Tissue sodium accumulation and peripheral insulin sensitivity in maintenance hemodialysis patients
Abstract
Background
Recent data suggest that sodium (Na+) is stored in the muscle and skin without commensurate water retention in maintenance hemodialysis (MHD) patients. In this study, we hypothesized that excessive Na+ accumulation would be associated with abnormalities in peripheral insulin action.
Methods
Eleven MHD patients and eight controls underwent hyperinsulinemic–euglycemic–euaminoacidemic clamp studies to measure glucose (GDR) and leucine disposal rates (LDR), as well as lower left leg 23Na magnetic resonance imaging to measure Na+ concentration in the muscle and skin tissue.
Results
The median GDR and LDR levels were lower, and the median muscle Na+ concentration was higher in MHD patients compared with controls. No significant difference was found regarding skin Na+ concentration between group comparisons. Linear regression revealed inverse relationships between muscle Na+ concentration and GDR and LDR in MHD patients, whereas no relationship was observed in controls. There was no association between skin Na+ content and GDR or LDR in either MHD patients or controls.
Conclusions
These data suggest that excessive muscle Na+ content might be a determinant of IR in MHD patients, although the causality and mechanisms remain to be proven.
Introduction
Insulin resistance (IR) is prevalent in patients with chronic kidney disease, especially in ones with end-stage renal disease (ESRD).1 The exact mechanisms, by which IR develops, specifically in patients on maintenance hemodialysis (MHD), are not fully elucidated. Studies suggest that while insulin excretion is altered by the loss of tubular function during progressive kidney disease, there is appropriate compensatory adjustment in beta cell function suggesting that decreased peripheral tissue sensitivity to insulin is the primary mechanism.2, 3
In addition to abnormalities in carbohydrate metabolism, we have previously reported that the severity of IR associates with enhanced muscle catabolism in MHD patients, suggesting that understanding the underlying mechanisms could lead to ways to improve their nutrition status.4, 5 Dahlmann et al. have recently reported that MHD patients have significantly elevated skin and tissue Na+ content when measured by magnetic resonance imaging (23Na+MRI) as compared with healthy controls.6 The metabolic consequences of higher tissue Na+ accumulation in MHD patients, especially on peripheral insulin action on the muscle tissue, are unknown.
In this study, we hypothesized that increased tissue Na+ may be associated with local derangements in peripheral insulin sensitivity to carbohydrate and protein levels leading to resistance to its glycogenic and anabolic actions. We tested this hypothesis in 11 MHD patients and eight controls without known kidney disease by simultaneous measurements of tissue Na+ and markers of insulin sensitivity using a dual glucose and amino acid clamp technique.
Materials and methods
Study population
Eleven MHD patients from the Vanderbilt University Outpatient Dialysis Unit and eight controls without kidney disease were recruited between November 2011 and December 2015. Primary inclusion criteria for the MHD group were treatment with thrice-weekly MHD program with single pool Kt/V over 1.2 for at least 6 months with well-functioning hemodialysis access. Exclusion criteria included patients with active infectious or inflammatory disease (i.e. vascular access infections, active connective tissue disorder, active cancer, HIV, and liver disease) and hospitalization within the last month prior to the study. Patients receiving steroids (>5 mg/day) and/or immunosuppressive agents and patients with Type 1 DM and type 2 DM who were using insulin or insulin-sensitizing medications were also excluded. The Institutional Review Board of Vanderbilt University approved the study protocol, and written informed consent was obtained from all study patients. The procedures were in accordance with the Declaration of Helsinki Principles regarding ethics of human research.
Study procedures
Hyperinsulinemic–euglycemic clamp protocol
All clamp studies were performed on a non-dialysis day after an overnight 8 h fasting period at the General Clinical Research Center at Vanderbilt University Medical Center (VUMC).
First, the dialysis shunt was accessed using 15-gauge fistula needles in opposite direction at least four fingerbreadths apart. The arterial side was used to obtain arterial blood samples and the venous side used for the infusion of tracers, insulin, and dextrose. Then, an IV was placed in the contralateral forearm vein in a retrograde direction to obtain venous blood samples. For the control subjects, an IV was inserted into an antecubital vein for the infusion of all test substances. Second and third IVs were placed antegradely and retrogradely in the opposite arm for blood sampling. The antegrade IV was placed distal to the retrograde IV, and the hand was kept in a heated box to achieve arterialization of the venous blood. The remaining IV was used for venous sampling.
After obtaining baseline blood samples, primed infusion of regular human insulin at the concentration of 2.0 mu/kg/min was started and maintained throughout the study procedure to obtain constant hyperinsulinemia. The goal plasma insulin concentration was 100 μU/mL. Following initiation of insulin, target plasma glucose levels were 90 ± 5 mg/dL, achieved by adjustment of 20% dextrose infusion. Constant monitoring of plasma glucose concentration was performed every 5 min and of plasma leucine levels every 10 min using rapid bedside high-performance liquid chromatography methodology. Once steady state was reached, GDR (mg/kg/min insulin-mediated glucose disposal rate) was calculated from samples taken during the last 30 min. This served as an index of in vivo insulin sensitivity. For reasons of comparison, the value was normalized to body weight.7
Hyperinsulinemic–euglycemic–euaminoacidemic (HEAC) clamp protocol
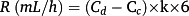
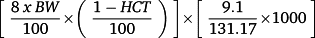
Total body blood volume is 8% of body weight. Multiplication by 1 minus hematocrit equals the total plasma volume for the total body; 9.1 mg is the total leucine amount in 1 mL of Freeamine III solution. Division by 131.17 (molecular weight of leucine) and further multiplication by 1000 convert the leucine amount from milligram per litre to micromoles per litre.
When the steady-state condition for leucine was achieved, the leucine infusion rate was assumed to be equal to the leucine incorporation rate into protein because endogenous leucine release from protein is assumed to be zero under steady-state conditions. Leucine disposal rate (LDR) was obtained by taking the leucine infusion rate (mg/min) during the final 30 min of the steady-state period and then normalizing by body weight (kg).7
Body composition
Within one week of each study, participants underwent dual-energy x-ray absorptiometry (DEXA) offering a rapid, non-invasive three-compartment evaluation that quantifies fat mass, LBM, and bone mineral content with minimal radiation exposure. All DEXA measurements were completed on a non-dialysis day using a Lunar Prodigy iDEXA machine v.11.40.004 (software versions 2003 to 2011, General Electric, Madison, WI).
23Na-magnetic resonance imaging technique
All MRI studies were performed on a non-dialysis day to keep consistency with clamp studies. The quantification of Na+ content in tissue level by 23Na-MRI in man was validated and recently published.6, 8, 9 Na+ content was measured in lower left leg muscle and skin using a 23Na+ knee coil (Rapid Biomedical GmbH, Rimpar, Germany) and a 3.0 T MR scanner (Philips Healthcare, Cleveland OH, USA) before the clamp study protocol. Four reference solutions (NaCl aqueous solutions of 10 mM, 20 mM, 30 mM, and 40 mM) served as calibration standards and were scanned together with sections through subject's calf muscles. Each subject was required to rest for 30 min before imaging to reduce possible physiological effects on tissue sodium (such as due to exercise), then the left lower leg (the widest part of calf region) was scanned with the skin closely in contact with the hard surface (a concave cover) of the phantom holder. MR scans primarily included a proton mDixon scan and a sodium scan. The mDixon provides a high-resolution anatomical image to allow muscle ROI definition, with parameters of FOV = 192 × 192 mm2, resolution = 1 × 1 mm2, five slices at a thickness of 6 mm. TR = 200 ms, TE = 1.15/2.3 ms. Four types of images (water, water fat in-phase, water fat out-of-phase, and fat) were generated resulting in a total of 20 images. Scan time = 3 min 52 s. Sodium imaging was performed using an optimized 3D gradient-echo sequence, FOV = 192 × 192 × 210 mm3, voxel size = 3 × 3 × 30 mm3, seven slices, TR/TE/FA = 130 ms/0.99 ms/90°, bandwidth = 434 Hz/pixel, acquisitions: 26, scan time = 15 min 54 s. After acquisition, data were extracted and processed off-line with homemade Matlab (R2013a) codes. Image analysis was performed using ImageJ (NIH, version 1.49v) software. Relative sodium tissue concentrations were obtained by comparing tissue sodium signal intensities to the calibration standards without regard to differences in their relaxation times.
Laboratory analysis
All blood sampling was performed at the General Clinical Research Center and analyzed at VUMC central laboratories. After blood draw was performed, samples were transported on ice and centrifuged at 3000 rpm for 15 min before being kept frozen at −80° Celsius. Plasma fasting glucose concentrations were analyzed using the glucose oxidase method (Glucose analyzer 2; Beckman Coulter, Brea, CA). Biochemistry measurements were analyzed at the VUMC Pathology Laboratory. High sensitivity C-reactive protein (hs-CRP) concentrations were measured by high-sensitivity particle-enhanced turbidimetric UniCel DxI Immunoassay system (Beckman Coulter).
Statistical analysis
Descriptive statistics were expressed as median (IQR) for continuous variables, and as frequencies and percentages for categorical variables. To compare sodium concentrations in MHD patients and healthy controls, a plot was generated showing the comparison between data points. The differences between cases and controls were tested with Wilcoxon rank-sum test. Linear regression analysis was performed to estimate simple associations between insulin sensitivity markers (GDR, LDR) and tissue sodium concentrations on log scale. Plots of the estimated slopes (with 95% confidence intervals) were generated. The variables were log scaled in multivariate models, and the confidence interval was obtained using bootstrapping. Analyses were performed using R, version 3.2.3 (http://www.r-project.org/).
Results
Characteristics of study population
The demographic characteristics of the study participants are shown in Table 1. In general, the MHD patient group was slightly older (51 (35, 61) vs. 49 (47, 51) years; median, IQR) and had more African Americans compared with controls. Hypertension was the most common primary disorder leading to ESRD (n = 6) and median time on MHD was 50 (IQR, 15, 76) months with median single pool Kt/V of 1.49 (IQR, 1.43, 1.54). The dialysate Na+ concentration was 138 mmol/L, and no Na+ modeling was applied in any of the MHD patients. The controls had no known acute or chronic or medical conditions. The median creatinine level was 0.9 (IQR, 0.9, 0.99) mg/dL, and median glomerular filtration rate was 97 (IQR, 81, 114) mL/min/1.73 m2 in control participants.
| MHD
(n = 11) |
Controls
(n = 8) |
|
|---|---|---|
| Age (years; median, IQR) | 51 (35, 61) | 49 (47, 51) |
| Gender (male/female, n) | 7/4 | 5/3 |
| Race (African American/Caucasian, n) | 9/1 | 6/2 |
| Duration of hemodialysis (months; median, IQR) | 50 (15, 76) | — |
| Kt/v (mean ± SD) | 1.49 (1.43, 1.54) | — |
| Etiology of ESRD (n, %)
Hypertension Nephrolithiasis Glomerulonephritis Unknown |
6 (55%)
1 (9%) 2 (18%) 2 (18%) |
— |
- MHD, maintenance hemodialysis; PCKD, polycystic kidney disease.
Table 2 depicts laboratory and metabolic characteristics of two study groups. The body mass index (BMI) was higher in MHD patients compared with controls with the median difference of 6 (95% CI; −3, 14) kg/m2. The MHD patients also tended to have a higher fat mass and truncal fat percentage [median difference, 10 (95% CI; −7, 28) and 14 (95% CI; −3, 35), respectively] and lower lean body mass [median difference, −5 (95% CI; −20, 11)] compared with the controls. MHD patients had higher hs-CRP levels compared with controls [median difference, 4 (95% CI; −0.04, 8)] (Table 2).
| MHD
(n = 10) |
Controls
(n = 8) |
Median difference (CI) | |
|---|---|---|---|
| Body composition | |||
| BMI (kg/m2; median, IQR) | 30 (23, 35) | 24 (21, 27) | 6 (−3, 14) |
| Lean body mass (kg; median, IQR) | 51 (43, 55) | 56 (45, 65) | −5 (−20, 11) |
| Fat mass (kg; median, IQR) | 32 (18, 42) | 22 (14, 30) | 10 (−7, 28) |
| Truncal fat (%; median, IQR) | 45 (25, 50) | 30 (21, 38) | 14 (−3, 35) |
| Albumin (g/dL; median, IQR) | 3.9 (3.8, 4.3) | 4 (3.8, 4.2) | −0.05 (−0.5, 0.4) |
| Carbohydrate metabolism | |||
| Fasting glucose (mg/dL; median, IQR) | 88 (87, 95) | 97 (91, 103) | −9 (−30, 12) |
| Glycated hemoglobin (g/dL; median, IQR) | 5.0 (4.6, 5.2) | 5.5 (5.4, 5.7) | −0.5 (−0.8, −0.07) |
| hs-CRP (mg/L; median, IQR) | 4.7 (2.6, 7.0) | 0.9 (0.6, 2.7) | 4 (−0.04, 8) |
- BMI, body mass index; hs-CRP, high sensitivity C-reactive protein; MHD, maintenance hemodialysis.
Insulin sensitivity markers derived by insulin clamp
In order to measure insulin sensitivity at the carbohydrate level, we performed hyperinsulinemic–euglycemic clamp (HEGC) studies. The median glucose disposal rate (GDR) level was lower for MHD patients compared with controls, 4.6 (IQR, 4.2, 5.1) mg/kg/min vs.6.3 (IQR, 3.5, 10.1) mg/kg/min, respectively, p = 0.86. For quantification of insulin sensitivity at the protein level, we performed hyperinsulinemic–euglycemic–euaminoacidemic (HEAC) clamps. The median LDR was significantly lower in MHD patients compared with control subjects [0.09 (IQR, 0.06, 0.1) mg/kg/min vs. 0.11 (IQR, 0.09, 0.15) mg/kg/min, respectively, p = 0.04].
Sodium content of muscle and skin
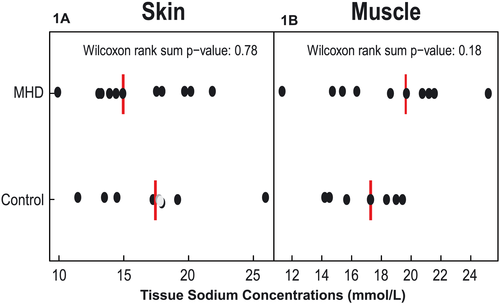
No significant difference was found regarding skin Na+ concentration between groups [15 (IQR, 13, 20) mmol/L for MHD vs.17 (IQR, 14, 19) mmol/L for control, p = 0.18] (Figure 1A). Although median muscle Na+ concentration was numerically higher in MHD patients compared with controls [20 (IQR, 15, 22) mmol/L vs.17 (IQR, 15, 19) mmol/L, respectively, p = 0.78], the difference was not statistically significant (Figure 1B).
The association between tissue sodium content and insulin sensitivity
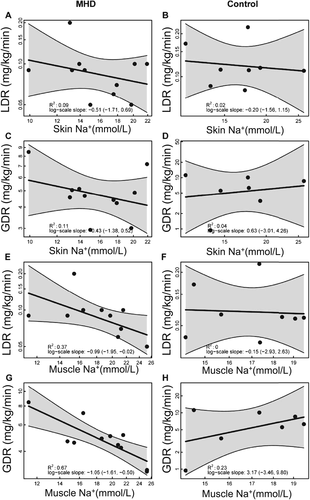
There were no significant associations detected between skin sodium content and LDR (Figure 2A and 2B) or GDR (Figure 2C and 2D) in either MHD patients or controls in univariate linear regression analysis. Similar results were obtained after individually adjusting for age, gender, BMI, hs-CRP, fat mass, lean mass, and truncal fat percentage (Table 3).
| LDR | GDR | |
|---|---|---|
| Β (95% CI) | Β (95% CI) | |
| Skin Na+ + study group + age | −0.16 (−0.73, 0.31) | 0.04 (−0.67, 0.73) |
| Skin Na+ + study group + gender | −0.24 (−0.67, 0.31) | −0.004 (−0.71, 0.87) |
| Skin Na+ + study group + BMI | −0.30 (−0.80, 0.39) | 0.15 (−0.71, 1.19) |
| Skin Na+ + study group + hs-CRP | −0.23 (−0.73, 0.20) | 0.01 (−0.63, 0.95) |
| Skin Na+ + study group + lean mass | −0.16 (−0.53, 0.29) | 0.01 (−0.52, 0.78) |
| Skin Na+ + study group + fat mass | −0.29 (−1.00, 0.32) | 0.10 (−0.67, 1.00) |
| Skin Na+ + study group + truncal fat % | −0.27 (−1.05, 0.23) | 0.10 (−0.82, 1.14) |
- Na+; sodium, LDR; leucine disposal rate, GDR; glucose disposal rate. The variables are log scaled, and the confidence interval was obtained using bootstrapping.
Higher muscle sodium concentration was associated with reductions in both LDR [R2 = 0.37, β: −0.99 (95% CI, −1.95, −0.02)] (Figure 2E) and GDR [R2 = 0.67, β: −1.05 (95% CI, −1.61, −0.50)] (Figure 2G) in MHD patients in univariate analysis. Very low GDR and LDR occurred predominantly in MHD patients with excessive muscle Na+ levels of >25 mmol/L. In healthy controls, which did not show similar muscle Na+ excess, we found no statistically significant relationship between muscle Na+ content and GDR or LDR (Figure 2F and 2H).
After individually adjusting for age, gender, BMI, hs-CRP, fat mass, lean mass, and truncal fat percentage, the association between muscle sodium and LDR remained statistically significant only for fat mass and truncal fat percentage (Table 4). The statistically significant association between muscle Na+ and GDR was not present after individually adjusting for age, gender, BMI, hs-CRP, fat mass, lean mass, and truncal fat percentage (Table 4).
| LDR | GDR | |
|---|---|---|
| Β (95% CI) | Β (95% CI) | |
| Muscle Na+ + study group + age | −0.45 (−0.91, 0.11) | −0.13 (−0.73, 1.23) |
| Muscle Na+ + study group + gender | −0.52 (−1.15, 0.02) | −0.17 (−0.84, 1.32) |
| Muscle Na+ + study group + BMI | −0.50 (−1.00, 0.00) | −0.21 (−0.88, 1.29) |
| Muscle Na+ + study group + hs-CRP | −0.47 (−0.99, 0.12) | −0.17 (−0.79, 1.37) |
| Muscle Na+ + study group + lean mass | −0.46 (−1.08, 0.13) | −0.16 (−0.82, 1.40) |
| Muscle Na+ + study group + fat mass | −0.50 (−0.94, −0.02) | −0.19 (−0.95, 1.36) |
| Muscle Na+ + study group + truncal fat % | −0.50 (−0.96, −0.02) | −0.24 (−0.98. 1.35) |
- Na+; sodium, LDR; leucine disposal rate, GDR; glucose disposal rate. The variables are log scaled, and the confidence interval was obtained using bootstrapping.
Discussion
In this pilot study, we report for the first time that muscle Na+ content measured by 23Na MRI is significantly and inversely associated with measures of peripheral insulin sensitivity in MHD patients. Notably, the association between muscle Na+ content is equally prominent for leucine and glucose disposal rates suggesting that muscle Na+ accumulation could be one of the primary mechanisms by which protein energy wasting develops in MHD patients. The cellular mechanisms leading to diminished tissue sensitivity to insulin in the setting of excessive tissue Na+ accumulation could be quite distinct and require further examination.
The clinical and research implications of these findings are highly relevant. First and foremost, these data provide novel in vivo evidence that Na+ balance has significant metabolic effects above and beyond volume management in stable MHD patients. Second, because one of the primary functions of maintenance dialysis therapy is to normalize volume and salt balance, the standard hemodialysis prescription employed in ESRD patients does not successfully normalize tissue Na+ content. Finally, these data suggest that the Na+ overloaded microenvironment in skeletal muscle of MHD patients is mechanistically coupled with glucose and energy metabolism, including but not limited to protein energy wasting.
The mechanism by which excess tissue Na+ accumulation may interfere with insulin signaling is not clear. Large amounts of Na+ are stored in the skeletal muscle in experimental salt-sensitive hypertension,10 in humans with secondary hypertension,8 and with increasing age with9 or without6 loss of kidney function. Titze et al. established that the large amount of Na+ that is stored inside skeletal muscle cells displays pro-inflammatory immune cell depolarization to provide local electrolyte clearance.11-14 In the setting of prolonged exposure, such as ESRD, Na+ accumulation could similarly promote a local inflammatory response. In our study, the statistically significant association between Na+ content in the muscle and markers of insulin sensitivity was not affected after adjustment by age or hsCRP. While this would suggest that these underlying conditions are not significantly mediating the process, we have previously shown that markers of systemic inflammation are strongly correlated with glucose homeostasis in MHD patients. Regarding the age, the difference between groups was minimal, median difference of 2 years, suggesting an unlikely explanatory mechanism.15-17 Alternatively, ultra-long-term balance studies in humans suggest that increasing salt intake and thus elevating body Na+ content result in salt-induced subclinical increases in glucocorticoid excretion.18 It is also possible that the disappearance of statistical significance could be the result of insufficient sample size, potentially leading to unreliable conclusions. Consistent with the latter explanation, the differences in odds ratio after each individual adjustment are relatively small and likely to have limited biological consequence. In any case, future studies extending this pilot study are needed to explore these hypotheses in more detail.
Another notable finding in our study was that the positive association between higher Na+ content in muscle and lower GDR and LDR was primarily observed in MHD patients and not in controls. There was also a substantial difference in tissue Na+ accumulation between MHD patients and controls, which has been previously reported.6 While these data suggest that in order to interfere with insulin signaling, significant tissue Na+ accumulation is necessary, it is also possible that there might be a Na+ muscle saturation threshold that needs to be reached before Na+ accumulation can impair insulin signaling. It is also possible that certain recognized and unrecognized uremic toxins present in MHD patients may act either alone or synergistically with Na+ muscle concentrations to reduce the responsiveness to insulin action. Further studies are necessary to determine the relative importance of clinical and demographic characteristics and of uremic toxins for insulin signaling in the presence of significant tissue Na+ accumulation.
While earlier studies have reported significant differences in GDR between MHD patients compared with healthy controls, recent data by our group and others suggest that resistance to glycogenic effects of insulin is determined by mechanisms besides the uremic milieu in CKD patients, including ones on maintenance dialysis.4, 5, 19 On the other hand, we have shown that resistance to anabolic actions of insulin is a direct consequence of underlying CKD, unlike insulin's glycogenic actions.7 These observations are based on methodology that is considered to be the gold standard for assessment of insulin sensitivity. Glucose disposal rate is already established gold standard for assessment of peripheral insulin sensitivity for carbohydrate metabolism. LDR used in this study is based on the same principal but directly assesses insulin sensitivity in terms of amino acid metabolism. We have reported that there is excellent correlation between LDR and whole body protein metabolism estimated by stable isotope studies in both healthy individuals and patients on MHD.7 The data reported herein suggest that the mechanisms by which insulin sensitivity is altered due to muscle tissue Na+ accumulation share a common signaling pathway for both carbohydrate and amino acid metabolism, which occurs predominantly when skeletal muscle is excessively Na+ overloaded. Accordingly, therapeutic mobilization of muscle Na+ might be a promising approach to improve not only protein-energy status of MHD patients, but also their metabolic and vascular health.
There are several strengths in this study. Tissue Na+ accumulation as a determinant of insulin action is novel and represents a potentially modifiable risk factor that can reduce derangements in protein and glucose metabolism observed in MHD patients. This study also employed the gold standard to provide the most precise measurements of IR available. Although not previously reported, the apparent association between excessive muscle tissue Na+ accumulation and peripheral tissue insulin sensitivity is expected. The skeletal muscle is the primary target for insulin action and a major source of glycogen storage, but skin is less involved in the regulation of protein and glucose metabolism, if at all. The associations observed in the skeletal muscle, but not in the skin, are consistent with the biologic role of muscle as the primary tissue reservoir for peripheral insulin action. Finally, inclusion of the control population without kidney disease also enabled us to examine a range of IR and tissue Na+ accumulation.
There are also several limitations of this study. First, the cross-sectional design limits any casual inference between tissue Na+ content and insulin sensitivity. Second, the small sample size precludes our ability to definitively explore covariates and predictors that might explain the associations observed. Accordingly, extreme caution should be exerted to make any reliable inference from these results. This novel, albeit preliminary, data should be considered as a basis for further research into the cellular Na+ networks and insulin signaling pathways in MHD patients.
In conclusion, the results of this pilot study demonstrate that higher muscle Na+ concentrations are associated with IR in MHD patients that has potential implications in the pathogenesis of protein-energy wasting and glucose metabolism in ESRD. Further research is needed to validate these findings, to identify the mechanisms underlying these associations, and to determine whether they are modifiable.
Acknowledgements
This study was supported in part by the following: US Department of Veterans Affairs under Award Number 1I01CX000414, Clinical Translational Science Award UL1TR000445 from the National Center for Advancing Translational Sciences of the NIH, Vanderbilt Diabetes Research and Training Center Grant P30 DK020593, Vanderbilt O'Brien Mouse Kidney Center Grant P30 DK079341, K24 DK62849 from the National Institute of Diabetes and Digestive and Kidney Diseases, Vanderbilt Center for Kidney Disease Grant RO1 HL118579-01, and the American Heart Association award 14SFRN20770008, as well as grants from Baxter Healthcare and Renal Research Institute. Serpil Muge Deger is currently a fellow of the American Heart Association. Edward D. Siew was supported in this work by the Vanderbilt Center for Kidney Disease. Data from this manuscript were presented in abstract form at the 53rd Congress of European Renal Association–European Dialysis and Transplant Association, May 2016, Vienna, Austria. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of the Cachexia, Sarcopenia, and Muscle: update 2015.20
Conflict of interest
None.




