Cardiac remodeling from the fetus to adulthood
Funding information: Fundación Mutua Madrileña; Fundació Jesus Serra; Cerebra Foundation for the Brain Injured Child; Fondo Europeo de Desarrollo Regional (FEDER); ISCIII-Subdirección General de Evaluación; Instituto de Salud Carlos III, Grant/Award Numbers: INT21/00027, PI20/00246
Abstract
Prenatal cardiac remodeling refers to in utero changes in the fetal heart that occur as a response to an adverse intrauterine environment. In this article, we will review the main mechanisms leading to cardiac remodeling and dysfunction, summarizing and describing the major pathological conditions that have been reported to be related to this in utero plastic adaptive process. We will also recap the current evidence regarding the persistence of fetal cardiac remodeling and dysfunction, both in infancy and later in adult life. Moreover, we will discuss primary, secondary, and tertiary preventive measures and future clinical and research aspects.
1 INTRODUCTION/RATIONALE
The human fetal heart is one of the first organs to develop and perform its function during the process of embryogenesis. By the end of the third week of gestation, fetal heart is capable of achieving its primary role -blood circulation and supply- to provide oxygen, nutrients, and waste products to and from the fetus.1, 2 The fetal heart is a kinetic organ whose shape changes considerably during development in an effort to achieve its duty in the optimal way. Several studies conducted on animal models, have shown that the formation of the embryonic heart results from a dynamic interplay between genetic and environmental factors, such as pressure loading and blood flow. Hemodynamic forces induce the expression of genes that allow cardiac tissues to react to physical environmental stimuli. In this way, cardiac function, development, and remodeling are strongly linked.3, 4
2 DEFINITION OF CARDIAC REMODELING AND DYSFUNCTION
In the framework of these complex interactions, the heart is likewise the key organ by which the fetus fits an adverse intrauterine environment.5 When an insult occurs, the fetal heart responds and adapts to it by changing its structure, shape, and size, allowing myocytes to work in their most efficient (mechanical) conditions; all in order to ensure an optimal blood supply to main organs, such as brain, coronary arteries, or suprarenal glands. The fetal heart adapts at an organ, tissue, and sub-cellular level.6 This process of structural and functional adaptation is termed as cardiac remodeling.5, 7
Beyond the macrostructure of the heart -shape and size-, the process of cardiac remodeling also determines subclinical microstructural alterations, at the tissue and subcellular level, such as fiber orientation, number of cardiomyocytes, sarcomere length, or mitochondrial rearrangement.6-8 In the early stages of an insult, before manifest heart failure occurs, alterations in cardiac shape and architecture lead to a phase of subclinical cardiac dysfunction, either of systolic and/or diastolic function.7, 9, 10 In recent decades, improved diagnostic technologies for evaluation of the fetal heart have allowed, along with cardiac morphologic assessment, the study of these fine subclinical functional changes.11, 12
2.1 Determinants of fetal cardiac (dys-)function
As an adverse working condition sets in, the impairment of cardiac function occurs gradually.
In the early stages of an insult, a phase of subclinical cardiac dysfunction is established before the development of an overt heart failure condition.10
- The orientation of cardiac fibers in the context of the layered heart muscle architecture, which itself determines the complex three-dimensional motion leading to myocardial contraction. Distinguished longitudinal endocardial fibers account for longitudinal deformation (in the base-to-apex direction), radial fibers in the middle portion of the cardiac wall are responsible for the radial one (from endo- to epicardium) and circumferential fibers, perpendicular to both of the above, involved in rotational movements (along the short-axis circumference).11 Hence, myocardial contractility influences both the force development of the myocardium and its regional deformation.12
- The ventricular loading, differentiated into volume loading and pressure loading. The first, called preload, is mostly determined by venous return, which is essentially the blood volume filling the ventricles at the end of diastole. The second, the afterload, represented by the pressure in the outflow of the ventricles.12
- The plasticity of myocardial tissue, related to the in-utero natural maturation of the cardiac organ and the peculiar fetal circulation typified by the physiological left/right shunts.13
2.2 Main techniques and indices used to assess fetal cardiac function
Once defined these extrinsic and intrinsic determinants of cardiac (dys-)function, its gradual onset is also provable with the progressive alteration of specific instrumental parameters that represent the different aspects of cardiac function and of these determinants themselves.11 In recent decades, improved diagnostic technologies for evaluation of the fetal heart (from the well-known conventional Doppler, M-mode and tissue Doppler imaging to the most innovative 2D speckle tracking and 4D spatio-temporal image correlation [STIC] techniques) have also allowed, along with cardiac morphological assessment, the study of fine subclinical functional changes of the early phase of an insult.14
These parameters can be categorized into indicators for the evaluation of systolic cardiac function and those for the evaluation of diastolic cardiac function.9 The most commonly used systolic parameters comprise: ejection fraction and cardiac output to obtain an esteem of blood volume, respectively assessed by 2D, M-mode or 2D speckle tracking and 2D conventional Doppler or STIC15; mitral and tricuspid annular displacement evaluated with M-mode or 2D speckle tracking15 and systolic annular peak velocity with spectral or color tissue Doppler imaging (TDI) techniques, for the study of cardiac motion; strain and strain rate for the evaluation of myocardial deformation using color TDI or 2D speckle tracking. Diastolic function parameters, conversely, include: precordial veins pulsatility index (DV and others), early diastolic filling/atrial contraction E/A Ratio, diastolic annular peak velocities and isovolumetric relaxation time; all of which can be measured by conventional Doppler and, in the case of the latter two, by TDI.12, 14, 15
3 CARDIAC REMODELING IN FETAL LIFE: CONCEPT OF FETAL PROGRAMMING
Recent data demonstrated that cardiac remodeling and dysfunction that developed in response to an adverse intrauterine environment could not regress when the insult disappears postnatally.
This could be explained by the “fetal programming hypothesis”: an adaptive response to an adverse environment during fetal life can cause structural, functional, and metabolic changes that can persist into postnatal life and increase susceptibility to certain diseases in adulthood.16 Indeed, fetal life represents a critical, “plastic” period of development in which key organs are programmed to adapt and match specific environments. Epigenetic modifications might explain the most important mechanism underlying fetal programming, the phenotypic effects of which may be manifest even later in adulthood. A mismatch between phenotype established in utero during the process of fetal programming and the characteristics of the current environment in adult life, increases the risk of cardiovascular diseases.17
Especially, as regards cardiovascular diseases, fetal programming seems to act following two main processes: metabolic programming and primary cardiovascular programming.6, 8, 18 The metabolic programming hypothesis is the consolidated one postulated by David Barker's group -and further confirmed by a large number of epidemiological and animal studies-6, 18 in the early 1990s. According to this theory, an intrauterine environment with restricted nutrient intake induces the manifestation of genetic pathways for adapting to such environment; if in the postnatal period the availability of nutrients becomes normal, the expression of these genes predisposes to a higher incidence of metabolic diseases (obesity, diabetes mellitus, and metabolic syndrome) that represent known risk factors for cardiovascular disease.16
4 MECHANISMS OF PRENATAL CARDIAC REMODELING
As mentioned earlier, the fetal heart is a fundamental organ to hemodynamic response to permanent or transient insults (i.e., chronic hypoxia, pressure/volume overload, toxins, infections, etc.). An insult can directly damage to the myocardium or overload it. These mechanisms may act separately, consecutively, or simultaneously to each other. In the following paragraphs, we will discuss the most common mechanisms related with prenatal cardiac remodeling.
4.1 Myocardial damage/toxicity
The developing myocardium could be directly affected by exogenous toxins19, 20 or hypoxia inducing cell loss/damage and a reduction of contractility.21-23 Genetic cardiomyopathies could also associate abnormal myocardial contractility. The decrease in myocardial contractility is usually compensated by myocardial hypertrophy.
4.2 Pressure overload
Pressure overload occurs when the heart needs to develop high pressures in order to pump the blood against an increased afterload, for instance in aortic or pulmonary valve stenosis/atresia,24 increased placental resistance (i.e., preeclampsia and/or fetal growth restriction),25, 26 thoracic tumors, assisted reproductive technologies and other conditions. Increased intracardiac pressure induces higher wall stress and triggers cardiac remodeling. First, the heart changes to a more spherical shape -with a lower radius of curvature- in order to reduce wall stress. In addition, the myocytes will increase their contractile capacity by hypertrophying. Atria -unable to hypertrophy- will react to pressure overload by dilating.
4.3 Volume overload
Volume overload occurs when the heart needs to pump a larger than normal amount of blood. An increase blood volume usually triggers atrial enlargement, ventricular dilation, and cardiomegaly. Common prenatal causes of volume overload are fetal anemia,27-29 vascularized tumors (sacrococcygeal teratoma30 or aneurism of vein of Galen31), precordial venous anomalies,32, 33 and twin-to-twin transfusion syndrome (TTTS) recipient twin.
5 TYPES/EXAMPLES OF FETAL CARDIAC REMODELING
Over the past decade, several studies have been carried out to demonstrate how intrauterine adverse environments can induce direct cardiac changes, leading to primary cardiovascular remodeling and dysfunction.6, 8, 18
5.1 Fetal growth restriction
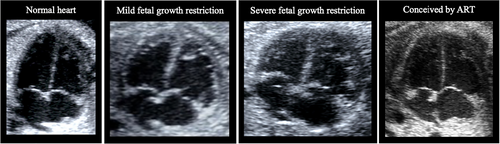
Fetal growth restriction embodies the best and most studied model of fetal cardiac remodeling and dysfunction.34, 35 The main culprit in fetal growth restriction is placental insufficiency. Indeed, the reduced oxygen and nutrients supply to the growing fetus may directly affect cardiomyocytes and at the same time, the increased placental resistance to blood flow leads to an increase in cardiac afterload. Therefore, the fetal heart at the beginning develops a more spherical shape to reduce wall stress (this is the main pattern in late-onset fetal growth restriction). However, if the insult continues and/or it is more severe, increased sphericity may not be sufficient: the heart becomes hypertrophic to increase contractility and decrease local wall stress (This typically occurs in early-onset fetal growth restriction). To note, volume overload would also explain the cardiomegaly and mild pericardial effusion in severe fetal growth restriction cases. This substantial remodeling is often accompanied by changes in heart function. Figure 1 shows prenatal echocardiographic images of mild and severe fetal growth restriction. Moreover, these changes persist postnatally in the form of subclinical cardiac and vascular remodeling and dysfunction,6, 36, 37 posing an increased risk for cardiovascular diseases later in life in those children/adults who were born with a low birthweight due to growth restriction during the fetal life.38, 39
5.2 Preeclampsia
Preeclampsia, a clinical condition that is often associated with fetal growth restriction and prematurity, has also been independently associated with fetal cardiac remodeling.40 Preeclampsia and fetal growth restriction share some of the same pathophysiologic mechanisms related to placental insufficiency and the predominantly anti-angiogenic environment.41 However, a prospective observational study by Youssef et al. has shown that fetuses of preeclamptic mothers show signs of cardiac remodeling and dysfunction, regardless of their growth pattern.40 These signs included observing larger hearts, more spherical right ventricles, and thicker ventricular walls.40 This consequently exposes such individuals to an increased cardiovascular risk in adulthood, in particular to have hypertension42 and an increased risk of stroke.43 Lazdam et al. hypothesized that the same placental factors responsible for preeclampsia in the mother also account for vascular dysfunction in the offspring.44
5.3 Preterm birth
This is another well-known condition that has been seen correlated with cardiac remodeling.45 So far, there are no studies on fetal heart in cases of preterm birth due to the difficulty in predicting preterm birth and performing fetal echocardiography once the diagnosis of preterm labor is made. A prospective study by Lewandowski et al. demonstrated the presence of alterations in left ventricular structure and function in adults born preterm; the changes were directly proportional to the degree of prematurity. Prematurity was shown to be an independent risk factor for cardiac remodeling. Obviously, its association with maternal preeclampsia exhibited worse changes in left ventricular function as well as a greater degree of endothelial dysfunction than when prematurity occurred isolated.44
5.4 Assisted reproductive technologies
Fetuses conceived by assisted reproductive technologies (ART) also display a cardiac phenotype of concentric ventricular remodeling in conjunction with atrial dilatation, reduced longitudinal motion, and poor diastolic function (Figure 1).46, 47 These changes might be due to a predominantly right pressure overload. Moreover, they are in line with studies reporting elevated pulmonary pressure in children conceived by ART. Dilated atria have been suggested as a result of a ventricular early filling problem that requires a greater atrial contribution to compensate; increasing pressure also promotes dilated atria. A recent study by Boutet et al. compared fetal echocardiography parameters at 28–33 weeks of gestation in pregnancies conceived by fresh and frozen embryo transfer to spontaneously conceived pregnancies.48 Surprisingly, the results demonstrated that cardiac remodeling is more prominent in cases of in vitro fertilization with fresh embryo transfer.
5.5 Exposure to toxic drugs
The use of antiretroviral drugs during pregnancy, specifically zidovudine, which induces cardiac mitochondrial toxicity and consequently lower intrinsic contractility, has been linked to a concentric hypertrophy pattern with larger hearts, thicker myocardial walls, and more spherical ventricles.19, 20 García-Otero et al. reported that 35% of the cases had a modest pericardial effusion, and the majority of them have systolic and diastolic dysfunction (reduced longitudinal motion and impaired relaxation). These cardiac changes are assumed to be a compensatory fetal cardiac hypertrophy response to toxicity-related myocardial damage.
5.6 Monochorionic diamniotic pregnancies and twin-to-twin transfusion syndrome
Fetuses from monochorionic diamniotic (MCDA) twin pregnancies show also signs of fetal cardiac remodeling. These signs were mainly observed in pregnancies complicated by TTTS or selective fetal growth restriction.49, 50 In TTTS, both donor and recipient fetuses present significant cardiac changes associated with the state of hypovolemia in the donor and hypervolemia and pressure overload in the recipient.49 In selective fetal growth restriction, Ortiz et al. showed echocardiographic signs of pressure/volume overload in both twins.50 Indeed, fetuses from complicated MCDA pregnancies are at risk of right ventricle outflow tract obstruction postnatally.25, 51 Moreover, a recent study by Torres et al. reported larger atrial areas and signs of concentric hypertrophy in fetuses from uncomplicated MCDA when compared with singletons.52
Among the other conditions that have also been well studied and associated with fetal cardiac remodeling are maternal diabetes and obesity53-56 and congenital heart disease.4, 57-59
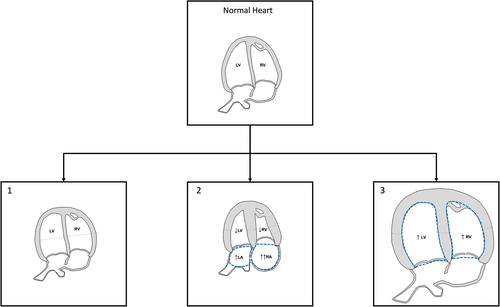
Interestingly, with the use of ultrasound, several patterns of fetal cardiac remodeling have been identified, depending on the time and length of exposure to an insult and, naturally, the type of this insult, such as toxins, hypoxia, malnutrition, and pressure/volume overload.5 According to that, cardiac adaptation may affect the whole heart (hypertrophic/dilated cardiomegaly) or with a preference for left/right cavities. Patterns of cardiac remodeling are usually defined according to the ventricular size and shape, the presence of myocardial hypertrophy and the ratio of myocardial mass to ventricular cavity.
- Non-hypertrophic globular shape with a normal size in response to mild pressure overload, described in mild late-onset cases of fetal growth restriction with mild placental insufficiency.34, 38 This type is usually subclinical since it is associated with a mild cardiac dysfunction, mainly reduced longitudinal motion, and impaired relaxation.
- Myocardial hypertrophy with normal size or reduced ventricular cavities in response to moderate/severe pressure overload, as in fetuses affected by pulmonary stenosis58 or conceived by ART.46
- Myocardial hypertrophy with cardiomegaly usually secondary to combined mechanisms (myocardial toxicity and/or pressure/volume overload), which has been reported in association with maternal diabetes,53, 54 antiretroviral drugs,19 cases of early-onset fetal growth restriction with severe placental insufficiency34, 38 and preeclampsia.40 A variable degree of impaired cardiac function has been described in those conditions.
6 PERSISTENCE OF CARDIAC REMODELING IN CHILDHOOD
The usual scenario in postnatal life is that cardiac remodeling could improve/revert after the insult is removed. However, fetal programming hypothesis postulates that prenatal cardiac remodeling can persist postnatally, even after removal of the initial insult. Here, we summarize the evidence of the persistence into childhood of prenatal cardiac remodeling.18, 36, 60-62
6.1 Fetal growth restriction
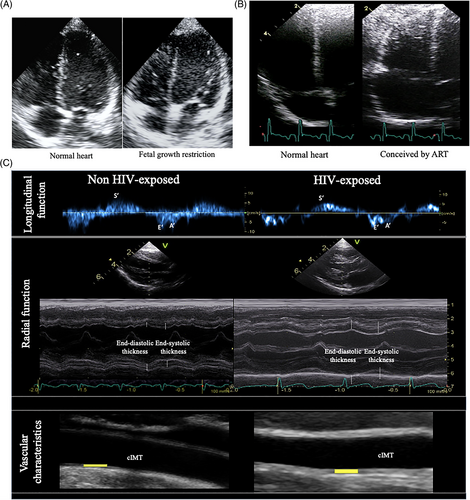
A prospective cohort study by Crispi et al. demonstrated that children (mean age 5 years), born growth restricted have a distinct cardiac geometry and shape, with less elongated and more globular ventricles as shown in Figure 3A.18 Morphometric measurements confirmed quantitatively an overall increase in transverse cardiac diameters, which led to apparent dilatation of the ventricular cavities. This study also highlighted the importance of longitudinal follow-up of this population and has been able to demonstrate not only an association, but also to better documented the persistence of its effect in postnatal life into childhood and pre-adolescence.36 The same research group demonstrated that not only fetal growth restriction but also small-for-gestational-age infants presented cardiovascular changes; highlighting the importance of the severity and/or time of appearance of these fetal conditions.34, 63 These studies also evaluated vascular changes, reporting higher carotid intima media thickness than controls and diastolic blood pressure in children born small.36
Posteriorly, and contrary to the former reports, Mäkikallio et al. studied a fetal growth restriction group at 1 week and 6 months of age, reporting that they had no detectable changes on cardiac dimensions and function at birth or at 6 months of age, only reported a higher increase in the brachial pulse wave velocity measurements suggesting that abnormalities related to growth restriction with cardiac changes could start later in childhood.64 Premises that were refuted by studies such as Akazawa et al. where they stratified into stages of severity according to infant birth weight in low, mild, or severe fetal growth restriction, demonstrating left ventricle remodeling, and increased IMT and stiffness in growth restricted neonates across a range of growth restriction severities during neonatal period.65 Another study carried out by Sehgal et al. demonstrated cardiac dysfunction in fetal growth restriction infants with lower left ventricular output; diastolic function that is, reduced E and A wave velocities and prolonged isovolumic relaxation time.66 Their vascular measurements reported thicker aortic intima media as well as arterial wall stiffness in the growth-restricted group.
6.2 Preterm birth
In the early postnatal life of preterm babies, a pattern of myocardial hypertrophy with restricted ventricular cavities has been documented, which is most likely due to relative pressure overload during the first days of life of preterm newborns.67
6.3 Assisted reproductive technologies
Another group of fetuses that have been studied postnatally, are those conceived by ART. Valenzuela-Alcaraz et al. in a prospective cohort study from fetal life through after birth and 6 months of age, demonstrated the persistence of cardiovascular changes in ART infants showed increased right atrial size, lower right sphericity index and thicker right ventricular wall as illustrated in Figure 3B.46 ART population also showed signs of both, systolic and diastolic dysfunction as measured by decreases in ring displacement, E deceleration time and tissue Doppler velocities and prolonged isovolumic relaxation time. The same cohort were followed until 3 years of age with cardiovascular changes persisting.61 These results were in line with other studies such as Zhou et al. showed signs of left ventricular hypertrophy and dysfunction in children between 2 and 6 years old.68 Liu et al. reported left ventricular reduced motion and diastolic dysfunction at 5 years of age, together with non-significant trend for more spherical ventricles.69 Von Arx et al. demonstrated right ventricular dysfunction under stressful conditions of high-altitude exposure in preadolescents. Although most studies report significant cardiovascular changes associated to ART, the pattern of cardiac remodeling and dysfunction differs among studies.70 Valenzuela-Alcaraz et al. reported dilated atria and more spherical and less efficient ventricles (both left and right), while Zhou et al. described left ventricular hypertrophy, Liu et al. left ventricular dysfunction and Von Arx et al. right ventricular dysfunction under stressful conditions.
Blood pressure was significantly higher in the ART group compared with controls. Intima media thickness was also significantly increased, even after normalizing by infant weight and adjustment for gestational age at delivery, birth weight percentile, and preeclampsia.46, 61 Many other cohorts have reported vascular impairment and raised blood pressure in ART children.71-74
A recent study including 8–9-year-old conceived after frozen, fresh embryo transfer and natural conceived children report no differences regarding arterial stiffness assessed from blood pressure and aortic ascendant distensibility between groups reported also no altered cardiovascular function.75 Results that seem reassuring.
6.4 Exposure to toxic drugs
García-Otero et al., in a prospective cohort including HIV-exposed uninfected infants versus non-HIV-exposed infants that were evaluated from fetal life up to 6 months postnatally reported cardiovascular changes in these infants.62 These changes included relative systolic dysfunction with decreased mitral ring displacement and decreased tricuspid S′ together with relative diastolic dysfunction showed by prolonged left isovolumic relaxation time. Vascular assessment showed higher systolic and diastolic blood pressure and thicker carotid intima media thickness (Figure 3C). This study goes in line with previous pediatric studies that also reported subclinical cardiac changes, yet significant differences in specific left ventricle diastolic indices.76, 77 Interestingly, together with subclinical cardiac impairment, higher blood pressure and thicker intima media thickness were observed in those infants; half of them presented hypertension, supporting a possible increased cardiovascular risk.
6.5 Monochorionic diamniotic pregnancies and twin-to-twin transfusion syndrome
Torres et al. recently reported cardiac dysfunction in fetuses from MCDA pregnancies diagnosed with TTTS, performing cardiac evaluation at different time periods: before laser treatment, 2–3 days after surgery, at 28–30 weeks of gestation and at 6–12 month after birth compared to uncomplicated MCDA twins.78 Postnatally, cardiac remodeling such as left relative wall thickness, persisted in recipients, whereas donors mainly presented decreased longitudinal motion with low tricuspid annular plane systolic excursion. Demonstrating not only that fetal surgery improve several cardiac parameters, but the remodeling and dysfunction persist postnatally.
In general, most of these changes are subclinical, with most cardiovascular indexes lying within normal ranges explaining that most children are asymptomatic without clinical signs of disease. However, subclinical changes in cardiovascular structure and function in the early stages of life might represent an underlying mechanism for increased cardiovascular risk later in life. In fact, some of cardiovascular differences, such as those reflected by significant increases in blood pressure and IMT, are recognized as potential risk factors for subsequent cardiovascular disease.
7 PERSISTENCE OF CARDIAC REMODELING IN ADULTHOOD: SECOND HIT THEORY
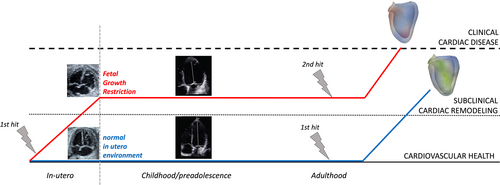
In 1971, Professor Alfred Knudson proposed the “2-hit” theory to demonstrate cancer predisposition using a retinoblastoma model. In this theory, a genetic mutation at conception (first hit) predisposes the cancer development if the same gene is exposed to an environmental trigger (second hit). Using this concept in placental mediated diseases, mainly fetal growth restriction appears as a logical approach. The first hit occurs during fetal life, where the developing heart adapts to an adverse environment (low nutrients and oxygen concentration, with increased vascular resistance) by increasing ventricular size and wall-thickness (Figure 4), to ensure a proper oxygen supply to all developing organs. Even more, theses fetuses also present signs of subclinical cardiac dysfunction when new methods, such as tissue Doppler or 2D-strain are used.79, 80 Postnatally, the insult usually is eliminated (i.e., the placenta is delivered, the fetus is not exposed any more to maternal toxic drugs or diabetes, etc.). However, some cardiovascular changes may persist postnatally even after insults' removal. These changes are usually minor and subclinical, and most children and adults can follow an apparent normal life. Several decades after birth, all individuals are exposed to different environmental risk factors: tobacco, sedentarism, high fat diet, obesity, and so forth (second hit). Any of these risk factors affect the cardiovascular system irrespective of birthweight. However, the second hit hypothesis postulates that a predisposed individual -that has already suffered a first hit prenatally- will more easily fail to cope with a second hit and go into disease/failure. Therefore, fetal cardiac remodeling could be considered a first hit that increases susceptibility to cardiovascular disease.
Several studies have evaluated the effect of postnatal stressors -second hit- on individuals who already suffered an in utero adverse environment -first hit-. Similarly, left ventricular structure and function in preterm adults worsens with systolic blood pressure elevation.81 Epidemiological studies demonstrate that obesity after being born small-for-gestational-age further increases the risk of coronary events, insulin resistance, and raised blood pressure.82 In a recent study Crispi et al. studied a cohort of 81 young adults (30 to 40 years-old) born small and compared to 77 adults with birthweight within normal ranges.83 All participants were assessed with a cardiac magnetic resonance (CMR) and also challenged using an exercise test. Analysis of cardiac structure and function at rest could only reveal subtle changes in right ventricular geometry. However, when cardio-respiratory systems were assessed under intense exercise, adults born small showed less exercise capacity with decreased maximal load and a reduced oxygen consumption compared with adults with normal birthweight. Likewise, Huckstep et al. showed similar baseline values, but a marked reduction of ejection fraction under exercise in young adults born preterm.84 Moreover, Scherrer et al. showed that children conceived by assisted reproductive technologies had similar baseline measures but remarkable differences in pulmonary pressure when exposed to low environmental oxygen.73
Conversely, a healthy postnatal environment seems to protect at-risk adults born after a suboptimal prenatal life. In particular, breastfeeding and healthy-fat dietary intake seem to improve cardiovascular outcomes in individuals born small-for-gestational-age or preterm.85, 86
Therefore, after the above-mentioned evidence, it seems that prenatal cardiovascular remodeling induces subtle changes in order to keep fetal cardiac performance, but at a high long-term cost by increasing susceptibility to disease later in life.
8 PREVENTION (WINDOW OF OPPORTUNITY)
Given the long-term consequences of fetal cardiac remodeling, research should focus on strategies to prevent these prenatal conditions or its consequences. Over the last few decades, an increasing amount of effort has been invested in recognizing preventive measures that could reduce the cardiac remodeling in the fetus (Table 1).
| Primary prevention | Secondary prevention | Terciary prevention | |
|---|---|---|---|
| Fetal growth restriction | Improve maternal diet or reduce stress to prevent the occurrence of fetal growth restriction. |
|
|
| Preeclampsia |
|
|
|
| Preterm birth |
|
|
|
| ART |
|
Fetal echocardiography. | Avoid adding subsequent risk factors. |
| Exposure to toxic drugs |
|
Fetal echocardiography. | |
| MCDA twin pregnancy | Fetoscopy laser surgery. |
Marker of cardiac dysfunction in MCDA twin pregnancy (i.e., Left MPI) | |
Maternal Diabetes and obesity |
|
Fetal echocardiography. |
- Abbreviations: ART, assisted reproductive technologies; MCDA, monochorionic diamniotic; MPI, myocardial performance index; NICU, neonatal intensive care unit; PUFAs, polyunsaturated fatty acids.
8.1 Primary prevention
Primary prevention aims to prevent the disease or injury before it ever occurs. That would be for example, preventing or treating the fetal conditions that associate in utero cardiac remodeling.
8.2 Secondary prevention
Secondary prevention aims to reduce the impact of a disease or injury that has already occurred. This is done by detecting and treating disease or injury as soon as possible to halt or slow its progress, encouraging personal strategies to prevent reinjury or recurrence, and implementing programs to return people to their original health and function to prevent long-term problems. This allows, in those fetuses of pregnancies considered to be at higher risk, an early diagnosis of possible cardiac remodeling and its subsequent careful monitoring.
8.3 Tertiary prevention
Tertiary prevention aims to soften the impact of an ongoing disease or injury that has lasting effects. This involves avoiding or reducing postnatal risk factors that could potentially represent the ‘straw that breaks the camel's back’, or the ‘second hit’ necessary for the onset of a cardiovascular disease in individuals with predisposing physiological and epigenetic conditions.
Herein, we provide a review of preventive measures in the different conditions associated with fetal cardiac remodeling.
8.4 Fetal growth restriction
As mentioned above, fetal growth restriction is one of the most investigated clinical conditions. Thus, for this condition we have the greatest amount of evidence even in terms of prevention.
8.4.1 Primary prevention
A recent randomized clinical trial has been conducted from February 2017 to October 2019 in the city of Barcelona by Crovetto et al. in singleton pregnancies recruited between 19 and 23 weeks of gestation at high anamnestic risk for fetal growth restriction. The trial demonstrated that, compared to routine obstetric care, adopting interventions based on a structured Mediterranean diet or a mindfulness-based stress reduction program, resulted in a significant reduction in infants with birth weight < 10th percentile.87 Additional data showed that, fetuses born from mothers assigned to the Mediterranean diet group, showed fewer signs of cardiac remodeling than the control group (unpublished data). However, further studies need to be conducted to better investigate this evidence with the perspective of making it suitable for all women, regardless of the presence of risk factors for intrauterine growth restriction and starting from the onset of gestation. These are interventions aimed at educating mothers to adopt specific behaviors during pregnancy, recognizing at this stage a window of opportunity for cardiovascular prevention of offspring in adulthood.16
8.4.2 Secondary and tertiary prevention
Nowadays, therefore, we have numerous scientific studies confirming the correlation between primary cardiac remodeling -and metabolic programming- and growth restriction during intrauterine life, giving to the latter the title of best-known prenatal risk factor for cardiovascular diseases. This has been observed in newborns born from pregnancies complicated by fetal growth restriction, both severe/early and mild/late, as well as in small-for-gestational-age fetuses with no Doppler alterations.18, 63 Since fetal growth restriction is a clinical condition with a prevalence of about 7% to 10% of pregnancies,88 recognizing and knowing children, adolescents and adults with a positive history of fetal growth restriction and implementing preventive healthcare measures and cardiological follow-up for them, could reduce the impact of cardiovascular diseases in the general population, with a real benefit on public health.
Once the diagnosis of fetal growth restriction has been made, it is necessary to find clinical tools that can help identify which affected fetuses have greater signs of cardiac remodeling and are at greater cardiovascular risk. As we described in the previous paragraphs, fetal echocardiography and the new methods associated seem to embody this role. Certain altered echocardiographic parameters or higher values of blood pressure6 might detect cardiovascular dysfunction in a subclinical phase, identifying fetuses that could benefit from early screening programs and preventive measures for cardiovascular risk factors since childhood.6, 89
Some of the preventive measures, recognized to have a concrete role in improving cardiovascular function in individuals with cardiac remodeling background, can be carried out in infancy and childhood. These early years of life, actually, embody the optimal period to implement actions leading to successful and persistent effects, embracing the philosophy of “the sooner, the better”. These include nutritional precautions such as promoting prolonged breastfeeding (longer than 6 months) and introducing polyunsaturated fatty acids (PUFAs) supplements into the diet since infancy. These, in a cohort study conducted in children aged 4–5 years born with a birth weight < 10th centile, were seen to be associated with less globular (less remodeled) hearts and reduced carotid intima-media thickness, respectively.85 Other previous studies have shown how breastfeeding also plays a protective role against other cardiovascular risk factors such as hypertension, obesity, diabetes and dyslipidemia.90
In addition to dietary precautions, healthy lifestyle rules, such as the prevention of excessive growth catch-up in the first months of life and overweight in childhood, play an important role as protective factors for hypertension and vascular remodeling at a young age.38, 85 Certainly, it is important to maintain healthy habits through adolescence and adulthood, associating them with the already known and confirmed good rules of cardiovascular risk prevention such as the promotion of physical activity and the demonization of smoking. Although fetal growth restriction is not yet officially recognized in the list of cardiovascular risk factors, the knowledge acquired and ongoing and upcoming studies will allow a greater characterization of it and its greater consideration in the effort to identify individuals at increased risk of cardiovascular morbidity and mortality.
8.5 Preeclampsia
Hypertensive disorders in pregnancy have a prevalence of about 5%–7%. Specifically, preeclampsia complicates 2% to 4% of pregnancies. Thus, identifying preventive measures to reduce the associated cardiovascular remodeling mechanisms would result in a reduction of long-life cardiovascular risk of preeclampsia offspring.
Several studies have been conducted to understand the pathogenesis of preeclampsia in order to develop feasible treatment schedules; nevertheless, the obstetric scientific community is still far from holding a complete and undisputed view of its underlying pathophysiology. In preeclampsia, the only medical intervention having a resolutive effect continues to be the delivery of the baby. Therefore, as the impact of preeclampsia is particularly severe, over the past decades there have been increasing medical efforts to develop measures that can prevent its onset. A very recent review published in the New England Journal of Medicine by Magee et al. summarized the latest and most important evidence on this subject.91
8.5.1 Primary prevention
With the goal of identifying patients who might benefit from preventive measures, risk prediction tools for preeclampsia are increasingly being used in clinical practice.92 The traditional approach involves screening based on the assessment of the patient's anamnestic and obstetric risk factors; this is a simple method, but with reduced sensibility (40% for preterm preeclampsia and 35% for term preeclampsia, with a predictive positive value of 10%).93 The multivariable model approach -of which the most widely used is the Fetal Medicine Foundation's competing-risks model- is based on the pooled assessment of anamnestic factors, clinical parameters, ultrasound, and angiogenic biochemical markers. When performed at 11–13 weeks of gestation, they can identify up to 90% of women who will develop early preeclampsia (<34 weeks) and up to 75% of those who will have preeclampsia at term, with a PPV of 10%.94 The 90% of patients who are found to be at low risk, conversely, could benefit from rescreening in the second and third trimester of gestation in order to restratify their risk; this is based on performing an ultrasound scan at 19–24 weeks of gestation and 32 weeks of gestation, identifying, respectively, almost all women who will develop preeclampsia at 32 weeks and 90% of those who will have it at 32–35 weeks. Regarding the use of angiogenic markers, specifically soluble fms-like tyrosine kinase 1 (sFlt-1), it represents a feasible screening method at 35–36 weeks to identify the risk of developing preeclampsia at term (detection rate of 75%–85%, PPV of 10%–20%).91
Having identified women at higher risk in the obstetric population, it will be possible to implement primary preventive measures on them. These are essentially based on avoiding the occurrence of conditions that have been recognized, individually or in concert, as potential triggers of preeclampsia: angiogenic imbalance, endothelial activation, oxidative stress, inflammatory status, and vasoconstriction. The evidence supports encouraging physical activity in pregnancy, consisting of moderate exercise (at least 140 min of walking per week) from the earliest weeks of gestation; this would reduce the risk of preeclampsia by about 40%.95 Aspirin intake by high-risk patients, from prior to 16 to 36 weeks of gestation, at a dosage of at least 100 mg/day, in the context of the ASPRE Trial, was seen to reduce by more than 60% the risk of early preeclampsia, but without any significant reduction in the term pathology, as well as in patient with chronic hypertension.93 Playing a role in the prevention of both early and late preeclampsia, with a 50% risk reduction, is calcium supplementation (at least 500 mg/day) in the second half of pregnancy when intake deficiency (<900 mg/day) is detected.91 Finally, low-risk nulliparous women would appear to benefit from induction of delivery between 39 + 0 and 39 + 4 weeks' gestation, with a 35% reduction in the risk of preeclampsia and gestational hypertension compared with an expectant conduction.96
Optimal maternal and fetal surveillance strategies have not yet been identified in patients found to be at high risk on screening tests. These patients could be encouraged to self-monitor for warning symptoms of preeclampsia, both maternal and fetal, as well as for checking blood pressure; however, the actual effectiveness of this method in predicting early disease remains uncertain. Scheduling more frequent outpatient visits, repeated hematochemical examinations to detect organ dysfunction typical of preeclampsia and performing ultrasound screening with assessment of fetal growth and/or Doppler velocimetry could be of greater benefit.
8.5.2 Secondary and tertiary prevention
If preeclampsia arises, despite screening of the patient and implementation of the measures described above, secondary prevention tools are based on monitoring -and eventual antihypertensive treatment- of the woman and the fetus, in order to reduce as much as possible, the short- and long-term adverse effects that this obstetrical syndrome can have in both the maternal and fetal domains. With regard to fetal monitoring, the only tool available is ultrasound, including growth monitoring, Doppler velocimetry and fetal echocardiography to detect any signs of fetal distress. Maternal and fetal monitoring will make it possible to orient the therapeutic course and eventual termination of pregnancy, by performing a balanced assessment of maternal-fetal risks and benefits (maternal aggravation, fetal suffering/remodeling signs vs. fetal prematurity) and reasoned on the basis of the gestational age at which the pathology manifests.
Future perspectives include the identification, in women at risk of or diagnosed with preeclampsia, of predictive models that associate cardiovascular and placental biomarkers (sFlt-1/PlGF ratio) with clinical echocardiographic parameters, in order to quantify the risk of cardiovascular remodeling/dysfunction in both mother and fetus, during and after gestation. The prospective cohort study ANGIOCOR is ongoing on this topic.41 Also in children of preeclamptic mothers has been observed that the adoption of dietary strategies (promotion of prolonged maternal breastfeeding and high dietary intake of PUFAs) and weight control represent tertiary preventive measures that can decrease the onset of cardiovascular disease development.85
8.6 Preterm birth
Since 11% of the births worldwide occur preterm,97 cardiac alterations resulting from this condition could also have an impact on the health of the general population; therefore, interventions to prevent or reduce this remodeling are necessary. Primary prevention of prematurity birth is, if not impossible, difficult to implement. Preterm birth, in fact, is an almost unpredictable event, except when associated with maternal pathological conditions such as preeclampsia or fetal growth restriction. A recently conducted IPD meta-analysis on the prevention of preterm birth has shown that the administration of prophylactic progesterone in patients with short cervical length reduces the incidence of prematurity.98 Although routine measurement of cervical length is not clearly recommended by all obstetrician-gynecologic societies, its monitoring as a screening in the second trimester could reasonably be implemented in all women to identify those at increased risk.99 For the most part, secondary prevention tools for preterm birth have been introduced in recent decades. These include the use of progesterone and the application of pessary or cervical cerclage in patients with a history of spontaneous and early prematurity and/or cervical length < 25 mm. In contrast, tocolytic therapy combined with corticosteroids in symptomatic patients has generally failed to prolong gestation and reduce related neonatal complications, although it is still largely performed in clinical practice.98
A prospective study conducted on infants born before 30 weeks, has shown how signs of cardiac remodeling can be detected very early after preterm birth and that most of these occur during the period of hospitalization in the neonatal intensive care unit. These findings support a possible opportunity for neonatologists to act during the first weeks of life, with the possibility of modifying/reducing the impact or even preventing the establishment of permanent pathological cardiovascular patterns, such as cardiomyopathy of the premature.100
As described for fetal growth restriction, also in preterm infants, public health policies that promote prolonged maternal breastfeeding, increased intake of healthy fats combined with avoidance of overweight and excessive growth catch-up represent optimal preventive strategies.85
8.7 Assisted reproductive technologies
Pregnancies achieved by ART, particularly in vitro fertilization and in vitro fertilization with intracytoplasmic sperm injection, amount to 1%–4% in developed countries. Children conceived by ART have been proven to show signs of cardiovascular remodeling/dysfunction both in utero and persisting into childhood, exposing them to increased cardiovascular risk later in life, as well as metabolic syndrome and type 2 diabetes.46, 101 Although, the detected dysfunctions were found to be minor, just above normal limits, and further studies are needed to confirm their permanence throughout life.
Over the years, long-term outcomes have been improved significantly in children conceived with ART due to the preference for single embryo transfer and fresh rather than frozen embryos.101 Increasing efforts and further studies need to be conducted in order to identify techniques that have a lower impact on fetal cardiovascular development and remodeling and fetal programming in general. (As well as educating offspring conceived through ART to avoid or reduce as much as possible cardiovascular risk factors or take preventive measures in childhood, adolescence, and adulthood).
8.8 Exposure to toxic drugs
Maternal intake of some drugs has been seen to be associated with direct damage of fetal myocardium. As described previously, a representative example is antiretroviral drugs, the use of which has been associated with primary fetal cardiac remodeling/dysfunction. The drug most correlated with these changes is Zidovudine, particularly when taken from the first trimester.19 The choice of alternative therapeutic regimens to Zidovudine in pregnancy could represent a measure to prevent fetal cardiac remodeling, as it has been shown that the latter is correlated to the drug rather than to HIV infection per se.102 In the absence of therapeutic alternatives, follow-up of these pregnancies with serial fetal echocardiography is highly recommended.
8.9 Monochorionic diamniotic twin pregnancy and twin-to-twin transfusion syndrome
TTTS complicates 10%–15% of MCDA pregnancies. Significant hemodynamic changes related to the establishment of pressure overload are detected in both donor and recipient103 justifying primary cardiovascular remodeling in these fetuses. Fetoscopy treatment by laser coagulation of placental anastomoses has been demonstrated to provide improved cardiac function in both fetuses, with normal cardiac function detected also in the postnatal period. However, there are articles in the literature reporting evidence of persistence in heart and vascular dysfunction among survivors of pregnancies complicated by TTTS.9
Studies are needed to identify tools to recognize MCDA pregnancies at risk for TTTS or to estimate the fetal cardiac prognosis before and after fetoscopic treatment, also in order to define the postnatal risk. An indicator of potential use for this purpose has been identified by Ortiz et al. in the left myocardial performance index, considering it a sensitive marker of cardiac dysfunction and hemodynamic alterations in MCDA pregnancies.50
8.10 Maternal diabetes and obesity
Numerous studies have demonstrated the association between maternal diabetes and the presence of cardiac remodeling/dysfunction in the offspring.55 Therefore, implementation of measures to control maternal diabetes during pregnancy are critical to prevent cardiovascular risk in the offspring.
As additional confirmation that maternal lifestyle plays a key role in determining offspring cardiovascular risk, several authors have also demonstrated that maternal obesity is an independent risk factor for long-term cardiovascular morbidity in offspring104 related to the associated primary cardiovascular105 and metabolic remodeling. Increasing efforts will be required by health care professionals during prenatal counseling and at the beginning of pregnancy itself in obese women, in order to prevent not only the complications -already well known- that may aggravate gestation and delivery, but also the effects that maternal obesity may have on fetal well-being in the short and long term.
8.10.1 The placenta as the window to congenital heart disease
A review of the literature on the relationship between structural and functional abnormalities of the placenta and their possible connection with the development of heart disease was conducted in 2021. It has been observed that placental pathologies are more frequent in fetuses with congenital cardiac abnormalities. Living with a dysfunctional placenta, moreover, would induce the fetus to assimilate the cardiac remodeling, keeping it persistent in extrauterine life. The maternal-placental-fetal vascular axis may embody a key aspect for our understanding of congenital cardiac anomalies. Future efforts to refine our ability to identify, characterize, and quantify placental insufficiency and dysfunction during gestation, could become a useful tool to improve the outcomes of patients with congenital heart disease, which continues to represent the most frequent congenital anomaly.59
9 FUTURE ASPECTS
The evidence discussed in this review proves the consistent prenatal and postnatal cardiovascular consequences of an adverse in utero environment. Cardiac remodeling and dysfunction can occur at any stage of life. However, while postnatal cardiac remodeling reverts after treating the cause, cardiac remodeling occurring in utero might persist postnatally even after the trigger has disappeared. Considering the high prevalence of prenatal conditions associated with cardiac remodeling and the improving prenatal and neonatal care that favors high survival rates of offspring from complicated pregnancies, it is critical to detect cardiovascular risks as early as possible, to introduce timely preventive interventions. Therefore, based on the collective research data known today, the incorporation of prenatal conditions (i.e., Fetal growth restriction, preeclampsia, prematurity, etc.) as a recognized cardiovascular risk factors in clinical guidelines is key for implementing a specific cardiovascular follow-up and management that could benefit their future health. Early-life preventive measures may have a strong impact on the future health of these children and future adults, having a remarkable effect on public health that merit to be carefully considered.
ACKNOWLEDGMENTS
The research leading to these results has received funding from the Instituto de Salud Carlos III (PI20/00246; INT21/00027) within the Plan Nacional de I + D + I and cofinanced by ISCIII-Subdirección General de Evaluación together with the Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa”, and the Cerebra Foundation for the Brain Injured Child (Carmarthen, Wales, UK). In addition, it has been funded partially by grants from Fundació Jesus Serra and Fundación Mutua Madrileña (Spain).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.