A systematic review and meta-analysis of telephone vs in-person genetic counseling in BRCA1/BRCA2 genetic testing
Abstract
Pathogenic variants in the BRCA1 and BRCA2 genes increase the risk of breast and ovarian cancer. Individuals with identified pathogenic variants in the BRCA1 or BRCA2 gene can benefit from cancer risk-reducing strategies. In the recent years, there has been an increase in the demand of genetic services. In light of the ongoing COVID19 pandemic, alternatives to face-to-face consultations have had to be considered and adopted, including telemedicine. Informed consent is necessary for genetic testing. Studies have suggested that increased levels of cancer-specific distress may impair the patient's ability to retain information, therefore, providing informed consent. This systematic review and meta-analysis aimed to answer if telephone genetic counseling for BRCA1 and BRCA2 genetic testing is non-inferior to in-person genetic counseling for the outcomes of cancer-specific distress and genetic knowledge. Databases of Medline, Embase, PsycINFO, CINAHL, SciELO, Web of Science, CENTRAL, ProQuest Dissertation & Theses Database, Clinicaltrials.gov, EU clinical trials register were accessed to identify any published or unpublished relevant literature. Random-effects models were used for the meta-analysis. Four studies were included in the qualitative synthesis of the results. Three studies were included in the quantitative synthesis of the results. Telephone genetic counseling was non-inferior compared to in-person genetic counseling for the outcomes of cancer-specific distress and genetic knowledge. Sensitivity analysis corroborated the main results. Telephone genetic counseling for BRCA1/BRCA2 genetic testing may be an alternative model of delivering genetic services in front of the increased demand/or when required by social context. However, the paucity of the evidence prevents from drawing strong conclusions regarding the generalizability of these results. Further research is needed to strengthen the conclusions.
What is known about this topic
Pathogenic variants in BRCA1 and BRCA2 confer a high lifetime risk of cancer in male and female carriers, and different models of service provision including telephone-based counseling may help expand access to genetic services.
What this paper adds to the topic
This systematic review and meta-analysis demonstrate that telephone-based genetic counseling is non-inferior compared to in-person genetic counseling with respect to the outcomes of cancer-specific distress and genetic knowledge.
1 INTRODUCTION
Pathogenic variants in the BRCA1 and BRCA2 genes are associated with an increased risk of hereditary breast and ovarian cancer (HBOC) in women, and an increased risk of breast and prostate cancers in men (Petrucelli et al., 2016). Germline pathogenic variants in the BRCA1 and BRCA2 genes are estimated to cause approximately 5% of breast cancers and between 8% and 17% of ovarian cancers in the population (Lang et al., 2017).
Advances in the understanding of the function of BRCA1 and BRCA2 have allowed development of targeted therapy for treatment of BRCA-deficient cancers, and for risk-reducing strategies to manage the increased risk of associated cancers and decrease morbidity and mortality (Eccleston et al., 2017).
Prior to genetic testing, informed consent should be obtained (NIH U.S. National Library of Medicine, 2020). For an informed consent to be valid, patients must have been given the information in an understandable way, conveying the implications, alternatives, benefits and risk of the genetic test (Samuel et al., 2017).
High levels of cancer-specific distress and low genetic knowledge have been found to impair the comprehension of genetic risk information, therefore, impairing the capacity to give informed consent (Martinez et al., 2015). High levels of cancer-specific distress and low levels of genetic knowledge have also been found to negatively impact the decision-making in risk-reducing management options in patients at risk of HBOC (Erblich et al., 2005; Price et al., 2010). Research has shown that addressing patients’ concerns and distress improved the comprehension of genetic information (Lerman et al., 2005) and decisions regarding risk management strategies (Price et al., 2010).
Different delivery models of genetic counseling have been described to cope with the increased demand of genetic counseling services and to overcome the limitations and barriers of face-to-face clinics, which may be impaired due to patient factors, limited health services resources, or social context factors (Pierle & Mahon, 2019). Some of these service delivery methods are video-conferencing genetic counseling or telephone genetic counseling (McCuaig et al., 2018; Terry et al., 2019). Most recently, the international COVID19 pandemic has prompted a paradigm shift in delivery of all types of medical consultations, including genetic counseling services (Contreras et al., 2020).
These suggested service delivery models have some advantages and limitations. Telephone genetic counseling removes the barriers of time and travel costs and it could be an accessible resource for genetic centers and patients worldwide; however, it limits the ability to perceive patients’ body language and non-verbal cues, and is not suitable for patients with hearing difficulties (Burgess et al., 2016). Video-conferencing allows for a digitalized face-to-face appointment that removes the barriers of time and travel costs. However, video-conferencing involves an investment in technology and equipment for delivery of the appointment. Patients are also required to have a working computer, internet access, and technological literacy, which may lead to health inequity. Difficulties in establishing rapport with patients and challenges associated with use of technology have also been reported (Hilgart et al., 2012). Telephone genetic counseling does not require a big financial investment to be implemented in hospitals. For these reasons, the present study focused on telephone genetic counseling as an alternative delivery model.
This systematic review and meta-analysis aimed to explore the current research evidence to answer if telephone genetic counseling for BRCA1 and BRCA2 genetic testing is non-inferior to in-person genetic counseling considering the outcomes of cancer-specific distress and genetic knowledge.
2 METHODS
A systematic review and meta-analysis were conducted following the guidance provided by the Cochrane Handbook (Higgins et al., 2019).
2.1 Search strategy
An electronic search was conducted to identify published relevant research until April 2020, using keywords ‘genetic counseling’ and ‘telephone’. No language or date limit filters were applied. Using the Wolters Kubler platform Ovid, the following scientific literature databases were accessed: Ovid Medline(R), Embase, and PsycINFO. Additional searches were conducted in the following scientific databases: CINAHL, Web of Science, ScIELO, Cochrane database, Cochrane Central Register of Controlled Trials. A search was also conducted in the dedicated journal to genetic counseling research ‘Journal of Genetic Counseling’. In order to maximize the results, a high sensitivity and low precision approach search was completed; unpublished literature was included and identified from the following sources: ProQuest Dissertation & Theses Database, Clinicaltrials.gov and EU clinical trials register. The search strategy was designed as ((genetic AND counseling) OR (genetic counseling)) AND (telephone OR telemedicine). Controlled vocabulary for each database and free text was combined in the search strategy for each concept. Wildcards were used to convey American and British English spelling. No date or language filters were applied.
2.2 Inclusion criteria
The study included randomized controlled trials including males or females aged over 18 years old, comparing telephone genetic counseling to in-person genetic counseling. Studies had to have specified cancer-specific distress and/or genetic knowledge among the measured outcomes.
2.3 Selection of studies
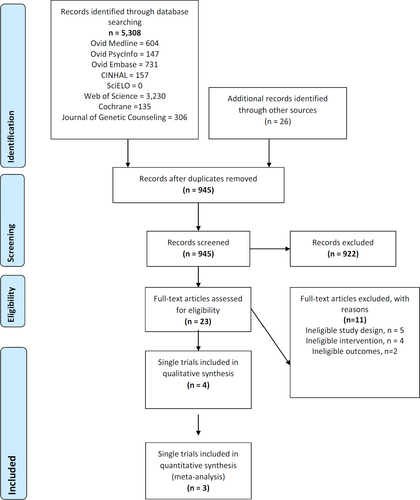
Retrieved results from different databases were merged in citation manager EndNote v7.1 (Figure 1). Manual curation by a single author removed duplicate articles. Thereafter, a review of the titles and abstract of the remaining articles was manually undertaken to screen for eligibility. Finally, full-texts were reviewed to confirm eligibility for inclusion. When a study had more than one article reporting different outcomes, articles from the same study were grouped and analyzed as a single study. Data were collected for type of study, type of intervention, demographic characteristics, and outcomes of cancer-specific distress and genetic knowledge.
2.4 Critical appraisal and risk of bias
Critical appraisal and risk of bias were assessed using The Cochrane Collaboration tool (Higgins et al., 2011).The Cochrane Collaboration tool consists of six domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported results. Each domain aims to make a judgment on the adequacy of the study and the risk of bias for each domain-item. The risk of bias is classified as low risk, some concerns or high risk of bias. The overall risk of bias is the same as the highest risk of bias scored by any of the domain-items.
RevMan5 software was used to illustrate the appraised risk of bias for each study.
2.5 Qualitative and quantitative synthesis of the results
Qualitative synthesis refers to the collection and critical appraisal of all studies meeting eligibility criteria as described above. Studies were appraised for risk of bias, and those found not to be at high risk of bias were included in the quantitative synthesis. Quantitative synthesis refers to combination of the results of eligible studies in a pooled estimate for the outcomes of cancer-specific distress and genetic knowledge.
2.6 Statistical analysis
Mean difference and standardize mean different were used to combine results of single trials into a pooled result. Meta-analysis was undertaken using random-effects model. Chi-square test was used to measure heterogeneity among studies, and I2 test to measure inter-study variability. A sensitivity analysis was undertaken to corroborate the results against the arbitrary decisions of the analysis. Sensitivity analysis included: (a) fixed-effect model analysis for cancer-specific distress and genetic knowledge, (b) a random-effects model meta-analyses for the outcomes of cancer-specific distress and genetic knowledge including those studies excluded from the primary analysis due to high risk of bias, and (c) a fixed-effects meta-analyses for the outcomes of cancer-specific distress and genetic knowledge including those studies excluded from the primary analysis due to high risk of bias. The statistical analysis was done using R software version 3.6.0. R software was run using the integrated development environment platform RStudio 1.2.1335.
3 RESULTS
Five thousand three hundred and eight studies were identified through database searching. Twenty-six additional records were identified through other sources. Four studies were included in the qualitative synthesis. The studies by Jenkins et al. (2007), Kinney et al. (2014), Schwartz et al. (2014), and Bradbury et al. (2018) met the eligibility criteria after full-text assessment. Of those, three studies were included in the quantitative synthesis (Figure 1). The studies of Schwartz et al. (2014), Kinney et al. (2014), and Bradbury et al. (2018) had more than one article reporting outcomes (Table 1). The first chronological article was selected as the main article for the study. For narrative purposes, the in-text references of the studies are made using the main article. Characteristics of the studies are outlined in Table 1.
| Study | Articles reporting study findings | Study design | Intervention | Control group | Visual aids | Delivery of the intervention | Number of participants, total (standard care: telephone) | Geographical location |
|---|---|---|---|---|---|---|---|---|
| Bradbury et al. (2018) | Beri et al. (2019) | randomized multicenter non-inferiority controlled trial | telephone genetic counseling | in-person genetic counseling | Visual aids used in both groups | board-certified genetic counselors and genetic nurses |
970 497:473 |
USA |
| Jenkins et al. (2007) | Single article | single-center randomized equivalence controlled trial | telephone genetic counseling | in-person genetic counseling | Not used | combination of physicians and advanced nurse practitioners |
102 50:52 |
USA |
| Kinney et al. (2014) |
Kinney et al. (2014) Kinney et al. (2016), Chang et al. (2016), Steffen et al. (2017) |
randomized single-center non-inferiority controlled trial | telephone genetic counseling | in-person genetic counseling | Visual aids used in both groups | board-certified genetic counselors |
1,012 510:502 |
USA |
| Schwartz et al. (2014) |
Schwartz et al. (2014) Butrick et al. (2015), Jacobs et al. (2016), Interrante et al. (2017) |
randomized multicenter non-inferiority controlled trial | telephone genetic counseling | in-person genetic counseling | Visual aids used in both groups | board-certified genetic counselors |
669 334:335 |
USA |
Note
- Some studies had more than one article reporting findings. The first published article chronologically was selected as the main article of the study as a reference.
The studies that met the eligibility criteria had a total of 2,753 participants, with an average of 619 participants and median of 669 (102–1012).
The vast majority of participants were non-Hispanic white (83.43%) and females (92.94%). Participants age ranged from 18 to 85 years old. The average weighted age of the studies was 47.55 years old. Most of the participants were married (68%), with college education or higher (55.26%) and with a personal history of cancer (62.45%).
All the studies that met the eligibility criteria focused on patients at risk of HBOC (Bradbury et al., 2018; Jenkins et al., 2007; Kinney et al., 2014; Schwartz et al., 2014).
3.1 Domain 1 risk of bias: bias arising from randomization process
The study of Schwarz et al. (2014) was judged as to raise some concerns arising from the randomization process, as allocation of participants was done using fixed randomized blocks; thus, last block allocation could be predicted by the staff involved.
The study of Kinney et al. (2014) did not raise concerns in regarding risk of bias in the randomization process.
The study of Bradbury et al. (2018) and Jenkins et al. (2007) were judged as to raise some concerns in the randomization process, as randomization components or concealment strategies were not described in the manuscript or study protocol.
3.2 Domain 2: Bias due to derivations from intended interventions
The studies of Schwartz et al. (2014), Kinney et al. (2014), and Bradbury et al. (2018) were judged as low risk of bias in the second domain. This judgment was based on the fact that there were no deviations from the interventions in the studies, intention to treat analysis was complemented with a sensitivity analysis, and non-inferiority margins used for the analysis were defined an justified in the reports.
The study of Jenkins et al. (2007) was judged as high risk of bias in the second domain for the following reasons: the type of analysis was not described, not enough information was given about non-adherence analysis, and statistical analysis was not corrected for multiple comparisons.
3.3 Domain 3: Bias due to missing data outcome
The studies of Schwartz et al. (2014) and Kinney et al. (2014) did not have a high dropout of participants; thus, these studies were judged as low risk of bias.
The study of Bradbury et al. (2018) reported low adherence to in-person genetic counseling; thus, the risk of bias was judged as to raise some concerns.
The study of Jenkins et al. (2007) did not report adherence to the intervention groups; thus, the risk of bias was judged as high.
3.4 Domain 4: Bias in measurement of the outcome
The measurement of cancer-specific distress in all studies was done using a validated tool with good psychometric properties; thus, the risk of bias was judged as low.
The measurement of genetic knowledge in the studies of Schwartz et al. (2014), Kinney et al. (2014), and Bradbury et al. (2018) was done using validated tools; thus, the risk of bias was judged as low. No articles were found evaluating the psychometric properties for the tool used in the study of Jenkins et al. (2007) to measure the outcome of genetic knowledge; thus, risk of bias was judged as to raise some concerns.
3.5 Domain 5: Bias in selection of the reported results
The studies of Schwartz et al. (2014), Kinney et al. (2014), and Bradbury et al. (2018) did not provide enough information to determine whether multiple analysis of the data was done in the studies. Variations of reporting of the same outcome have been found in the different published reports of studies, which suggest post hoc derived analysis of the data. For this reason, the risk of bias was judged as to raise some concerns.
The study of Jenkins et al. (2007) was a secondary analysis using a cohort from another main study. Furthermore, the study was not powered for any of the main outcomes. For these reasons, the risk of bias was assessed as high.
3.6 Overall risk of bias
The studies of Schwartz et al. (2014), Kinney et al. (2014), and Bradbury et al. (2018) were judged as to raise some concerns regarding overall risk of bias. The study of Jenkins et al., 2007 was judged as high risk of bias (Supplementary information 1).
The studies conducted of Schwartz et al. (2014), Kinney et al. (2014), and Bradbury et al. (2018) were included in the qualitative and quantitative synthesis.
The study conducted by Jenkins et al. (2007) was included in the qualitative synthesis. However, based on the high risk of bias, it was excluded from the quantitative synthesis.
For a detailed explanation of each study's risk of bias domain, please refer to the Supplementary Information 2.
3.7 Cancer-specific distress: systematic review
Cancer-specific distress was a primary outcome for the studies of Schwartz et al. (2014) and Kinney et al. (2014). Cancer-specific distress was a secondary outcome for the studies of Jenkins et al. (2007) and Bradbury et al. (2018).
All the studies that met the eligibility criteria measured cancer-specific distress (Bradbury et al., 2018; Jenkins et al., 2007; Kinney et al., 2014; Schwartz et al., 2014), using a validated tool known as Impact of Event Scale (IES). The Impact of Event Scale is a 15 item scale that measures the subjective stress perceived by a patient regarding a specific event (Horowitz et al., 1979). The scores range from 0 to 75, with higher scores indicating higher perceived stress.
The psychometric properties of IES have been broadly validated in different types of patients (Sundin & Horowitz, 2018), specifically in women at risk of hereditary breast or ovarian cancer. The psychometric properties showed reliability and internal consistency when evaluated in patients at risk of HBOC (Thewes et al., 2001). Impact of Event Scale is considered a reliable tool to measure cancer-specific distress in BRCA1/BRCA2 genetic testing.
All studies measured cancer-specific distress in both groups at baseline.
Schwartz et al. (2014) and Kinney et al. (2014) measured cancer-specific distress one week after the pre-testing counseling session. Both studies found telephone genetic counseling non-inferior to in-person genetic counseling one week after the pre-test genetic counseling session for BRCA1/BRCA2 genetic testing (Kinney et al., 2014; Schwartz et al., 2014).
All studies (Bradbury et al., 2018; Jenkins et al., 2007; Kinney et al., 2014; Schwartz et al., 2014) measured cancer-specific distress after the result disclosure genetic counseling session.
Considering cancer-specific distress following result disclosures, telephone-based genetic counseling was deemed non-inferior compared to in-person genetic counseling, in all studies (Table 2).
| Study | Group | Baseline | Pre-results | Post-results | ||
|---|---|---|---|---|---|---|
| 1 week | ≤ 3 months | 6 months | 12 months | |||
| Bradbury et al. (2018) | In-person | 16.91(13.89) | – | 18.52(14.57) | – | – |
| Telephone | 17.21(13.70) | – | 17.43(14.23) | – | – | |
| Comparison | – | – | upper-bound one-sided 97.5% CI = 1.09; non-inferiority limit = 4 | – | – | |
| Jenkins et al. (2007) | In-person | 8.14(8.44) | – | 6.52(10.65) | 8.04(12–54) | 6.10(8.60) |
| Telephone | 11.96(14.18) | – | 7.34(9.87) | 7.38(9.46) | 5.83(8.98) | |
| Comparison | – | – | (p = .046) | (p = .042) | (p = .83) | |
| Kinney et al. (2014) | In-person | 15.5(14.18) | 12.5(14.18) | 14(27.24) | 13.4(14.75) | 10.06(12.46) |
| Telephone | 15.4(14.72) | 12.9(14.72) | 11(26.05) | 13.1(15.29) | 11.19(13.26) | |
| Comparison | – | – | upper-bound one-sided 97.5 CI = −3.96; non-inferiority limit = 4 | upper-bound one-sided 97.5 CI = 0.3; non-inferiority limit = 4 | upper-bound one-sided 97.5% CI = 0.66; non-inferiority limit = 4 | |
| Schwartz et al. (2014) | In-person | 20.7(15.5) | 17.0(15.5) | 14.0(14.7) | – | 13.1(14.3) |
| Telephone | 23.2(15.1) | 17.3(15.1) | 14.8(14.9) | – | 12.6(14.3) | |
| Comparison | – | – | upper-bound one-sided 97.5% CI = 1.16; non-inferiority limit = 4 | – | upper-bound one-sided 97.5% CI = −0.07; non-inferiority limit = 4 | |
Note
- Studies used Impact of Event Scale to measure the outcome of cancer-specific distress. Impact of Event scale ranges. 0–8: no significant impact. 9–25: possible impact. 26–43: moderate impact 44–74: severe impact.
3.8 Cancer-specific distress: meta-analysis
Pre-test genetic counseling meta-analysis showed that telephone genetic counseling was non-inferior compared to in-person genetic counseling for the outcome of cancer-specific distress (95% CI −1.15, 1.86; upper-bound non-inferiority margin: 4; X2 < 0.95, I2 0%) (Figure 2a).
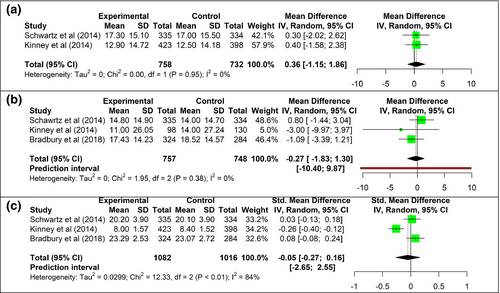
Post-test genetic counseling meta-analysis showed that telephone genetic counseling was non-inferior to in-person genetic counseling for the outcome of cancer-specific distress (95% CI −1.83, 1.3; upper-bound non-inferiority margin: 4, X2 = 0, I2 = 0%) (Figure 2b).
3.9 Genetic knowledge: systematic review
Genetic knowledge was a primary outcome for the studies of Schwartz et al. (2014) and Bradbury et al. (2018). Genetic knowledge was a secondary outcome for the studies of Jenkins et al. (2007) and Kinney et al. (2014).
Studies used different tools to measure genetic knowledge, precluding direct comparison.
Schwartz et al. (2014) used the ‘Breast Cancer Genetic Counseling Knowledge Scale’ (BCGCKS) to measure genetic knowledge. The BCGCKS is a 27-item questionnaire that evaluates knowledge of breast and ovarian cancer genetics (Erblich et al., 2005). The BCGKS showed excellent psychometric properties, with high validity, high reliability and it was validated in a cohort of women that underwent breast and ovarian cancer genetic counseling (Erblich et al., 2005). Kinney et al. (2014) used an adapted 10-item questionnaire developed by the University of Michigan to measure genetic knowledge. The adapted questionnaire showed good psychometric properties in breast cancer patients or patients at-risk of breast cancer (Wang et al., 2005). Bradbury et al. (2018) evaluated genetic knowledge using a 6-item adapted questionnaire from the Cancer Genetics Knowledge Scale (CGKS). The CGKS showed good psychometric properties in a cohort of patients at-risk of breast and ovarian cancer; however, the socioeconomic status of the participants was high, which reduces the generalizability of the scale use (Kelly et al., 2004). The participants in the study of Bradbury et al. (2018) had a high socioeconomic status, which is similar to the cohort of patients where CGKS was validated. Jenkins et al. (2007) used a modified 10-item true/false questionnaire developed by the National Human Genome Research Institute (NHGRI) Cancer Genetics Studies. No articles were found evaluating the psychometric properties of the questionnaire.
All studies measured genetic knowledge outcome as a baseline measure.
Kinney et al. (2014) measured genetic knowledge one week after pre-testing genetic counseling. Schwartz et al. (2014) measured genetic knowledge within two weeks after the pre-test genetic counseling session. Jenkins et al. (2007) measured genetic knowledge at three, six, and twelve months after result disclosure. Bradbury et al. (2018) evaluated genetic knowledge within one week after result disclosure genetic counseling session. The studies of Jenkins et al. (2007), Schwartz et al. (2014), and Kinney et al. (2014) did not show statistically significant differences between in-person compared to telephone genetic counseling for genetic knowledge. The study of Bradbury et al. (2018) found that genetic knowledge was inferior in the telephone group compared to the in-person group (Table 3).
| Tool used | Group | Baseline measure | Post genetic counseling measure | |
|---|---|---|---|---|
| Bradbury et al. (2018) | 6-item adapted questionnaire from Cancer Genetics Knowledge Scale (CGKS)a | In-person | 22.52(2.59) | 23.07(2.72) |
| Telephone | 23.1(2.58) | 23.29(2.53) | ||
| comparison | – | lower bound one-sided 98.33% CI = −0.3; non-inferiority margin = −0.247 | ||
| Jenkins et al. (2007) | 10-item, true–false scale modified from tool developed by the NHGRI Cancer Genetics Studies Consortiuma | In-person | 8.12(1.7) | 9.68(0.71) |
| Telephone | 7.83(2.08) | 9.79(0.46) | ||
| Comparison | – | 3 months (p = .62), 6 months (p = .56) or 12 months (p = .41) | ||
| Kinney et al. (2014) | 10-item questionnaire (university of Michigan)a | In-person | 7(1.66) | 8.4(1.52) |
| Telephone | 6.9(1.65) | 8(1.57) | ||
| Comparison | – | lower bound one-sided 97.5% CI = 0.188; non-inferiority margin = −1 | ||
| Schwartz et al. (2014) | 27-item questionnaire Breast Cancer Genetic Counseling Knowledge Scale (BGCKS)a | In-person | 17.0 (4.8) | 20.1(3.9) |
| Telephone | 17.3 (4.8) | 20.2(3.9) | ||
| Comparison | – | lower bound one-sided 97.5% CI = −0.61; non-inferiority margin = −1) |
- a Higher scores indicate higher knowledge.
3.10 Genetic knowledge: meta-analysis
Meta-analysis showed that telephone genetic counseling was non-inferior compared to in-person genetic counseling for the outcome of genetic knowledge (95% CI −0.27, 0.16, X2 p < .01, I2 84%) (Figure 2c).
3.11 Sensitivity analysis
Primary results were corroborated using a sensitivity analysis. Sensitivity analysis results were consistent with the primary meta-analysis results. Results for the sensitivity analysis and illustrative forest plots can be found in Supplementary information 3.
4 DISCUSSION
The systematic review and meta-analysis of the evidence showed that telephone genetic counseling was non-inferior to in-person genetic counseling for the outcomes of cancer-specific distress. The systematic review for the outcome of genetic knowledge found that telephone genetic counseling was non-inferior to in-person genetic counseling in three studies. One study showed that genetic knowledge gain was inferior in telephone genetic counseling compared to in-person genetic counseling. The meta-analysis synthesis of the studies showed that telephone genetic counseling was non-inferior to in-person genetic counseling for the outcome of genetic knowledge. These results were corroborated with a sensitivity analysis.
Only three studies were included in the meta-analysis. While this is a small number of studies, a meta-analysis allowed interrogation of results and creation of a pooled estimate from a total of 758 patients for the outcome of cancer-specific distress and 1,016 patient for the outcome of genetic knowledge, increasing the statistical power of the analyses. Some of the studies did not have cancer-specific distress or genetic knowledge as a main outcome; thus, the statistical analysis in those studies was not powered for the secondary outcomes. The results of this meta-analysis provide a further level of evidence for the use of telephone genetic counseling in circumstances where in-person genetic counseling is not recommended. Furthermore, this meta-analysis contrasts the differences in non-inferiority between studies for the outcome of genetic knowledge.
Bradbury et al. (2018) was the only study that showed that telephone counseling was inferior to in-person counseling for the outcome of genetic knowledge. This discrepancy with the other studies could be explained due to the fact that Bradbury et al. (2018) calculated the non-inferiority interval based on knowledge gain, rather than based on a one-sided confidence interval of the difference between the group means like the other studies did (Jenkins et al., 2007; Kinney et al., 2014; Schwartz et al., 2014). In Bradbury et al. (2018) study, the knowledge in the telephone group was higher at baseline than the post-consultation scores in the in-person group; hence, it could be that knowledge gain was limited in the telephone group due to a ceiling effect on the knowledge measurement (Wang et al., 2009). The post hoc analysis of the standardized mean difference undertaken in this meta-analysis suggested that there were no differences in the outcome of knowledge between telephone genetic counseling and in-person genetic counseling (95% CI −0.08; 0.24) (Figure 2c).
The outcome of genetic knowledge used different measurement tools in each trial. For this reason, the meta-analysis used standardized mean difference (SMD) as an indirect measure, comparing if there were differences in the magnitude of the effect between both interventions. This approach has some limitations that should be considered. First, comparing the magnitude of the effects of the interventions is different than non-inferiority, as originally intended in the research trials. Secondly, the magnitude of the effect size can be affected by the composition of the study sample, which may not reflect real world conditions. Despite the limitations of SMD, SMD allows to include a quantitative synthesis of the results in a meta-analysis, which can be used to complement the qualitative synthesis of the results. However, results should be interpreted with caution.
The included studies present some limitations that may affect the applicability of the evidence. All the studies that met the eligibility criteria of this review were conducted in the United States of America. The demographics of the study participants were mostly women, non-Hispanic white, with higher than average income, and with college education. The demographic characteristics of patients attending genetic services in other regions may be different. The studies did not look at the preferences of women that declined taking part in the randomization of telephone or in-person genetic counseling. Results for cancer-specific distress or genetic knowledge may have differed for people with a strong preference for in-person genetic counseling. Furthermore, in the reports of articles from the studies of Schwartz et al. (2014) and Kinney et al. (2014) there was a high prevalence of overlapping authors that could have added dependence to both studies results. This was not investigated in this review.
This systematic review has some potential limitations that should not be overlooked. The use of other search terms, such as such as ‘virtual’, ‘conference,’ or ‘remote’, might have yielded a higher number of results; thus, relevant studies might have not been retrieved with the search strategy implemented. The selection and assessments of studies eligible for this review were done by a single author potentially introducing a risk of bias. Another potential source of bias is intrinsic to the research methodology of the included studies, most of which were non-inferiority trials. If the studies included had other sources of bias that were overlooked, the nature of the design of the studies could create false-positive results of non-inferiority.
Some of the limitations and potential sources of bias in this review are inherent to the methodology of a meta-analysis. Poor quality of the studies included in a meta-analysis can yield results that are invalid. The present meta-analysis tried to minimize this limitation by excluding from the quantitative synthesis those studies at high risk of bias. The studies at high risk of bias were included in posterior sensitivity analysis to corroborate the main results. It was observed that all the included studies presented some concerns regarding the risk of bias assessment. Further studies at low risk of bias are needed to strengthen the quality of the evidence.
The evidence of the meta-analysis results for the outcome of cancer-specific distress was rated as moderate; and for genetic knowledge was rated low, according to the GRADE working group guidelines. All the trials included in the meta-analysis were randomized controlled trials, which gave a high level of quality. However, participants in the trials were primarily non-Hispanic white, women, with higher education, which is considered indirectness for population generalizability, which lowered the quality from high to moderate for both outcomes. The quality was further downgraded to low for the outcome of genetic knowledge due to imprecision. This is based on the discrepancy of the effect size in the studies of Kinney et al. (2014) and Bradbury et al. (2018). For more details about the quality of the evidence grading, please refer to Supplementary Information 4.
The qualitative synthesis of the data included four studies, believed to represent the only existing randomized clinical trials that compare cancer-specific distress and/or genetic knowledge in telephone genetic counseling and in-person genetic counseling for BRCA1/BRCA2 genetic testing. To the authors knowledge, this is the only study presenting a systematic review and meta-analysis comparing in-person versus telephone genetic counseling for the outcomes of cancer-specific distress and genetic knowledge in BRCA1/BRCA2 genetic testing to date.
5 CONCLUSION
The results from this systematic review and meta-analysis suggest that telephone genetic counseling could be an alternative delivery method to in-person genetic counseling for patients that are eligible for BRCA1/BRCA2 genetic testing. These results might be relevant to different health professional providing genetic counseling for BRCA1/BRCA2 genetic testing. The studies included genetic counseling provided by a range of different health professionals, such as: physicians, board-registered genetic counselors, genetic nurses and advance nurse practitioners. Telephone consultations might be a preferable option for some patients, such as patients with neutropenia, ill patients or geographically distant patients. Telephone genetic counseling could also complement trending genetic testing practices, such as mainstreaming genetic testing, for those patients that require extra support before consenting to genetic testing. Further research is needed to corroborate these results in other ethnic groups and/or levels of education.
AUTHOR CONTRIBUTIONS
Xavier Bracke conceived and developed the research, acquired the data, analyzed and interpreted the data, and drafted the manuscript. Dr. Terri McVeigh assisted with data interpretation, drafting and critical revision of the article. Dr. Jonathan Roberts acted as academic supervisor during the research, and helped design the study, and assisted in interpretation of the data. All authors approved the final draft of the manuscript and agreed upon its contents.
ACKNOWLEDGMENTS
The work was performed by Xavier Bracke as part of his training.
COMPLIANCE WITH ETHICAL STANDARDS
CONFLICT OF INTEREST
Xavier Bracke, Jonathan Roberts, and Terri McVeigh declare that they have no conflicts of interest.
HUMAN STUDIES AND INFORMED CONSENT
No human studies were conducted for this research.
ANIMAL STUDIES
No non-human animal studies were conducted for this research.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




