Preferences of parents from diverse backgrounds on genomic screening of apparently healthy newborns
Nina B. Gold and Jacklyn O. Omorodion should be considered joint first author.
Abstract
Genomic sequencing has been proposed as a strategy to expand newborn screening. Perspectives on genomic newborn screening from parents of diverse racial, ethnic, and socioeconomic backgrounds are needed to shape equitable implementation of this modality. We conducted 20 semi-structured interviews (15 English, 5 Spanish) and seven focus groups (4 English, 3 Spanish) with parents from diverse backgrounds to assess their perspectives regarding which disorders and variants might be screened, data privacy, and barriers to pursuing specialized care. Parents felt that genomic newborn screening would provide them with improved understanding of their children's health and had the potential to yield health and personal benefits. Themes that became evident included: interest in childhood and family health risks, the value of emotional preparation and personal planning, understanding of uncertain and low-risk results, concerns regarding data privacy, and concerns about support following the receipt of a positive newborn screening result. The expected benefits and concerns expressed by parents of diverse backgrounds regarding genomic newborn screening should guide future policy decisions. Their preferences should be considered prior to the implementation of large-scale genomic newborn screening programs.
What is known about this topic
As the number of treatable genetic conditions grows, it is important to consider the views of stakeholders, especially parents, on the future expansion of newborn screening through genomic sequencing.
What this paper adds to the topic
Interviews and focus groups with parents from diverse backgrounds revealed enthusiasm for expanding newborn screening using genomic sequencing. They expressed comfort with uncertain and low-risk results, but had concerns about barriers to care following the receipt of a positive result.
1 INTRODUCTION
Newborn screening (NBS), heralded as one of the most significant public health interventions of the twentieth century (Centers for Disease Control and Prevention, 2011), identifies infants at risk for a range of childhood-onset, treatable disorders. At present, 37 core conditions and 26 secondary conditions are recommended for national inclusion in NBS, but these represent only a fraction of the approximately 700 genetic disorders for which targeted treatments are available (Health Resources & Services Administration, 2023; Watson et al., 2006). As the cost of genomic technologies declines, the expansion of NBS using genomic sequencing (NBSeq) has been proposed as a strategy for screening for more genetic disorders simultaneously (Berg & Powell, 2015; Downie et al., 2021; Kerruish & Robertson, 2005; Kingsmore, 2016).
Prior studies have shown that parents support expanding the number of disorders included in NBS through NBSeq (Etchegary et al., 2012; Hayeems et al., 2015; Mak et al., 2012; Timmins et al., 2022; Waisbren et al., 2015), but have concerns about data privacy, unwanted results, and anxiety related to waiting for results (Hayeems et al., 2015; Joseph et al., 2016; Timmins et al., 2022). As a large proportion of rare disease physicians and genetic counselors have indicated support for NBSeq (Gold et al., 2023; del Rosario et al., 2024), and a growing number of research studies show clinical utility of NBSeq (Bodian et al., 2016; Ceyhan-Birsoy et al., 2019; Green et al., 2023; Roman et al., 2020), more data on parental perspectives, particularly those from minoritized groups, are needed. Prior studies among African American and Hispanic parents have demonstrated concerns about privacy, control over test results, and limited trust in the medical system and NBS program (Joseph et al., 2016; Timmins et al., 2022). In this study, we conducted interviews and focus groups with a diverse group of parents regarding their perspectives on NBSeq, particularly their expected benefits and concerns if this type of screening were implemented.
2 METHODS
Using a qualitative approach within a realist epistemological framework (Braun & Clarke, 2006), we conducted semi-structured interviews and focus groups with parents of diverse racial, ethnic, and socioeconomic backgrounds. This framework assumes a direct link between individuals' experiences, their meaning, and how they are expressed through language. We selected this framework for our study because we wanted to assess perspectives on NBSeq from parents of diverse backgrounds at a semantic level. Guides were developed by three of the authors, N.G., J.O., and C.H. for the interviews and for the focus groups. The content of these guides was based on prior literature assessing parental preferences regarding genomic testing and specifically genomic screening of newborns and children (Etchegary et al., 2012; Hayeems et al., 2015; Joseph et al., 2016; Pereira et al., 2019). The interview guides were all translated into Spanish by one of the study authors (G.R.C.) who is fluent and practices medicine in both English and Spanish. Interviews and focus groups were both performed in an effort to gain a more comprehensive understanding of participant views. It was expected that interviews might provide more depth on specific topics while the focus groups would give more insights into a breadth of opinions and also for generation of new ideas in the group. We also expected to triangulate information from both methods to enhance the validity of the themes that were identified. The study was approved by the Institutional Review Board at Boston Children's Hospital (BCH).
As a positionality disclosure, the study authors who conducted the interviews and focus groups (N.G., J.O., G.R.C., C.H.) are in favor of NBSeq for genes associated only with treatable childhood-onset conditions at this time. As more information about the sensitivity and specificity for this screening modality is gained, and new therapies become available, the study authors are in favor of adding additional genes to the NBSeq list.
A completed Consolidated Criteria for Reporting Qualitative Studies (COREQ) Checklist is included in the Appendix S1.
2.1 Participants and recruitment
Eligible participants included parents 18 years or older of infants 1–6 months of age recruited from three clinical sites: Children's Hospital Primary Care Center at BCH (Boston, MA), UAB (University of Alabama) Pediatric Primary Care Clinic at Children's of Alabama (Birmingham, AL), and Mount Sinai Pediatric Associates Practice (New York, NY). Since this study focuses on parents from diverse backgrounds, purposive sampling was used regarding the race and ethnicity of potential participants. Race and ethnicity were self-identified by participants. Once these criteria were met, convenience sampling was employed. Participants were identified by pediatricians at well-child checks and, if they agreed, their contact information was shared with study staff. At BCH, parents of children of any age who were enrolled in the BCH Biobank and had previously consented to be recontacted about research studies were also called. Initially, participants were assigned to interviews. Once 20 interviews were completed, additional participants were enrolled in focus groups.
The final number of participants was determined based on the information power it generated given the rather narrow aim of this study, the participant sample with targeted characteristics, the development of the interview and focus group guides to elicit very targeted answers and/or dialogue, and the type of analysis intended, a thematic analysis focusing on semantics (Malterud et al., 2016).
2.2 Study design
The interviews and focus groups were conducted by four of the study authors (N.G., J.O., G.R.C., C.H.). All are female. At the time, one was an attending physician practicing as a medical geneticist (N.G.), and two were physicians in residency programs, training to become medical geneticists (J.O., G.R.C.) One was a graduate student studying bioethics (C.H.). Two interviewers had prior experience conducting interviews and focus groups (N.G., C.H.). Two interviewers had no prior experience (J.O., G.R.C.) and were trained by another study author (S.Z.) with over 20 years of experience conducting interviews and focus groups. Participants had no prior relationship with the interviewers before this study. Only the interviewer's name and study location were provided to the participants following enrollment. During interviews, typically only one interviewer was present. Two to three moderators were present for the duration of each focus group.
Informed consent for participation was obtained verbally. Interviews and focus groups were conducted using Zoom online videoconferencing software. Participants were asked to turn their cameras on but it was permissible for them to keep the cameras off if they preferred. The interviewers and focus group moderators were either located at home or in their office while all participants logged in from various locations. No other people besides the interviewers or moderators and participants were present. Interviews were completed over approximately 30–60 min and focus groups were completed over approximately 60–75 min. There were no repeat interviews completed and transcripts were not returned to participants. As the topics of the interviews and focus groups differed, participants could participate in an interview as well as one focus group. Participants were offered a $50 gift card for participating in an interview or focus group.
Interviews included questions addressing five interrelated topics: understanding of current NBS practices, preferences regarding the inclusion of various types of genetic conditions, coping with uncertain genetic results, familial testing related to NBSeq results, and barriers to care for infants receiving positive NBSeq results (Appendix S2). One set of focus groups centered on preferences for screening of different types of genetic conditions (Appendix S3) and a second set of focus groups centered on privacy of genetic information (Appendix S4).
2.3 Data analysis
The interviews and focus groups were recorded in Zoom and the audio-recordings were transcribed by an outside service. The English audio-recordings were transcribed directly (Mulberry Studios, Cambridge, MA) and the Spanish audio-recordings were transcribed and translated (Always on Time, Las Vegas, NV). All identifying information was removed from the transcripts, which were uploaded into the software program, Dedoose.
No field notes were taken, as there was only one interviewer present during the interviews, and all facilitators wanted to appear attentive to participants during the focus groups. The data analysis was completed from transcripts from the audio-recordings. The transcripts were evaluated using a semantic analytical approach that involves the interpretation of the participants' explicit meaning of their words. Using a deductive approach, we developed two codebooks, one centered on parental preferences regarding disorders to include and variant types to report (used for individual interviews and preferences focus groups) and a second codebook for the focus groups that discussed privacy. Once the codebooks were developed, codes were assigned to the participant responses. There were codes for all response types (yes, no, unsure). For questions that had more variable responses (for example, barriers to follow-up care) we had many potential responses covered by codes, including an option for “other” (in the above case, “other barriers”).
Coding was initially completed by two members of the research team (M.D.R., S.Z.) on the same randomly selected set of two interviews and one focus group to assess inter-rater reliability (Overall Gwet's Agreement Coefficient: 0.945) (Gwet, 2016). After clarifying one group of codes and making small changes (Gwet, 2016), the remainder of the interviews and focus groups were coded by one of two members of the research team (J.O., M.D.R.). Themes were generated inductively based on the codes and discussed by the research team. We did not contact participants to provide feedback on these findings.
3 RESULTS
We conducted 20 semi-structured interviews (15 English, 5 Spanish) and seven focus groups (4 English, 3 Spanish); four focus groups (2 English, 2 Spanish) centered on preferences for screening of different types of genetic conditions and three (2 English, 1 Spanish) centered on privacy of genetic information. There were 20 interview participants and 23 focus group participants. There were 7 individuals who participated in both an interview and a focus group (4 English-speaking and 3 Spanish-speaking).
All participants identified as female. The majority (11 of 20 participants in the interview group and 16 of 23 participants in focus groups) were 30–40 years of age. Ten of the 20 participants who completed interviews identified as Black, African American, or African, as did 9 of the 23 individuals in the focus groups. The majority in both groups identified as Hispanic (11 of 20 participants in the interview group and 18 of 20 participants in the focus groups). Annual household income varied significantly across all participants (interview group: <$15,000 to >$150,000, focus groups: $15,000 to >$150,000). These characteristics are detailed in Table 1.
| Interview participants, N = 20 (%) | Focus group participants, N = 23 (%) | |
|---|---|---|
| Place of enrollment | ||
| Boston Children's Hospital | 10 (50) | 20 (87) |
| Mount Sinai Hospital | 6 (30) | 2 (9) |
| University of Alabama Hospital | 4 (20) | 1 (4) |
| Gender | ||
| Female | 20 (100) | 23 (100) |
| Age (years) | ||
| <30 | 4 (20) | 3 (13) |
| 30–35 | 8 (40) | 9 (39) |
| 36–40 | 3 (15) | 7 (31) |
| 41–45 | 4 (20) | 3 (13) |
| 46–50 | 1 (5) | 1 (4) |
| Number of children | ||
| 1 | 9 (45) | 6 (26) |
| 2 | 5 (25) | 6 (26) |
| 3 | 1 (5) | 4 (17) |
| 4 | 2 (10) | 3 (13) |
| 5 | 2 (10) | 2 (9) |
| 6+ | 1 (5) | 2 (9) |
| Race | ||
| Black, African American, or African | 10 (50) | 9 (39) |
| White | 10 (50) | 14 (61) |
| Ethnicity | ||
| Non-Hispanic | 9 (45) | 5 (22) |
| Hispanic | 11 (55) | 18 (78) |
| Education level | ||
| Some High School | 1 (5) | 3 (13) |
| High School Graduate or GED | 4 (20) | 4 (17) |
| Some College | 6 (30) | 9 (39) |
| Bachelor's Degree or Equivalent | 9 (45) | 6 (26) |
| Master's Degree | 0 | 1 (5) |
| Household income ($) | ||
| <15,000 | 0 | 3 (13) |
| 15,000–24,999 | 3 (15) | 8 (35) |
| 25,000–34,999 | 5 (25) | 2 (9) |
| 35,000–49,999 | 3 (15) | 3 (13) |
| 50,000–74,999 | 0 | 0 |
| 75,000–99,999 | 4 (20) | 4 (17) |
| 100,000–150,000 | 2 (10) | 0 |
| >150,000 | 2 (10) | 1 (4) |
| Unknown | 1 (5) | 2 (9) |
Thirteen individuals (7 English-speaking, 6 Spanish-speaking) who expressed interest in participating in an interview ultimately did not schedule one. Several of these individuals cited time constraints due to the holidays and school breaks. Eleven additional individuals (8 English-speaking, 3 Spanish-speaking) did not attend their scheduled focus groups.
Several themes were identified through semantic analysis of the interviews and focus groups: (1) strong interest in childhood and family health risks, (2) emphasis on emotional preparation and personal planning, (3) comfort with uncertain and low-risk results, (4) mixed reactions regarding data privacy, and (5) concerns about support and resources following the receipt of a positive NBSeq result (Table 2).
| Theme | Interview excerpt |
|---|---|
| Interest in pediatric and family health risks | “It's just now you know, for sure, that there's a chance that they might have [this condition]. So it's something that their doctors will know. And it's something that, when they have their physical, they will check.” (IBCHE13) |
| “I feel that, with my child, my children, I want to test for everything underneath the sun, due to the fact that I just want to make sure that if there is anything that I can do to further along their health, prolong their life, and just be able to help them in any kind of way possible, then I would want to.” (IBCHE07) | |
| Importance of emotional preparation and personal planning | “I feel like, if I know, I'll feel comfortable accepting what's coming my way, because I'm prepared. I'm prepared in all aspects. And I could also prepare their siblings if anything and make them understand.” (F3E07) |
| “I can at least try to spend the time that I have with my child, you know, make it memorable for the time that they're here.” (IUABE06) | |
| Mixed reactions regarding uncertain and low-risk results | “I feel like it might be helpful, and wouldn't be too much different than just being aware of like increased risks that you already know you have.” (IUABE08) |
| “Because the risk of getting whatever it is, is very low. …I don't think I would want to know about something that was at that low range as far as the possibility.” (IUABE06) | |
| Caution regarding data privacy | “I feel the same way. …I'm good at keeping documents and stuff, but not very good. It's better that [the lab] have it because if a different doctor or a different specialist needs that card or that information, then it's readily available to them. They could just go to the lab and get it.” (F4E14) |
| “To be included in the child's medical record would be good so that any doctor can know what to do, but not share it with the state or the federal government.” (F7S23) | |
| Concerns about support and resources following the receipt of a positive newborn screening result | “But someone they also can cry to, talk to, someone they could have a minute with. Because I could only imagine a lot of it would be hard to stomach, especially if you have a lack of support, or not enough people in your corner.” (IBCHE02) |
| “Medications are quite expensive in this country, as you know. Treatments are quite expensive, and we would need assistance with that. As I said, as a mother, I would move heaven and earth to ensure my children are well.” (F5S19) |
3.1 Strong interest in childhood and family health risks
As a parent, [you] could sit down your child and say, ‘Hey, when you was younger, you got tested for this. And it came back that there was a chance. Let's start getting you testing at this age. Let's see what we can do.’ Like take the right steps and cover our bases. (F3E07)
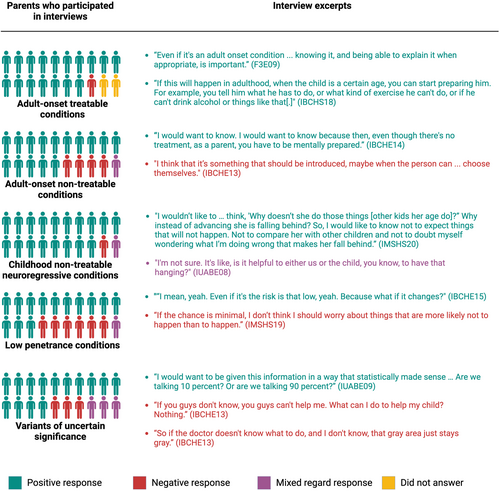
Although he won't be affected in childhood, the risk is present, and you can monitor the condition as he grows up. (IBCHS17)
The majority of parents who were interviewed (18 of 20 participants) supported the inclusion of non-treatable forms of childhood-onset neuroregression. One parent who was interviewed felt that an early diagnosis provided a pathway to future treatment, stating: “The only way to help someone is to know what he has” (IMSHS19). Others felt that the potential for medical benefits may not outweigh the stress of receiving a positive screening result for a non-treatable disorder. One parent who was interviewed implied that such information might have the potential to interfere with parent-infant bonding: “The first year is so much happiness and so much joy going into your little one … [S]omething like that, I don't think that you should worry about until, like, it kind of starts to show up” (IMSHE05). Others agreed, noting that they were unsure how this information would be helpful to themselves or their child.
3.2 Importance of emotional preparation and personal planning
Many parents spoke of ways in which genomic information might allow them to educate and prepare their children for future health risks. In an interview, one parent described preparing their daughter by creating a “strong mind”: “I think that it will help her…to let her know that she's strong, that she can do it” (IMSHE10). Another parent said that they would ask the child's pediatrician to educate the child about his risks.
I know I could prepare myself and everyone around me as well. And they could help me live better. Like, you know, cope with the situation better, have the right people around me. (F3E07)
It'd be nice to know if I'm possibly going to maybe end up burying my child before I actually get buried as well. It would give me time to sit with that, but also not to be a better parent, but to probably enjoy my child a lot more, knowing that I don't have a whole lot of time. (IBCHE02)
3.3 Understanding of uncertain and low-risk results
There's going to be a point where you all actually are able to identify what [that variant] would cause or what may happen with that type of [variant]. So, yes. I would want to know it. (IUABE06)
A minority of parents, however, felt that VUS would not provide medical or personal utility, because neither the parent nor the child's doctors would know if the child was at risk of a genetic condition. Of note, many parents in the Spanish-speaking focus groups were concerned or unsure about the disclosure of VUS.
I don't need to put anything in my head that doesn't need to be there, to take up space. (IMSHE05)
3.4 Caution regarding data privacy
[Saving the data in the medical record] wouldn't hurt. Easy access to anyone who needs it, you know, different departments usually work together…It's unique to the child, so it should be ready and available. (F4E16)
3.5 Concerns about resources after a positive newborn screening result
Despite the medical condition that my child might have, I still have to make sure my child has a roof over their head. (IUABE06)
The challenge then is sitting for hours in the hospital, lack of help, lack of food, wanting to take a break. But then mentally and physically [you] can't take a break because you want to be there…to understand what's going on. (IBCHE02)
Another parent who was interviewed suggested that each family who receives a positive screening result should be paired with a patient advocate who could attend visits with them.
4 DISCUSSION
The expansion of NBS using genomic sequencing is being explored in a growing number of research studies (Adhikari et al., 2020; Bodian et al., 2016; Green et al., 2023; Holm et al., 2018; Jian et al., 2022; Kingsmore et al., 2022; Pichini et al., 2022; Roman et al., 2020). To ensure ethical implementation of NBSeq, it is crucial to obtain the perspectives of a wide range of parents who will be impacted. We conducted interviews and focus groups with a racially, ethnically, and socioeconomically diverse group of parents to explore their perspectives on the expansion of NBS to include genomic sequencing. Two other studies interviewed parents from similar populations about their views on NBSeq (Joseph et al., 2016; Timmins et al., 2022). Our study population differed, however, as there is a higher percentage of Black/African American/African participants, and the average educational level was lower (only one participant had a degree above a bachelor's degree) compared to the two prior studies. Our findings show that parents from diverse communities also broadly support NBSeq, as has been shown previously (Joseph et al., 2016; Timmins et al., 2022). In addition, our findings add new perspectives from diverse communities on the types of disorders to screen for and variants to return, perceived barriers to care following a positive screening result, and demonstrate less participant concern regarding data privacy.
In this study, parents identified a range of medical and personal benefits of NBSeq, including early diagnoses and treatment for children, as well as appropriate surveillance for adult-onset conditions as children age. Additionally, many parents were in favor of screening for some conditions for which no treatments are currently available, emphasizing the personal and emotional utility of this information, a common theme among prior studies (Peinado et al., 2020; Pereira et al., 2019; Timmins et al., 2022). However, participants in this study had fewer concerns about data privacy than those in prior studies (Joseph et al., 2016), and instead felt that data would be most appropriately stored in the electronic health record.
VUS pose several challenges with regard to genomic screening, but parents in this study demonstrated understanding of this category of variant and broadly supported their return. VUS are of particular relevance to healthcare inequities because of their higher prevalence in individuals of non-European descent (Downie et al., 2021). Genomic screening research protocols have varied in their handling of VUS, with some returning only likely pathogenic and pathogenic variants to participants (Ceyhan-Birsoy et al., 2019) and others, like the ongoing GUARDIAN study (https://guardian-study.org/), returning VUS for autosomal recessive conditions when in trans with a disease-associated variant. Parents in our study supported the return of VUS provided that their meaning and limitations were clearly communicated.
Our study also adds new information about parents' perceived concerns about care following a positive result on NBSeq. A prior randomized controlled trial of exome sequencing in ostensibly healthy newborns found that approximately 11% had a reportable genetic variant (Ceyhan-Birsoy et al., 2019). Several parents here highlighted the need for financial, work, and childcare support to improve their understanding of medical information and facilitate emotional coping.
4.1 Study limitations
This study has several limitations. First, only mothers participated, limiting the representativeness of this sample. Some parents were recruited through a hospital biobank or participated in other research studies and may not represent the views of parents who are more reluctant to engage in research related to genomic screening. Also contributing to this selection bias, we were unable to enroll as many Spanish-speaking participants as planned, in part because some prospective participants had concerns about what to expect in the interviews and chose not to participate. Additionally, while our study included a large proportion of Black and Hispanic mothers, the perspectives of other minoritized individuals who have historically been excluded from biomedical research were not represented.
Finally, although the authors attempted to remain neutral during each interaction with participants, their own positive attitudes and biases regarding NBSeq may have affected the development of the interview and focus group scripts, answers provided by the participants, and interpretation of the participant data. The framing of the questions and hypothetical scenarios may have led participants to underestimate the potential complexities of NBSeq, including uncertainty related to genomic results or the inability to unlearn information that has been shared. Participants may have provided different responses had these questions been developed or framed by a study author who had negative views of NBSeq. Relatedly, in our focus groups, participants may have collectively endorsed a positive view of NBSeq as they viewed this as the socially desirable answer.
Our findings also reflect participants' reactions to hypothetical NBSeq scenarios. More work must be done in the future to explore participants' real-world responses to this screening modality, such as psychosocial reactions and decisional regret. As NBSeq research programs expand, new research questions related to parental experience will be identified and can be studied in more detail.
5 CONCLUSIONS
This study adds to a growing understanding of stakeholder perspectives on NBSeq. Our findings reinforce that parents of diverse backgrounds are interested in receiving genomic information related to their child's future health risks, and further delineates the types of disorders and variants for which parents might find medical and personal utility, while also highlighting the need for parental support programs following the receipt of positive screening results. Such information should be used to inform future NBSeq programs that can improve childhood health without exacerbating existing healthcare disparities.
AUTHOR CONTRIBUTIONS
Dr. Gold contributed to the concept and design of the study, conducted interviews and focus groups, contributed to the data analysis, drafted the initial manuscript, and reviewed and revised the manuscript. Dr. Omorodion contributed to the concept and design of the study, conducted interviews and focus groups, contributed to the data analysis, and reviewed and revised the manuscript. Ms. del Rosario contributed to the design of the study, coordinated participant recruitment, contributed to the data analysis, and reviewed and revised the manuscript. Dr. Rivera-Cruz conducted interviews and focus groups and reviewed and revised the manuscript. Ms. Hsu contributed to the design of the study, conducted focus groups, and reviewed and revised the manuscript. Dr. Ziniel contributed to the design of the study, contributed to the data analysis, provided expert opinion on qualitative research techniques, and reviewed and revised the manuscript. Dr. Holm received funding for the study, conceptualized and designed the study, oversaw the study, contributed to the data analysis, and reviewed and revised the manuscript. Dr. Holm confirms that she had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All of the authors gave final approval of this version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGMENTS
This work was supported by the Greenwall Foundation (Dr. Holm, PI). Drs. Holm and Gold received support from grant U01TR003201 from the National Center for Advancing Translational Sciences and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Gold received support from grant K08HG012811 from the National Human Genome Research Institute. Dr. Rivera-Cruz received support from grant T32GM007748 from the National Institute of General Medical Sciences.
CONFLICT OF INTEREST STATEMENT
Dr. Gold is a paid consultant to RCG Consulting. Dr. Omorodion, Ms. del Rosario, Dr. Rivera-Cruz, Ms. Hsu, Dr. Ziniel, and Dr. Holm declare that they have no conflict of interest.
ETHICS STATEMENT
Human studies and informed consent: This study was approved by and conducted according to the ethical standards of the Boston Children's Hospital Institutional Review Board. Informed verbal consent for participation in this study was obtained from all participants prior to each interview and/or focus group.
Animal studies: No non-human animal studies were carried out by the authors for this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




