU6no.2 and FTC-hFIX-attB-(FRT)2 at a MOI of 300, 30 and 10. Control groups received pTHBV2 and pHBVU6no.2, the plasmid pTHBV2 only, or stuffer DNA. As an internal control and for normalization, all groups received the plasmid pRSV.hAAT.bpA, respectively. To analyse shRNA-mediated knockdown during an established HBV infection, 2.2.15 cells (stably transduced with the HBV genome) were infected with FTC/HBVU6no.2 and FTC-hFIX-attB-(FRT)2 using a MOI 100 and MOI 500. HBsAg levels in the supernatant were measured 3 days post-infection by enzyme immunoassay (Abbott Laboratories, Abbott Park, IL, USA).
Animal studies
C57Bl/6 mice (Jackson Laboratories, West Grove, PA, USA) and HBV transgenic mice 30 were kept under the Stanford rules and regulations. Due to the fact that varying starting levels of serum HBsAg levels are present when using this HBV transgenic mouse line, each individual was pre-screened for HBsAg levels before treatment. For transient HBV studies, C57Bl/6 mice (8 weeks old) were co-injected via high-pressure tail vein injection 11 with 12 µg of pTHBV2 and either 5 µg of pHBVU6no.2 or stuffer DNA. As an internal control for transduction efficiencies, all individuals received pRSV.hAAT.bpA. This plasmid encodes hAAT, which can be monitored by enzyme-linked immunosorbent assay (ELISA) in mouse serum. Adenoviral vectors were diluted in Dulbecco's phosphate-buffered saline (DPBS) and a total volume of 200 µl per mouse was injected. Blood samples were obtained by retro-orbital bleeding. For bioluminescent images and measurements of emitted light units, we used an in vivo imaging system (IVIS; Xenogen, Alemeda, CA, USA). To study transduction efficiencies after adenoviral infection, mice were injected with the GD-AdV HD28E4LacZ. Frozen sections of mouse liver were stained for β-gal activity as described previously 31.
HBsAg levels in mouse serum were monitored by enzyme immunoassay (Abbott Laboratories, Abbott Park, IL, USA). SGPT (alanine aminotransferase activity) assays were performed by using a diagnostic kit for colorimetric determination of SGPT (Sigma procedure no. 505-OP; Sigma, St Louis, MO, USA).
Mouse serum hFIX antigen levels 19 and serum hAAT levels were determined by ELISA. Normal hFIX serum levels were 5000 ng/ml.
Southern blot analyses
For genomic DNA isolation, mouse liver was removed and used for DNA extraction as previously described 19. Blots were hybridized with [α-P32]–deoxycytidine triphosphate (dCTP)-labelled cDNA lacZ probe (HindIII/StuI fragment from pRSV/β-Gal) and an HBV probe (EcoRI/AccI digested plasmid pTHBV2). To quantify the adenoviral vector genome copy number per cell and to generate a standard curve, we digested the plasmid pHD28E4LacZ19 with the restriction enzyme HindIII.
Northern blot analyses
To test for small hairpin RNA expression and to verify processing into mature small inhibitory RNA molecules, we performed a northern blot analysis. These in vitro studies were performed in 293 cells (human embryonic kidney cells) which were grown in DMEM media supplemented with L-glutamine, penicillin/streptomycin, and fetal bovine serum (10%). 293 cells were seeded in six-well plates and infected with the adenoviral vector FTC/HBVU6no.2 at a MOI of 20. As a positive control for HBVU6no.2 short hairpin RNA expression we transfected the previously described plasmid pHBVU6no.2 11 and the plasmid pBS/hFIX/HBVU6no.2. The plasmid pBS/hFIX/HBVU6no.2 represents a subclone of pFTC/HBVU6no.2. Both vectors contain the identical expression cassette for hFIX and HBVU6no.2. Other controls received either pBS/hFIX/lucRNAi expressing shRNA against luciferase and pBS/hFIX/empty without shRNA expression cassette. Forty-eight hours post-transfection and infection, cells were harvested and small RNAs isolated using a Trizol (Invitrogen, Carlsbad, CA, USA) based procedure. Total RNA (20 µg) was separated on a 17% polyacrylamide gel containing 7M urea in 0.5× tris-borate-ethylenediaminetetracetic acid (TBE) buffer. As a positive control also representing the length marker, we ran the reverse oligonucleotide of the first 21 nucleotides of the DNA oligo probe HBV-sense (see below) and the first 21 and 25 nucleotides of the DNA oligo probe Luc-antisense (see below). We performed a semi-dry transfer in 0.5× TBE onto a Hybond-N + membrane (GE Healthcare, Pittsburgh, PA, USA). Northern hybridizations were performed with the following radioactively end-labelled DNA oligonucleotide probes: HBV-sense (5′-CTC AGT TTA CTA GTG CCA TTT GTT C-3′), HBV-antisense (5′-GAA CAA ACG GCA CTA ATA AAC CGA G-3′), Luc-antisense (5′-GGA TTC CAA CTC AGC GAG AGC CAC CCG AT-3′), and as an internal control the oligonucleotide 5.8 S-rRNA 32 (5′-TTC ATC GAC GCA CGA GCC GAG TGA TCC-3′). Hybridization was performed in Denhardt's solution at 50 °C. Blots were washed twice for 10 min with 5 × SSC/0.1% SDS and once for 10 min with 1 × SSC/0.1% SDS at 50 °C.
Results
Inhibition of luciferase expression by luciferase RNAi-mediated RNA interference from a GD-AdV in vitro and in vivo
Various viral and nonviral vector systems have been studied in recent years for the delivery of shRNA expression cassettes against HBV transcripts 12-16. However, the in vitro and in vivo efficacy of GD-AdV lacking all viral coding sequences remains to be studies. To determine optimized conditions for shRNA delivery, we co-transduced HeLa cells with the GD-AdV FTC/lucRNAi expressing shRNA against luciferase (Figure 1B) and the fg adenoviral vector fgAdluc expressing luciferase (Figure 1A). We observed a 77% reduction in luciferase expression at a 1 : 1 ratio (fgAdluc: FTC/lucRNAi). At a 1 : 5, 1 : 10 and 1 : 50 ratio (fgAdluc: FTC/lucRNAi), we measured up to a 94% reduction in luciferase expression levels (Figure 2A). To demonstrate that this effect was specifically related to shRNA-mediated transgene silencing, control cells received an adenoviral vector with an unrelated shRNA expression cassette (FTC/HBVU6no.2; Figure 1C). As shown in Figure 2B, there was no reduction of luciferase expression in control cells.
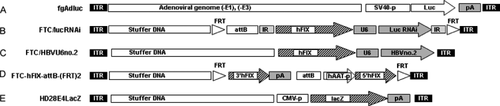
DNA sequences contained in the recombinant adenoviral vector genomes. (A) The fg adenoviral vector fgAdluc contained a luciferase expression cassette encoding luciferase under the control of Simian Virus 40 (SV40-p) promoter. pA, polyadenylation signal. (B) The adenoviral vector FTC/lucRNAi contained an expression cassette encoding a previously described shRNA against luciferase (Luc RNAi) under the control of the U6 promoter 11, 12. The stuffer DNA contained in the GD-AdV was based on the vector pAdFTC 19. In addition, this vector contained a previously described hFIX expression cassette 19 and the following DNA sequences: AttB, bacteriophage integrase phiC31 attachment site; IR, inverted repeats for Sleeping Beauty transposase; FRT, recognition sites for Flp-mediated recombination. (C) The adenoviral vector FTC/HBVU6no.2 contained the shRNA expression cassette HBVU6no.2 and the identical hFIX expression cassette as FTC/lucRNAi. (D) The control vector FTC-hFIX-attB-(FRT)2 without shRNA coding sequence contained a previously described split transgene expression cassette for hFIX under the control of the liver specific human alpha-1-antitrypsin promoter (hAAT-p) 24. (E) The GD-AdV HD28E4LacZ was described previously 26 and contained the β-galactosidase cDNA (lacZ) under the control of the murine cytomegalovirus (CMV-p) promoter
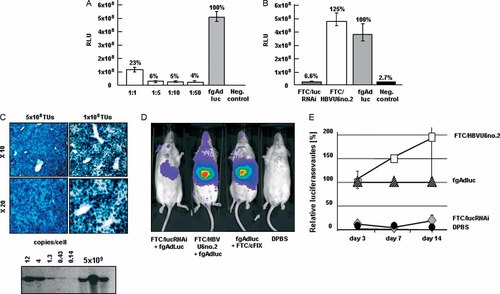
Inhibition of luciferase expression by luciferase RNAi-mediated RNA interference from a GD-AdV in vitro and in vivo. (A) Optimization of RNA interference-mediated post-transcriptional silencing by GD-AdV with a shRNA expression cassette against luciferase. HeLa cells were co-transfected with the first generation adenoviral vector fgAdluc at a MOI of 10 and the shRNA expressing adenovirus FTC/lucRNAi at a MOI of 10, 50, 100 and 500, respectively. Data are the mean ± SD (n = 3 per group). Luciferase levels were measured 3 days post-infection. (B) Inhibition of luciferase expression by the GD-AdV FTC/lucRNAi. The negative control group received the adenoviral vector FTC/HBVU6no.2 with an irrelevant shRNA expression cassette. Data are the mean ± SD (n = 3 per group). (C) Robust mouse liver transduction with a GD-AdV. Mice were injected with 1 × 109 and 5 × 109 TUs of the GD-AdV HD28E4LacZ (n = 3 per group). A representative picture of a liver section after X-gal staining is shown. To determine the vector genome copy number per cell, mouse liver was analysed by Southern blot analysis (bottom panel). (D) RNA interference in FVB mice (n = 3 per group). Representative images are shown of light emitted from mice that received the luciferase expressing vector and a second adenoviral vector that expressed short hairpin RNA against luciferase (FTC/lucRNAi). Control groups received an adenoviral vector with an irrelevant shRNA (FTC/HBVU6no.2), no shRNA (AdFTC/cFIX/ChMAR 25), or the vehicle control. Representative individuals of each group are shown. (E) Time course of luciferase inhibition by a GD-AdV expressing shRNA against luciferase in FVB mice. Data are the mean ± SD (n = 3 per group)
With respect to efficient shRNA-mediated post-transcriptional gene silencing in mouse liver and for the treatment of HBV infection, transduction of all hepatocytes may be required. Thus, C57Bl/6 mice were transduced with 5 × 109 and 1 × 109 transducing units (TUs) of the GD-AdV HD28E4LacZ (Figure 1E). This vector contained a transgene expression cassette encoding beta-galactosidase (β-gal) under the control of the cytomegalovirus promoter. Three days post-injection, livers sections were stained for β-gal expression. We observed a transduction rate of 100% of mouse hepatocytes after injection of 5 × 109 TUs of the GD-AdV HD28E4LacZ (Figure 2C). A dose of 1 × 109 TUs was not sufficient for transduction of all mouse hepatocytes (Figure 2C). We detected approximately 12 adenoviral vector genome copies per liver cell after administration of 5 × 109 TUs of the adenoviral vector HD28E4LacZ, as demonstrated by Southern blotting (Figure 2C). Thus, for the following experiments demonstrating RNA interference-mediated transgene silencing in vivo, we used a minimum of 5 × 109 TUs of the shRNA expressing vector.
To demonstrate in vivo efficacy, FVB mice (n = 3 per group) were co-injected with 1.2 × 1010 TUs of the adenoviral vector FTC/lucRNAi and 4 × 109 TUs of the fg adenoviral vector fgAdluc. Control mice were co-injected with 4 × 109 TUs fgAdluc and either 1.2 × 1010 TUs of the GD-AdV AdFTC/cFIX/ChMAR 22 without shRNA coding sequence or the GD-AdV FTC/HBVU6no.2 (Figure 1C) with an irrelevant shRNA coding sequence. Negative control mice received the vehicle for adenoviral delivery (DPBS). We found up to a 96% reduction of luciferase expression 7 days post-injection in mice that received the adenoviral vectors FTC/lucRNAi and fgAdluc. Figure 2D shows representative images of light emitted from mice in all groups. The effect of shRNA-mediated post-transcriptional silencing of luciferase expression lasted for 2 weeks (Figure 2E). Long-term studies were not feasible because luciferase expression levels from the fg adenoviral vector fgAdluc decreased to undetectable levels 4 weeks post-injection. We concluded that GD-AdVs are sufficient to mediate RNA interference-mediated post-transcriptional gene silencing in vitro and in vivo.
Inhibition of hepatits B virus infection in vitro
Using a nonviral gene therapy approach, it was demonstrated that a 25-mer shRNA against HBV (HBVU6no.2) driven by the polymerase III U6 promoter resulted in efficient knockdown of HBV infection in vitro (up to 94% reduction in HBsAg levels, a marker for progression of HBV infection) 11. HBsAg of HBVU6no.2 treated mice was reduced by 84.5% in a transient animal model for HBV infection 11. Interestingly, another study reported that the identical shRNA, HBVU6no.2, was lethal at high dose in HBV transgenic mice and regular mice in the context of an AAV2/8 vector 12. It is speculated that this phenomenon was due to oversaturation of the micro RNA/shRNA pathways.
The present study evaluated GD-AdV for delivery of HBVU6no.2. To verify expression and processing of the shRNA against HBV and luciferase contained in our shRNA expression plasmids and in the viral vector FTC/HBVU6no.2, we performed a northern blot analysis. As shown in Figure 3, we detected expression and processing of the highly efficient shRNA against luciferase (Figure 3A) and the shRNA against HBV (Figure 3B). Notably, in contrast to the shRNA expression cassette against luciferase, we found that the ratio of hairpin to processed siRNA for HBVU6no.2 appears to be shifted towards the hairpin (Figures 3A and 3B). This was in concordance with a previously described study showing the efficacy of HBVU6no.2., where the identical hairpins were delivered by AAV2/8 vectors 12 and analysed by northern blotting.
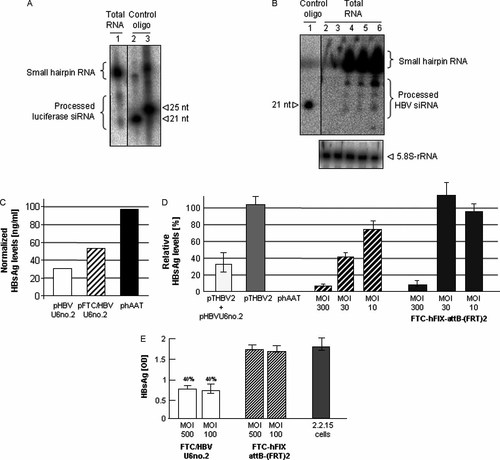
Expression of shRNA and inhibition of hepatits B virus infection in vitro. (A) Hairpin expression and processing of the shRNA against luciferase. Cells were transfected with the luciferase shRNA expression plasmid pBS/hFIX/lucRNAi. As a standard, we loaded the 21-mer and the 25-mer of the reverse sequence of the oligonucleotide Luc-antisense on the gel. The blotted membrane was probed with the radioactively labelled probe Luc-antisense. (B) Northern blot analysis to show expression and processing of shRNA against HBV. We infected 293 cells with the viral vector FTC/HBVU6no.2 at a MOI of 20 (lane 6) and performed a Northern blot analysis. Positive controls were transfected with the shRNA expression vectors pHBVU6no.2 11 (lane 4) and pBS/hFIX/HBVU6no.2 (lane 5). Negative controls received the plasmids pBS/hFIX/empty (lane 2) and pBS/hFIX/lucRNAi (lane 3) containing an irrelevant shRNA against luciferase. The membrane was hybridized with the radioactively labelled oligonucleotides HBV-sense and HBV-antisense. To show that equal amounts of total RNA were loaded on the gel, the membrane was subsequently hybridized with the 5.8S-rRNA oligonucleotide. As a standard, we loaded the 21-mer of the reverse sequence of the oligonucleotide HBV-antisense. (C) DNA sequences contained in the GD-AdV FTC/HBVU6no.2 induce silencing of HBsAg. Huh-7 cells were co-transfected with pTHBV2 and pHBVRNAno.2, or pFTC/HBVU6no.2 or stuffer DNA. As an internal control, all groups received the plasmid pRSV.hAAT.bpA 29. HBsAg levels in the supernatant were measured 2 days post-infection by enzyme immunoassay (n = 2 per group). (D) RNA interference induced silencing of HBsAg levels in the human hepatoma cell line Huh7. Huh7 cells were transiently transfected with the plasmid pTHBV2 containing the HBV genome. Twenty-four hours post-transfection, cells were transfected with the GD-AdV FTC/HBVU6no.2 or FTC-hFIX-attB-(FRT)2 at a MOI of 300, 30 and 10. Control groups were either co-transfected with the plasmids pTHBV2 and pHBVU6no.2, the plasmid pTHBV2 only or the negative control plasmid phAAT. HBsAg levels in the supernatant were measured 2 days post-infection by enzyme immunoassay. Data are the mean ± SD (n = 3 per group). (E) RNA interference induced silencing of HBsAg from an adenoviral vector in 2.2.15 cells (stably transfected with the HBV genome). Control groups either were untreated or received the GD-AdV FTC-hFIX-attB-(FRT)2. HBsAg levels in the supernatant were measured 3 days post-infection by enzyme immunoassay. Data are the mean ± SD (n = 3 per group)
To demonstrate that the shRNA sequences against HBV contained in our adenoviral vector FTC/HBVU6no.2 (Figure 1C) are functional, we performed an in vitro study solely based on plasmid transfection. Human hepatoma cells (Huh7) were co-transfected with pTHBV2 (a plasmid that contained the complete HBV genome for establishment of transient HBV infection) and either the plasmid pHBVU6no.2 or pFTC/HBVU6no.2. We observed a 68% and 47% drop in HBsAg levels, respectively (Figure 3C). In a further step, we evaluated the potency of the adenoviral vector FTC/HBVU6no.2 (Figure 1C). Huh7 cells were transiently transfected with the plasmid pTHBV2. This step was followed by infection with either the GD-AdV FTC/HBVU6no.2 or the control vector FTC-hFIX-attB-(FRT)2 (Figure 1D) at a MOI of 300, 30 and 10. For groups that received GD-AdV FTC/HBVU6no.2, we observed a 94%, 60% and 30% reduction in HBsAg levels in the supernatant of Huh7 cells, respectively (Figure 3D). We observed vector related cellular toxicity at the highest adenoviral vector dose (MOI = 300). This observation may explain the reduction in HBsAg levels in the group that received the highest dose of the control vector FTC-hFIX-attB-(FRT)2 (Figure 3D). The positive control group received the plasmids pTHBV2 and pHBVU6no.2 for RNA interference induced silencing of HBV transcripts. In concordance with results presented in Figure 3C, we observed a 75% reduction in HBsAg levels in the supernatant of Huh7 cells of this group (Figure 3D). All groups, including the negative control group, received the plasmid pRSV.hAAT.bpA encoding secreted hAAT for normalization of transfection efficiencies.
2.2.15 cells are stably transfected with the HBV genome and thus pre-existing HBV infection is present. To test susceptibility of 2.2.15 cells to superinfection with adenovirus, we infected these cells at various MOIs with the GD-AdV expressing the marker gene β-gal. We found that a MOI of 100 was sufficient to transduce 100% of 2.2.15 cells (not shown). Thus, 2.2.15 cells were transduced at a MOI of 100 with the GD-AdV FTC/HBVno.2. We observed up to a 60% reduction of secreted HBsAg levels in the supernatant (Figure 3E). This was in contrast to the control group that was transfected with the adenoviral vector FTC-hFIX-attB-(FRT)2 without shRNA expression cassette. The levels of HBsAg in this group were comparable to HBsAg levels in the supernatant of untransfected 2.2.15 cells.
A GD-AdV results in reduction of hepatits B surface antigen in small animal models for hepatitis B infection
In previous studies, it was demonstrated that hydrodynamic transfection of nonviral DNA containing the complete HBV genome represents a transient animal model for HBV infection 11, 28. In this animal model, blood antigen levels drop to undetectable concentrations as early as 7 days post-injection due to formation of neutralizing antibodies against HBV gene products 28. McCaffrey et al. 11 found that secreted HBsAg in mouse serum were significantly reduced after co-delivery of a plasmid containing the HBV genome (pTHBV2) and a second plasmid driving expression of the shRNA HBVU6no.2 (pHBVU6no.2). To test the efficacy of the GD-AdV FTC/HBVU6no.2 in this transient animal model, we sequentially injected pTHBV2 containing the HBV genome and the adenoviral vector FTC/HBVU6no.2 into C57Bl/6 mice (n = 4 per group). The plasmid pTHBV2 was injected by high-pressure tail vein injection followed by injection of 5 × 109 TUs of the GD-AdV FTC/HBVU6no.2. We measured HBsAg levels 2 and 4 days after adenoviral delivery and we observed a 68% drop in serum HBsAg levels (Figure 4A). By contrast to control mice, which solely received the HBV containing plasmid, we found that serum HBsAg levels were transiently increased in the group that received the GD-AdV FTC/HBVU6no.2 (Figure 4B). Control mice showed a 30% drop in serum HBsAg levels (Figure 4A). Mice that either received the GD-AdV HD28E4LacZ with a β-gal expression cassette or pHBVU6no.2 showed a reduction of 73% and 66% in HBsAg levels, respectively (Figure 4A). This finding suggests that the transgene product β-gal itself may be responsible for the reduction in HBsAg levels. To further investigate these findings in the transient animal model for HBV infection, we performed studies in HBV transgenic mice.
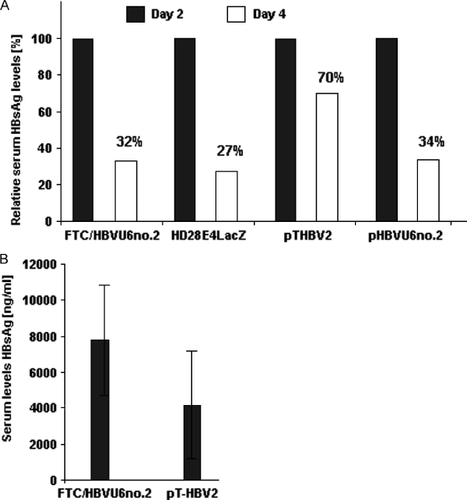
Reduction of HBsAg levels in vivo by a GD-AdV in a transient mouse model for hepatitis B infection. (A) Reduction of HBsAg levels in mouse serum. C57Bl/6 mice were injected with a plasmid containing the HBV genome (pTHBV2) by high-pressure tail vein injection. This step was followed by injection of 5 × 109 transducing units of either the GD-AdV FTC/HBVU6no.2 or HD28E4LacZ (Figure 1E). Control groups were injected with pTHBV2 or co-injected with pTHBV2 and pHBVRNAno.2. Serum HBsAg levels were measured 2 and 4 days after adenovirus injection by enzyme immunoassay. Data are the mean (n = 4 per group). (B) Serum HBsAg levels are transiently increased 2 days after superinfection with the GD-AdV FTC/HBVU6no.2. Data are absolute values of HBsAg 2 days after adenoviral infection
Recent studies by Uprichard et al. 15 and Carmona et al. 16 used fg adenoviral vectors (deleted for the adenoviral early genes E1 and E3) for delivery of HBV specific shRNAs. After systemic application of these adenoviral vectors into a transgenic mouse model for HBV infection, this approach resulted in reduced HBV infection levels for up to 26 and 12 days, respectively. Our goal was to evaluate GD-AdV lacking all adenoviral coding sequences in HBV transgenic mice. HBV transgenic mice (n = 4 per group) were either injected with 5 × 109 TUs of the GD-AdV FTC/HBVU6no.2 or the β-gal expressing GD-AdV (HD28E4LacZ). After a transient increase in HBsAg levels in both groups, we observed an up to 68% and 86% reduction in serum HBsAg levels compared to the vehicle control group (Figures 5A and 5B). The transient increase in serum HBsAg levels after adenovirus infection was in concordance with our study performed in a transient animal model for HBV infection (Figure 4B).
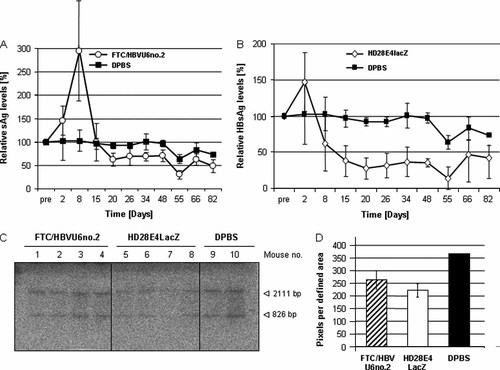
Evaluation of GD-AdV in a transgenic mouse model for HBV infection. (A) Relative HBsAg levels after injection of the adenoviral vector FTC/HBVU6no.2 into HBV transgenic mice. Data are the mean ± SD is shown (n = 4). Control mice solely received the vehicle (DPBS) used for diluting the adenoviral vector. Data are the mean ± SD (n = 3). Serum HBsAg levels were monitored by enzyme immunoassay. Data are the mean ± SD (n = 4). (B) Relative HBsAg levels after injection of the adenoviral vector HD28E4LacZ. Data are the mean ± SD (n = 4). Control mice were injected with the vehicle. Serum HBsAg levels were monitored by enzyme immunoassay. Data are the mean ± SD (n = 3). (C) Analysis of HBV genome replication after injection of the GD-AdV FTC/HBVU6no.2 and HD28E4LacZ. We performed Southern blot analysis of mouse genomic DNA. Blots were hybridized with a [α-P32]-dCTP-labelled HBV probe. (D) Quantification of viral HBV DNA. Depicted is the intensity of the obtained bands for all groups. The y-axis shows the number of pixels per defined area. Data are the mean
To analyse knockdown of HBV replication by other means, we performed a Southern blot analysis of HBV transgenic mouse liver genomic DNA. Compared to the control group that received the vehicle (DPBS), we found that HBV viral DNA was reduced in mice that received FTC/HBVU6no.2 and HD28E4LacZ (Figures 5C and 5D). In concordance with the obtained HBsAg levels, we observed a less pronounced reduction in HBV replication in FTC/HBVU6no.2 treated mice compared to individuals that received HD28E4LacZ.
Limited toxicity studies were performed. For mice that received FTC/HBVU6no.2, the highest liver enzyme levels were measured 20 days post-injection, which returned to normal levels 40 days post-injection (Figure 6A). By contrast, we observed two peaks of increased transaminase levels in individuals that received HD28E4LacZ (Figure 6A). We speculate that an immune response against the transgene product β-gal is responsible for the significant drop in serum HBsAg levels and increased liver enzyme levels for up to 106 days post-injection. To demonstrate that adenoviral vector genomes are maintained in treated mice displaying elevated transaminase levels, we monitored hFIX levels in mouse serum of individuals that received the GD-AdV FTC/HBVU6no.2. In concordance with our previous studies 19, we detected supraphysiological serum hFIX levels (normal levels: 5000 ng/ml) on days 8, 20 and 41 (Figure 6B).
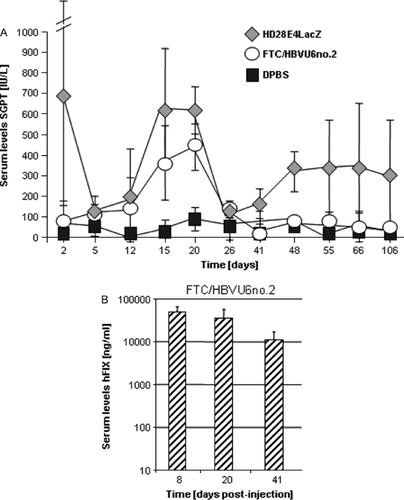
Analysis of liver enzymes and maintenance of adenoviral vector genomes. (A) Increased liver enzyme levels in HBV transgenic mice after injection of the GD-AdVs FTC/HBVU6no.2 and HD28E4LacZ. SGPT (alanine aminotransferase activity) levels in mouse serum were measured. Data are the mean ± SD (n = 4 per group). (B) GD-AdV genomes are maintained in tranduced mouse liver. Mouse blood samples from FTC/HBVU6no.2 treated mice were obtained by retro-orbital bleeding. Serum hFIX levels were monitored by ELISA
To rule out unspecific effects of GD-AdV on HBV replication, we performed a second experiment in HBV transgenic mice. Individual mice were either transduced with 5 × 109 transducing units of the GD-AdV FTC/HBVU6no.2 or 5 × 109 transducing units of the GD-AdV FTC/lucRNAi (Figure 1B). The latter group functions as a control with an irrelevant shRNA expression cassette. We observed a transient decrease in HBsAg levels up to 7 days post-injection followed by an increase in HBsAg levels (Figure 7A). This finding was in contrast to our first experiment in HBV transgenic mice (Figure 5A). One fatality was accounted for in the group that received FTC/HBVU6no.2, but the reason for this observation remains to be investigated. By contrast to FTC/HBVU6no.2 treated mice, only one mouse in the group that received the GD-AdV FTC/lucRNAi showed a slight decrease in HBsAg levels (Figure 7A). In concordance to our first experiment, we observed increased release of liver enzymes as demonstrated by SGPT levels (Figure 7B).
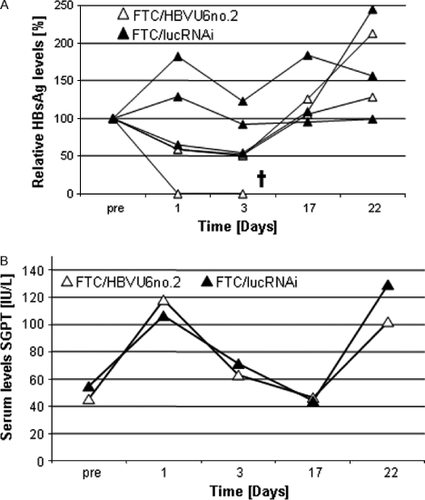
Evaluation of the GD-AdVs FTC/HBVU6no.2 and FTC/lucRNAi in HBV transgenic mice. (A) Analysis of HBsAg levels after injection of 5 × 109 trandusing units of the GD-AdV FTC/HBVU6no.2 and 5 × 109 trandusing units of the FTC/lucRNAi (n = 3 per group). Serum HBsAg levels were monitored by enzyme immunoassay. HBsAg levels of each individual mouse are depicted. (B) SGPT (alanine aminotransferase activity) levels in mouse serum. Data are the mean
Discussion
The present study demonstrates that GD-AdV deleted for all viral coding sequences have the potential to result in reduction of transient and preexisting HBV infection. However, the study also emphasizes that the design of DNA sequences contained in the vector and virus–host interactions during superinfection needs to be carefully considered.
The effect of shRNA-mediated RNA interference against luciferase lasted for 2 weeks in vivo (Figure 2E). A long-term study was not applicable because luciferase expression levels from the fg adenoviral vector fgAdluc decreased to undetectable levels 4 weeks post-injection. The persistence of transgene expression levels from fg adenoviral vectors depends on the promoter that controls transgene expression and the mouse strain 19, 33, 34. In the present study, luciferase expression was driven by the Simian Virus 40 (SV40) promoter. However, tissue specific promoters may result in stabilized transgene expression levels with a reduced toxicity profile 19, 34. Thus, future studies might investigate a GD-AdV with a liver specific promoter driving luciferase expression or transgenic mice for hepatic luciferase expression. With respect to long-term suppression of luciferase expression or therapeutically relevant target transcripts (e.g. viral transcription units or endogenous genes), shRNA may be delivered with recently developed GD-AdV for somatic integration 24. These vectors were shown to result in stabilized transgene expression levels, even during rapid cell cycling in vivo 24.
We observed a transient increase in serum HBsAg levels shortly after GD-AdV administration into the transient and the transgenic mouse model for HBV infection (Figures 4B, 5A and 7A). This was in contrast to studies performed by Uprichard et al. 15 and Carmona et al. 16. After adenoviral injection, there was no transient increase in HBsAg antigen levels. One explanation for these observed differences may be the fact that the two latter studies utilized fg adenoviral vectors. We speculate that the remaining adenoviral genes in fg adenoviral vectors and their gene products may have an additional inhibitory effect on HBV infection. Indeed, it was demonstrated that inflammatory cytokine induction after systemic administration of HBV transgenic mice with a fg adenoviral vector resulted in reduced HBV replication 35. By contrast to fg adenoviral vectors, GD-AdV lack all viral coding sequences. Thus, immunomodulatory adenoviral gene products are not present after infection with GD-AdV. However, the host response against the incoming adenoviral viral capsid proteins and the subsequent change in the expression profile of the host cells may directly or indirectly result in increased HBsAg levels.
In our first experiment performed in HBV transgenic mice, we found that HBsAg levels were transiently increased up to 2 weeks post-injection of the GD-AdV FTC/HBVU6no.2 (Figure 5A). This was in contrast to our second experiment (Figure 7A) in which we measured a decrease in HBsAg levels shortly post-injection. We speculate that this difference may be related to the HBV viral load of each individual pre-injection of the adenoviral vector. On average, HBV transgenic mice used in the first experiment had higher starting HBsAg levels (989 ng/ml, 548 ng/ml, 475 ng/ml and 365 ng/ml) pre-injection compared to individuals used in the second experiment (103 ng/ml, 323 ng/ml, 276 ng/ml).
Recently, it was reported that injection of a high dose of recombinant AAV vectors expressing various short hairpin RNAs caused lethality in mice 12. It was speculated that oversaturation of the shRNA/micro RNA pathways may be responsible. By contrast to superinfection with AAV2/8 vectors expressing the shRNA HBVU6no.2, we only observed one case of lethality in HBV transgenic mice after systemic administration of a high dose of a GD-AdV with the identical shRNA expression cassette (Figure 7A). However, it is important to point out that the reason for this fatality remains to be investigated and that further studies need to be performed. It was proposed that oversaturation of the micro/short hairpin RNA pathways may be responsible for lethality in AAV8 injected mice. Both recombinant AAV2/8 and adenoviral vectors have the ability to infect 100% of mouse hepatocytes. However, for sufficient transduction of all mouse hepatocytes using single-stranded AAV2/8 vectors, a high vector dose (7.2 × 1012 viral genomes per mouse) is required. It was demonstrated by Nakai et al. 31 that this dose resulted in up to 1000 vector genome copies per cell. Even for double-stranded AAV2/8 vectors with improved transduction efficiencies, 1 × 1012 AAV vector genomes per mouse are required to efficiently transduce all mouse hepatocytes 12. This feature of AAV vectors is in sharp contrast to adenoviral vectors. After transduction of 100% of mouse hepatocytes, we only detected up to 12 adenoviral vector genomes per hepatocyte (Figure 2C). We believe that this vector genome copy number per cell and subsequent shRNA expression levels were not sufficient to oversaturate the micro RNA/shRNA pathways. To further shed light on the differences between AAV and adenoviral vectors for shRNA delivery against HBV, additional studies remain to be performed. One might consider analysing and comparing additional blood parameters or major factors of the innate immune response.
It is established that, in patients with self-limited HBV infection, the innate and the adaptive immune responses to translated HBV antigens play important roles during pathogenesis and resolved HBV infection 1. It was demonstrated in a transgenic mouse model for HBV infection that induction of an innate immune response due to infection with other unrelated hepatotrophic viruses reduces replication of an existing HBV infection 35, 36. One of these hepatotrophic viruses was an E1 together with parts of E3 deleted recombinant adenoviral vector with a β-gal expression cassette. This vector was shown to significantly inhibit HBV replication in a transgenic mouse model due to induction of local cytokines in the liver 35. The present study utilized a GD-AdV with a β-gal expression cassette lacking all viral coding sequences. This vector also resulted in sufficient reduction of HBsAg levels (Figure 5B). Thus, the results presented here suggest that the transgene β-gal itself may have induced a cytokine response and predominantly be responsible for the reduction of HBsAg antigen levels. This hypothesis is further supported by the fact that there were no significant unspecific effects after administration of the control vector FTC/lucRNAi (Figure 7A). However, further parameters regarding immunostimulation with respect to the innate and the adaptive immune response remain to be investigated.
In summary, we believe that GD-AdV have the potential to mediate potent shRNA-mediated gene silencing in vivo but the design of DNA sequences including shRNAs contained in the vector needs to be carefully considered. In addition, we believe that it will be essential to further elucidate biological virus–host interactions during superinfection with HBV and adenovirus. Moreover, further understanding of the micro RNA/shRNA pathways will be required before pursuing these gene therapy approaches in a clinical setting.
Acknowledgements
The authors thank Philip Ng for providing the producer cell line and the helper virus for gene-deleted adenoviral production and Mark A. Kay at the Stanford University for providing materials. This work was supported by DFG grants SFB 455 and SPP 1230 to A.E. and the NIH grant DK49022 to Mark A. Kay. The authors declare that they have no conflict of interest.




