Prenatal genetic counseling challenges with indeterminate SMA results
Abstract
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular condition with complex genetic etiology. About 95% of individuals affected with this condition have a homozygous deletion of the SMN1 gene. One scenario that complicates risk is when a parent is identified as a possible silent carrier, meaning they have a [2 + 0] chromosome configuration. This configuration occurs when an individual has two copies of the SMN1 gene on one chromosome and no copies on the other chromosome. It is thought that 3.8–4.0% of the general population is a [2 + 0] carrier with a higher prevalence in African American and Hispanic populations. The [2 + 0] configuration makes it more difficult to calculate residual risk because testing cannot determine the difference between [2 + 0] carriers and [1 + 1] non-carriers, leading to indeterminate SMA carrier screening results. SMA was added to general population carrier screening in 2017, leading to an increase in the number of patients identified to have indeterminate results. Previous research has not examined how this addition has affected counseling practices involving indeterminate results. The purpose of this research was to gain a better understanding of the practices and challenges in this area, specifically within non-Ashkenazi Jewish (AJ) populations. This study utilized a quantitative survey with open-response questions. Responses from 49 prenatal genetic counselors from the United States and Canada were analyzed and it was found that genetic counselors face similar challenges when counseling indeterminate SMA results across all regions. These include negative patient emotions and both patient and referring provider misunderstanding, as highlighted in the qualitative data. Three major categories emerged including (1) challenges with patients, (2) challenges with referring providers, and (3) the effects of the 2017 addition to general population carrier screening. This study highlights the need for provider education surrounding indeterminate SMA results, the development of a visual aid, and future research from the patient and referring provider perspective.
What is known about this topic:
To date, previous research has not examined prenatal genetic counselor perspectives when counseling indeterminate SMA results.
What this paper adds to the topic:
This study provides quantitative and qualitative data on the perspectives of prenatal genetic counselors when counseling indeterminate SMA results, including the effects of the 2017 addition of SMA to general population carrier screening.
1 INTRODUCTION
Spinal muscular atrophy (SMA) occurs in every 1 in 10,000 livebirths and is the most common genetic cause of infantile death (Carré & Empey, 2016; Luo et al., 2014). There are five subtypes of the condition (0, I, II, III, IV) with type 0 being the most severe (Carré & Empey, 2016; Moultrie et al., 2016). The five subtypes are determined by their varying phenotypes, such as the age of onset and the clinical course of the disorder. Common characteristics include motor delays, low muscle tone, and proximal muscle weakness (Moultrie et al., 2016). Treatments have been developed and proven to show improvements in the features of individuals with SMA, but there is currently no cure for this condition (Nakevska & Yokota, 2023).
Spinal muscular atrophy is a condition complicated by the reduction of functional survival motor neuron protein (Carré & Empey, 2016). The SMN1 gene produces 80–90% of the SMN protein and the SMN2 gene produces the remaining 10–20% (Carré & Empey, 2016). SMA is caused by variants in the survival motor neuron 1 (SMN1) gene on chromosome 5, with the severity of the disease dependent on the number of survival motor neuron 2 (SMN2) copies present (Nakevska & Yokota, 2023). The relationship between SMN2 copy number and disease severity is complex, but previous studies have suggested the higher the SMN2 copy number, the less severe the phenotype (Carré & Empey, 2016).
This neuromuscular condition follows an autosomal recessive inheritance pattern, but the genetics of this condition are complex. About 95% of individuals affected with this condition have a homozygous deletion of the SMN1 gene. Most carriers, therefore, have a heterozygous deletion of the SMN1 gene (Carré & Empey, 2016). According to Sugarman et al., the pan-ethnic carrier frequency is 1 in 54 individuals. Carriers of SMA are healthy and do not present with features of the disorder. Typically, risk counseling is straightforward. If both parents are heterozygous carriers of a SMN1 deletion, then they have a 25% chance with each pregnancy of having a child with SMA. There are, however, multiple scenarios that can complicate this counseling.
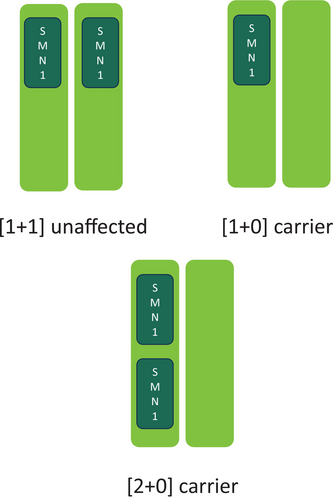
One scenario that complicates risk is when a parent has a [2 + 0] chromosome configuration, which is referred to as a silent carrier. This configuration (illustrated in Figure 1) occurs when an individual has two copies of the SMN1 gene on one chromosome and no copies on the other chromosome (Carré & Empey, 2016). Since these individuals possess two copies of the SMN1 gene, they do not show signs or symptoms of SMA but there is a risk to pass on the chromosome lacking the SMN1 gene to their children. It is thought that 3.8–4.0% of the general population is a carrier of the [2 + 0] configuration (Carré & Empey, 2016). It is more difficult to calculate residual risk of carriers with [2 + 0] configuration because current testing cannot decipher between the two carrier types, leading to indeterminate SMA carrier screening results (Carré & Empey, 2016).
Carrier screening is a testing methodology used to identify couples who are at an increased risk of having a child with a genetic disorder, specifically targeting autosomal recessive and X-linked conditions (Ghiossi et al., 2018; Gregg et al., 2021). SMA is a disorder identified in all ethnicities, so individuals of all backgrounds should be allowed the option of carrier screening (ACOG Committee Opinion No. 691, 2017b; Sugarman et al., 2012). In 2017, the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) updated their recommendation for carrier screening for SMA. Due to the severity of the disease and availability of new treatments, ACOG and ACMG recommend all women who are considering pregnancy or who are currently pregnant should be offered carrier screening for SMA, regardless of family history or ethnicity (ACOG Committee Opinion No. 690, 2017a; Gregg et al., 2021). These practice recommendations greatly increase the number of patients who are receiving carrier screening for SMA, which in turn increases the number of patients counseled by prenatal genetic counselors. Previous studies have not researched how the 2017 recommendation has changed the workload and patient population for prenatal genetic counselors.
Carrier screening detects about 96% of SMA carriers, but 4% of carriers remain undetected and are referred to as silent carriers (Ware et al., 2022). Ware et al. explain how past studies have shown silent carriers to be rare in the general population but more prominent in African American and Hispanic populations due to increase detection of a risk-modifying single nucleotide polymorphism (SNP). The current recommendation of pan-ethnic carrier screening has a high detection rate of about 90% for SMA carriers in AJ and Asian populations, but a detection rate of about 70% in African American and Hispanic populations due to silent carriers (Luo et al., 2014).
To increase the detection of these carriers, a risk-modifying SNP (c.*3+80T>G) was added to carrier screening in a study conducted by Luo et al. (2014) (Ware et al., 2022). This risk-modifying SNP had a positive correlation with detection of SMA carriers in a cohort of AJ individuals. A study by Ware et al. then identified that 46% of African American patients harboring two copies of SMN1 in cis configuration also harbored the risk-modifying SNP. The other minority populations had increased detection for SMN1 in cis configuration as well, but AJ and Caucasian (we recognize that this term is no longer supported by this journal, but we chose to include it because it is the language used in the study being discussed) populations were consistent with detection rates in previous studies. Prenatal genetic counselors are having to navigate counseling sessions involving indeterminate SMA results and are facing challenges unique to these results and their patient populations. Counseling practices for indeterminate [2 + 0] SMA results performed by prenatal genetic counselors and the challenges these counselors are facing have not been previously studied.
Genetic counselors are often the ones to report abnormal carrier and newborn screening results because they have the unique training to explain carrier status and the residual risk associated with a negative result (Gregg et al., 2021). A study by Leppert et al. (2018) assessed genetic counselors' experience with incidental carrier findings from abnormal newborn screening. From this study, researchers learned that counselors believe patients should be given educational materials to help absorb the overwhelming amount of information. To apply this finding to our study, the creation of educational materials pertaining to indeterminate [2 + 0] SMA results should be considered. If educational materials specific to indeterminate [2 + 0] SMA results were created, then counselors and referring providers could offer patients these materials before the counseling session. A study by Sagaser et al. (2023) commented on time being a barrier for healthcare providers. Genetic counselors are required to share a tremendous amount of information in a short amount of time. Implementing this workflow will hopefully decrease the amount of time spent with the patient and allow the session time to be used for questions. The counselors in the 2018 Leppert et al. study also expressed a desire for providers outside of the genetics specialty to be better educated on this information as well, possibly through continuing education opportunities. Incorporating these two ideas will help the overall workflow for the counselors so they can focus on what the patient truly needs in a limited amount of time.
The purpose of this research was to gain a better understanding of the prenatal genetic counseling practices and challenges involving indeterminate [2 + 0] SMA carrier screening results within non-AJ populations across the United States and Canada. For our first aim, we assessed if there are regional differences in prenatal genetic counselors' experiences when counseling indeterminate SMA results in underrepresented populations by comparing genetic counselors' patient volumes and demographics, perceived time spent with patients, referring providers, and the provider ordering the initial screening. We predicted that genetic counselors will have less time spent with patients, less utilization of materials, more challenges, and express decreased patient knowledge after the inclusion of the risk-modifying SNP to carrier screening and the 2017 recommendation. Our second aim assessed if there are common challenges counselors face involving patient knowledge, psychosocial issues, and patient access when counseling underrepresented populations on indeterminate SMA results. We predicted that genetic counselors in the regions with a higher percentage of underrepresented populations would have a more challenging time than genetic counselors where minority populations are not as prevalent.
2 METHODS
This study was approved by The University of Alabama at Birmingham's Institutional Review Board (IRB #300010630). This study utilized a survey to assess prenatal genetic counselors' experiences with indeterminate SMA results.
2.1 Participants
Participants were recruited through the National Society of Genetic Counselors (NSGC) listserv, the NSGC Prenatal Special Interest Group (SIG) discussion forum, and at the 2023 NSGC annual conference. Study announcements were purchased through the NSGC Student Research Survey Program to elicit responses in August and September of 2023. Flyers including a QR code to the survey were created and randomly distributed during the NSGC annual conference in October 2023.
Eligible participants of this study were genetic counselors who were NSGC members and/or who attended the annual NSGC conference. The NSGC listserv was utilized to send out a link to the survey. To properly answer the entirety of the survey, previous experience with indeterminate [2 + 0] SMA results was preferred, but it was not a requirement for participation. Participants also had to be English speaking. The genetic counselors did not have to be a current practicing prenatal genetic counselor and there were no specific years of experience required.
2.2 Instrumentation
The survey was developed by incorporating the experience and expertise of four ABGC board-certified genetic counselors, three of whom are prenatal genetic counselors who have varying levels of experience with indeterminate SMA results. The survey was built in REDCap, a secure web-based application designed to support data capture for research studies. The survey consisted of 19 multiple choice questions, which included four Likert-Scale format questions and three “select all” questions. The survey was divided up into four sections: genetic counselor demographics (8 questions), patient demographics (1 question), spinal muscular atrophy (10 questions), and open response (3 questions). For genetic counselor demographics, examples of questions include what year they graduated from their genetic counseling program and how long they have been actively seeing patients as a prenatal genetic counselor. For patient demographics, participants were asked about the ethnicity of their patients and the percentage of these patients that are seen for indeterminate SMA results. Examples of SMA-specific questions included patient knowledge, provider knowledge, percentage of patients seen for indeterminate SMA results, and use of educational materials. To allow participants to elaborate on their answers, branching logic was used for questions regarding patient demographics and visual aids. The voluntary open-response section involved questions about psychosocial concerns for these patients and any differences in this area noticed by genetic counselors over the past seve years after the addition of SMA to general population carrier screening. The survey can be found in Data S1.
2.3 Procedures
Survey responses were collected from August to December 2023. Study data were collected and managed using REDCap electronic data capture tools hosted at The University of Alabama at Birmingham Department of Medicine IT (Harris et al., 2009, 2019). Participants were required to consent to being involved in research before proceeding to the survey.
2.4 Data analysis
Data were summarized using descriptive statistics. Fisher's Exact Test, or the exact Cochran–Mantel–Haenszel test, was used to evaluate the association between different variables among the regions, including genetic counselors' patient volumes and demographics, perceived time spent with patients, patient and referring provider understanding, and the provider ordering the initial screening. Statistical analysis was performed with SAS 9.4 software (SAS Institute, Cary, NC, USA). Qualitative responses were analyzed using an approach similar to previous studies involving genetic counselor perspectives (Burzynski et al., 2024; Glessner et al., 2012). The open-response questions were evaluated using inductive content analysis to categorize the data. Responses with similar content were grouped together, followed by the identification of categories and subcategories. Frequencies of responses were calculated, and representative quotations were selected. The first author (MS) categorized the data, calculated the frequencies, and identified quotations. The second and last authors (LH and AG) reviewed the data separately and provided categories, which were compared with the first author's findings. Major categories and subcategories were developed to describe the psychosocial issues the counselors' patient populations are experiencing and how the addition of SMA to the general population carrier screening in 2017 has affected their counseling.
3 RESULTS
3.1 Genetic counselor demographics
According to NSGC's 2023 Professional Status Survey (PSS), the survey was sent to approximately 464 prenatal genetic counselors. Approximately 11% (n = 52) consented to complete the survey. Forty-nine participants' data were included in the study as three participants were removed due to incomplete survey instructions. Six participants completed the first nine questions of the survey only, which includes the participant and patient demographics sections. Forty-three participants completed all 19 required quantitative questions (see Table 1 for participant demographics). All participants (100%) reported having at least one year of experience working as a prenatal genetic counselor, with the majority (63%) having graduated between 2017 and 2023. Geographically, the highest survey participant representation was from region 2 (27%), region 3 (18%), and region 4 (35%).
| Variables | # | % |
|---|---|---|
| Currently working as a prenatal GC | ||
| Yes | 48 | 98 |
| No | 1 | 2 |
| Graduation year | ||
| 2020–2023 | 20 | 41 |
| 2017–2019 | 11 | 22 |
| 2010–2016 | 6 | 12 |
| 2000–2009 | 8 | 16 |
| Before 2000 | 4 | 8 |
| Years actively seeing patients as a GC | ||
| 1–3 years | 22 | 45 |
| 4–5 years | 5 | 10 |
| 6–10 years | 10 | 20 |
| 11–20 years | 6 | 12 |
| 20+ years | 6 | 12 |
| Years actively seeing patients as a prenatal GC | ||
| 1–3 years | 25 | 51 |
| 4–5 years | 5 | 10 |
| 6–10 years | 8 | 16 |
| 11–20 years | 7 | 14 |
| 20+ years | 4 | 8 |
| Region of patients being counseleda | ||
| Region 1- CT, MA, ME, NH, RI, VT, CN Maritime Provinces | 5 | 10 |
| Region 2- DC, DE, MD, NJ, NY, PA, VA, WV, PR, VI, Quebec | 13 | 27 |
| Region 3- AL, FL, GA, KY, LA, MS, NC, SC, TN | 9 | 18 |
| Region 4- AR, IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, OK, SD, WI, Ontario | 17 | 35 |
| Region 5- AZ, CO, MT, NM, TX, UT, WY, Alberta, Manitoba, Sask. | 6 | 12 |
| Region 6- AK, CA, HI, ID, NV, OR, WA, British Columbia, Yukon | 5 | 10 |
| Percentage of perceived time GC sees prenatal patients | ||
| 0–25% | 3 | 6 |
| 26–50% | 6 | 12 |
| 51–75% | 3 | 6 |
| 76–100% | 37 | 76 |
| Employment settinga | ||
| Hospital/Medical Facility- Academic Medical Center | 25 | 51 |
| Laboratory- Commercial | 3 | 6 |
| Hospital/Medical Facility- Public (including FQHC) | 11 | 22 |
| Hospital/Medical Facility- Private | 11 | 22 |
| Private Company- Telegenetics/Consulting/Utilization Management | 0 | 0 |
| MFM private practice | 1 | 2 |
| Counseling setting | ||
| Both in-person and telehealth | 35 | 71 |
| In-person | 9 | 18 |
| Telehealth (Video and/or Phone) | 5 | 10 |
- Abbreviation: GC, Genetic Counselor.
- a % does not add up to 100% as multiple answer choices were allowed.
3.2 Patient demographics per region
Indeterminate SMA patient demographics reported per genetic counseling region are detailed in Table 2. The most common reported patient demographic was black, African American, or of African descent (19%), followed by white/Caucasian (18%) and Hispanic/Latinx (17%).
| Region 1 | Region 2 | Region 3 | Region 4 | Region 5 | Region 6 | Totalb | |
|---|---|---|---|---|---|---|---|
| Asian | 2 | 8 | 2 | 10 | 2 | 3 | 27 |
| Black, African American, or of African Descent | 4 | 10 | 5 | 16 | 4 | 3 | 42 |
| Hispanic or Latinx | 4 | 10 | 4 | 13 | 4 | 3 | 38 |
| Pacific Islander | 1 | 3 | 2 | 7 | 2 | 1 | 16 |
| White/Caucasian | 4 | 11 | 5 | 14 | 4 | 3 | 41 |
| Ashkenazi Jewish | 2 | 6 | 1 | 8 | 3 | 2 | 22 |
| Middle Eastern or North African | 2 | 6 | 2 | 10 | 2 | 3 | 25 |
| American Indian, Alaskan Native, or Indigenous Peoples of Canada | 1 | 1 | 1 | 5 | 2 | 1 | 11 |
| Other | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Totalc | 21 | 55 | 22 | 83 | 23 | 19 | 223 |
- a Multiple answer choices were allowed to account for the patient demographics in each region.
- b Total number of participants who selected each ethnicity.
- c Total number of responses from each region given that participants were able to select multiple demographics to represent their region.
3.3 Experience counseling indeterminate SMA results
Genetic counselors were asked about their experience counseling patients on indeterminate SMA results. Altogether, genetic counselors provided responses representative of their experience counseling on indeterminate SMA results (see Table 3 for participant responses). Over half of the participants (58%) stated indeterminate SMA results take more time to counsel compared to other types of results, while 33% of participants stated these results take the same amount of time compared to others and 9% reported these results take less time. The majority of genetic counselors (75%) reported patient understanding of the results before the counseling session as very poor/poor, as well as referring provider's (74%) understanding to be very poor/poor. When asked how often genetic counselors encounter patients with misinformation, almost all (98%) reported they encounter patients with misinformation ranging from sometimes to always. Using Fisher's exact test, the genetic counselor experiences reported were not found to be statistically different among genetic counseling regions (Table S1).
| Variables | # | % |
|---|---|---|
| Percentage of patients referred for indeterminate results | ||
| 0% | 1 | 2 |
| 1–15% | 35 | 81 |
| 16–30% | 6 | 14 |
| 31–50% | 1 | 2 |
| >50% | 0 | 0 |
| Average perceived time counseling compared to other results | ||
| More time counseling | 25 | 58 |
| Same amount of time counseling | 14 | 33 |
| Less time counseling | 4 | 9 |
| Patient understanding of referral before appointment | ||
| Very poor | 12 | 28 |
| Poor | 20 | 47 |
| Average | 10 | 23 |
| Good | 1 | 2 |
| Excellent | 0 | 0 |
| Patient understanding of referral after appointment | ||
| Very poor | 1 | 2 |
| Poor | 3 | 7 |
| Average | 19 | 44 |
| Good | 16 | 37 |
| Excellent | 4 | 9 |
| How often gcs encounter patients with misinformation | ||
| Never | 0 | 0 |
| Rarely | 1 | 2 |
| Sometimes | 8 | 19 |
| Often | 27 | 63 |
| Always | 7 | 16 |
| Not applicable | 0 | 0 |
| Use of visual aids | ||
| Yes | 39 | 91 |
| No | 4 | 9 |
| Timing of genetic counseling session | ||
| Pre-test only | 0 | 0 |
| Post-test only | 24 | 56 |
| Both pre-test and post-test | 19 | 44 |
| Referring providers | ||
| OB/GYNs | 40 | 93 |
| Primary care providers | 2 | 5 |
| Otherb | 1 | 2 |
| GC perspective on referring provider knowledge | ||
| Very poor | 10 | 23 |
| Poor | 22 | 51 |
| Average | 6 | 14 |
| Good | 1 | 2 |
| Excellent | 0 | 0 |
| I don't know | 4 | 9 |
| Internal or outside carrier screening orders | ||
| Outside orders | 30 | 70 |
| Internal orders | 13 | 30 |
- Abbreviation: GC, Genetic Counselor.
- a Six respondents stopped answering the survey after demographics questions.
- b Mix of obgyn, nurse midwife, NP, GC, etc.
3.4 Inductive content analysis
Inductive content analysis was utilized to identify categories within the qualitative responses, which are represented in Table 4. Of the 49 participants who answered the survey, 38 completed at least one of the three open-response questions. Participants were asked to describe psychosocial issues patients experience during sessions discussing indeterminate SMA results, how these psychosocial issues compare to other carrier screening results, and if the participants have noticed any differences in counseling practices and/or workflow since SMA was added to the general population carrier screening in 2017. Inductive content analysis of the responses identified three main categories: challenges with patients, challenges with referring providers, and effects of the 2017 addition to carrier screening.
| Category | Subcategory | Representative quote |
|---|---|---|
| Challenges with Patients (N = 38) | Heightened emotions surrounding results (76%) | “Psychosocial issues include anxiety over being told they have an “abnormal” result by their referring provider, anxiety over referral to genetics because their provider cannot explain the result, concern about risk in the event that the reproductive partner is unknown/unavailable/unwilling to complete testing. Some patients look up SMA ahead of the appointment and come in afraid because they think they have the condition or their baby has the condition.” Participant 44 |
| “Indeterminate result patients are generally more anxious than other carrier screen result patients and find the uncertainty more distressing than the actual results” Participant 20 | ||
| Difficulty understanding results (68%) | “There is much more misunderstanding/misinformation to be addressed, and sometimes that is difficult to undo during the session.” Participant 12 | |
| “It's a difficult comprehend maybe being a carrier. It's also hard to discuss the silent carrier status in layman's terms and so it takes multiple tries for this to be understood. When I tell patients the actual percentage of their risk, they feel much better. Most come in thinking they either definitely are carriers or that their baby has SMA. They report high levels of stress and anxiety before our appointments.” Participant 4 | ||
| Challenges with referring providers (N = 38) | Result misunderstanding (37%) | “I don't encounter psychosocial issues as much as misunderstanding of the results both by patients and their referring providers. The ordering Obs typically tell the patient that they are definitely an SMA carrier when they receive indeterminate results and then refer them to genetic counseling. They also almost always put the diagnosis code “carrier of genetic disease” in the patient's chart.” Participant 16 |
| “SMA indeterminate results are more confusing to providers, who frequently misinterpret these results when communicating to patients. Other carrier results (e.g., carrier of CF) are more easily understood by providers & thus patients are more likely to have an accurate understanding of results prior to GC” Participant 31 | ||
| Effects of the 2017 addition to carrier screening (N = 15)a | Increased referrals (47%) | “Far more referrals to review carrier screening results” Participant 38 |
| “Increased volume of consults for these indications; increased referrals to specialty providers/clinics from OB/GYNs” Participant 5 | ||
| Impact on minority populations (11%) | “We have a large African-American patient population, so when the indeterminate SMA carrier results started being released, we had a huge increase in volume of patients referred to discuss this result.” Participant 33 | |
| “We have made multiple attempts to educate providers about residual risk especially associated with Hispanic ancestry since this represents a large portion of our referrals. Our referrals for this indication. Continue to increase however. Ultimately, we are hoping to change the referral so that Hispanic ancestry patients specifically are counseled about low residual risk and do not require GC” Participant 49 |
- a This question was for participants who were counseling before 2017, so the sample size decreased.
3.4.1 Challenges with patients
Indeterminate result patients are generally more anxious than other carrier screen result patients and find the uncertainty more distressing than the actual results (Participant 20)
There is much more misunderstanding/misinformation to be addressed, and sometimes that is difficult to undo during the session. (Participant 12)
[There are] not a lot of good resources online. (Participant 12)
3.4.2 Challenges with referring providers
SMA indeterminate results are more confusing to providers, who frequently misinterpret these results when communicating to patients. Other carrier results (e.g., carrier of CF) are more easily understood by providers & thus patients are more likely to have an accurate understanding of results prior to GC. (Participant 31)
3.4.3 Effects of the 2017 addition to carrier screening
Increased volume of consults for these indications; increased referrals to specialty providers/clinics from OB/GYNs. (Participant 5)
We have made multiple attempts to educate providers about residual risk especially associated with Hispanic ancestry since this represents a large portion of our referrals. Our referrals for this indication continue to increase, however. Ultimately, we are hoping to change the referral so that Hispanic ancestry patients specifically are counseled about low residual risk and do not require GC. (Participant 49)
4 DISCUSSION
This study examined prenatal genetic counselors' perspectives on counseling indeterminate SMA results and the challenges that subsequently arise. Previous research has not studied the perspectives of genetic counselors working with indeterminate SMA results, specifically in non-AJ populations in different regions of the United States and Canada. Other studies have also not considered the effect of the 2017 SMA recommendation for carrier screening on counseling practices and clinic management. The purpose of this research was to gain a better understanding of the counseling practices and subsequent challenges prenatal genetic counselors face when counseling indeterminate [2 + 0] SMA results. This study assessed if there were regional differences due to a higher number of minority populations, which was not observed in the data. The practices and challenges associated with indeterminate SMA results were distributed equally throughout all six regions and were reported in all suggested ethnic groups. Geographically, the highest respondent rate is from regions 2 and 4. The two most common minorities that are reportedly counseled are Black/African American/Of African Descent and Hispanic/Latinx. Previous literature supports this statement by identifying these minority groups as the two most common minorities found to have indeterminate SMA results (Luo et al., 2014; Ware et al., 2022).
The challenges faced by genetic counselors when counseling indeterminate SMA results can be found in all regions. Over half of the participants (58%) reported indeterminate SMA result counseling sessions taking more time to counsel. This is a significant finding when considering the time allotted for each appointment and restrictions on billing for genetic counseling. Genetic counselors are often already pressed for time during counseling sessions, so additional concerns about time management is not ideal (Sagaser et al., 2023). Indeterminate SMA results may need additional time for counseling because of the intense emotions and confusion associated with these appointments, which is a frequent challenge described by prenatal genetic counselors. When first meeting with a genetic counselor, patients feel anxious, stressed, and/or worried about the possibility of having a child with SMA. This can be attributed to incorrect information provided by the referring provider or from information the patients sought out themselves. Providing well-informed pre-test counseling either by genetic counselors or other providers may help improve these heightened emotions (Sagaser et al., 2023).
Another challenge genetic counselors face with patients is the misunderstanding of results. By nature, indeterminate results are not simple or straightforward, so patients being confused about what the results mean is understandable. This misunderstanding, however, often comes from referring providers delivering incorrect information when the results are initially discussed. An increase in understanding after the genetic counseling session was noted in the results, which is always a goal surrounding the education counselors provide. To help with understanding, participants reported trying to use educational materials, such as visual aids, in their sessions. Some participants noted they draw their own visual aid in the session or have created one over time that they use. These responses represent the need for a uniform visual aid genetic counselors can use to explain the complex genetics of SMA.
Referring provider misunderstanding was described by the participating genetic counselors as another challenge when counseling indeterminate SMA results. Providers who do not have a background in genetics may struggle to understand indeterminate results as they are not intuitive. This misunderstanding from providers, however, creates a problem when patients receive their results. ACMG's 2021 practice resource states that carrier screening counseling should be conducted by a knowledgeable and well-trained healthcare professional (Grody et al., 2013). Participants described experiences where referring providers delivered incorrect risks and/or diagnoses to patients, such as telling the patients they are carriers for SMA. Incorrect information adds to the patients' intensified emotions entering the genetic counseling session and makes the counselors' job of correcting misinformation harder. Educating referring providers on indeterminate SMA results may help decrease the amount of misinformation patients receive.
Participating genetic counselors who have been counseling patients before and after the 2017 addition of SMA to general population carrier screening commented on a noticeable difference in the increase in referrals for SMA results. This is not a surprising finding, but it is useful when evaluating clinic workflow and management. Educating referring providers on indeterminate SMA results adds on additional labor-intensive task to genetic counselors' already busy schedule (Gregg et al., 2021). Clinics dealing with an influx of referrals that may require more complex education may need to adjust their referral process and develop time management tools, such as educational videos and group counseling sessions (Sagaser et al., 2023).
Several participants (11%) commented on indeterminate SMA results in relation to Black/African American/Of African Descent and Hispanic/Latinx populations. The risk-modifying SNP added to general population screening is not as useful for certain minority populations because exact risk calculations are not well known. This poses a challenge to the interpreting providers. Due to the ambiguity of these results, it was suspected that more participants would comment on the effect of these results in relation to minority populations. Future research can focus on the effects of indeterminate SMA results in relation to minority populations.
4.1 Limitations
A limitation of this study is that the survey questions were not validated, which may contribute to measurement error. In relation to the survey questions, there were questions used that are subjective. For example, the participating genetic counselors were asked about their perspective on the understanding of their patients and the referring providers. This style of question requires a subjective answer but poses an opportunity for patient and referring provider knowledge to be examined in future research. Potential response bias is another limitation due to the high response rate from regions 2 and 4. Important to note, however, is how these two regions contain a high proportion of minority groups that correlate with the populations often affected by indeterminate results. Participants' experiences are challenging to represent when they practice in more than one region compared to the experiences of participants who practice in only one region. Finally, of the 49 responses used in the analysis, 43 completed all required questions. The lack of completion from six respondents may have been due to response fatigue as they all stopped answering after the same question.
4.2 Practice implications
The frequency of misinformation genetic counselors encounter emphasizes the need for proper education on indeterminate SMA results for referring providers prior to any referrals that are sent to genetics. Finding time to educate other providers can be a challenge, so creating an educational handout or video to send may be helpful. Proper education on these results can also lead to the possibility of decreased referrals. If referrals continue to increase, then clinics will have to reevaluate their intake system and/or clinic workflow. To help decrease patient misunderstanding, a visual aid can also be developed for prenatal genetic counselors to use in sessions. Offering visual aids and educational materials to patients is supported in the 2021 ACMG Practice Resource on carrier screening during pregnancy and preconception (Grody et al., 2013). An ethnicity specific visual aid may be useful in sessions due to the complexity of risk counseling for minority populations.
4.3 Research recommendations
Future studies can help address the patient and referring provider perspectives on indeterminate SMA results. For patients, additional studies that focus on obtaining direct responses from patients on the emotions they feel surrounding the results before and after the counseling sessions, as well as their overall understanding may be helpful. Specific minority populations can be focused on to gain a better understanding of how the ambiguity of the results affects them. Future research with referring providers can include piloting educational materials to improve understanding and assessing their understanding of the results before and after the additional education. Providers can also attempt to track the incidence of 2 + 0 results, uptake of amniocentesis, and the birth rate incidence of SMA via newborn screening data. This data can then attempt to comment on the benefits and limitations of adding this result to carrier screening, specifically for minority populations.
5 CONCLUSION
This study investigated prenatal genetic counselors' perspectives on counseling indeterminate SMA results and the challenges counselors encounter involving both patients and referring providers. Our study did not demonstrate a significant difference in the experience of genetic counselors counseling indeterminate SMA results based on region of practice in the United States and Canada. This study highlights genetic counselors are facing similar challenges of heightened emotions and lack of understanding by patients and referring providers, as well as increased referrals since the 2017 addition of SMA to general population carrier screening throughout the country. The effect of these results on minority populations was mentioned but should be explored further in future research. This study shows the need for referring provider education and the development of a visual aid to help in the explanation of indeterminate SMA results.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. All authors made contributions to the conception and design of this study. Molly Spangenberg was responsible for obtaining the data. Guihua Zhai was responsible for the descriptive statistics. Molly Spangenberg, Alicia Gomes, and Laura Hendon contributed to the drafting of the manuscript, as well as the analysis and interpretation of the data. All of the authors gave final approval of this version to be published and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
The authors would like to thank Ashley Cannon for her assistance in reviewing the manuscript. The research presented in the paper was conducted while the first author was in training or to fulfill her Master's in Genetic Counseling.
CONFLICT OF INTEREST STATEMENT
Molly Spangenberg, Laura Godfrey Hendon, Dana H. Goodloe, Fallon Brewer, Guihua Zhai, and Alicia Gomes state no conflicts of interest to report.
ETHICS STATEMENT
Human Studies and Informed Consent: This study was reviewed and granted an exemption by the University of Alabama at Birmingham Institutional Review Board (IRB #300010630). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants electronically prior to the survey.
Animal Studies: No non-human animal studies were conducted for this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings in this study are available upon reasonable request.




