Ca2+-activated K+ channels regulate cell volume in human glioblastoma cells
Abstract
Glioblastoma (GBM), the most lethal form of brain tumors, bases its malignancy on the strong ability of its cells to migrate and invade the narrow spaces of healthy brain parenchyma. Cell migration and invasion are both critically dependent on changes in cell volume and shape driven by the transmembrane transport of osmotically important ions such as K+ and Cl−. However, while the Cl− channels participating in cell volume regulation have been clearly identified, the precise nature of the K+ channels involved is still uncertain. Using a combination of electrophysiological and imaging approaches in GBM U87-MG cells, we found that hypotonic-induced cell swelling triggered the opening of Ca2+-activated K+ (KCa) channels of large and intermediate conductance (BKCa and IKCa, respectively), both highly expressed in GBM cells. The influx of Ca2+ mediated by the hypotonic-induced activation of mechanosensitive channels was found to be a key step for opening both the BKCa and the IKCa channels. Finally, the activation of both KCa channels mediated by mechanosensitive channels was found to be essential for the development of the regulatory volume decrease following hypotonic shock. Taken together, these data indicate that KCa channels are the main K+ channels responsible for the volume regulation in U87-MG cells.
Abbreviations
-
- BKCa
-
- Ca2+-activated K+ channel of large conductance
-
- GBM
-
- glioblastoma
-
- ICl,swell
-
- swelling-activated Cl− current
-
- IKCa
-
- Ca2+-activated K+ channel of intermediate conductance
-
- KCa
-
- Ca2+-activated K+ channels
-
- RVD
-
- regulatory volume decrease
-
- VRAC
-
- volume-regulated anion channel
1 INTRODUCTION
All animal cells have evolved a complex machinery to control and regulate their volume, both to respond to osmotic changes of the environment and to enable vital functions as proliferation, migration, differentiation, and death (Hoffmann et al., 2009). Experimentally, the mechanisms of cell volume regulation can best be appreciated by studying the regulatory volume decrease (RVD), as a prototype mechanism of volume regulation shared by virtually all cells. RVD is a complex process whereby cells recover their original volume after an osmotic shock-induced swelling. It has been widely demonstrated that cell volume re-establishment is due to the concerted activation of Cl− and K+ channels that mediate a net efflux of KCl from the cell, followed by osmotic loss of water and decrease of cell volume. Previous observations suggest that intracellular Ca2+ signals, that occur following hypotonic shock-induced cell swelling as a result of both Ca2+ entry from the extracellular space through mechanosensitive channels (Arniges et al., 2004; Benfenati et al., 2011; Brignone et al., 2022; Kim et al., 2017; Lanciotti et al., 2012; Liedtke, 2005; Numata et al., 2021; Toft-Bertelsen et al., 2017) and Ca2+ release from internal stores (Liu & Zhang, Men, et al., 2019; Park et al., 2002; Sheader et al., 2001a), play a central role in cell volume regulation and RVD in some cell types (Hazama & Okada, 1990; Hoffmann & Dunham, 1995; Lang et al., 1998; McCarty & O'Neil, 1992). However, many other cell types do not require Ca2+ to fully activate the RVD process, rendering this Ca2+ dependency highly variable among cell types (Pasantes-Morales & Morales Mulia, 2000).
The understanding of the mechanisms underlying cell volume regulation was significantly improved by studying tumor cells, as they show evident alterations of all the biological processes listed above. One of the tumor cell models that has offered a major contribution to the study of cell volume changes in migration and invasion is glioblastoma (GBM), the most common and aggressive human brain tumor, with very poor prognosis and median survival of about 15 months (Davis et al., 1998; Davis, 2018). GBM malignancy is primarily due to the strong ability of tumor cells to invade the peritumoral parenchyma, leading to tumor re-growth outside the central mass (Claes et al., 2007). Invasion requires GBM cells to adjust their volume and shape, which they do by controlling several processes including transmembrane water fluxes. In this context, ion channels are critically involved as determinants for both dynamic volume regulation and intracellular Ca2+ signals. Intensive investigation has shown that the Cl− efflux involved in GBM cell volume changes is mainly sustained by the volume-regulated anion channel (VRAC), a heteromeric channel made of the leucine-rich repeat containing 8 (LRRC8) protein subunits, which is highly expressed in GBM cells where it mediates the so-called swelling-activated Cl− current (ICl,swell) (Catacuzzeno et al., 2014; Ransom et al., 2001; Saberbaghi et al., 2019; Sforna et al., 2017; Caramia et al., 2019; Sontheimer, 2008; Soroceanu et al., 1999; Varricchio et al., 2023). Much less is known about the molecular identity of the K+ channels involved in the RVD process. It is widely appreciated that GBM cells express abundant levels of Ca2+-activated K+ (KCa) channels of intermediate (IKCa) and large (BKCa) conductance type, which are both activated by increases in cytoplasmic Ca2+ concentrations, but also by depolarization in the case of BKCa channels. Both these channels have been shown to play a significant role in GBM migration and invasion, processes where cell volume regulation is essential (Catacuzzeno et al., 2015; Rosa et al., 2017, 2018; Ruggieri et al., 2012). The shared view is that KCa channels promote cell migration/invasion either by controlling cell volume or by modulating the membrane potential, that is, the driving force for Ca2+ influx, thus regulating intracellular Ca2+ signals.
A reasonable scenario would be that during cell migration/invasion, KCa channels sustain the K+ efflux that, in combination with Cl− efflux through VRAC, promotes the osmotic water loss underlying cell shrinkage (Catacuzzeno et al., 2014; Turner & Sontheimer, 2014). However, the precise involvement of KCa channels in the RVD of GBM cells has never been conclusively proven.
Many studies using fluorescent dyes have shown that hypotonic-induced cell swelling triggers increases in intracellular Ca2+ concentrations, which represent the signal for KCa channels activation. We have then hypothesized that IKCa and BKCa channels respond to hypotonicity by increasing K+ conductance necessary for cell volume regulation in GBM cells. To clear this point, we studied human U87-MG cells, as they show volume regulatory mechanisms in a very pronounced way. We conducted electrophysiological experiments to identify the K+ currents activated by the hypotonic shock, and videoimaging recordings to evaluate the RVD process, taken as benchmark for the assessment of cell volume regulation. We show that both IKCa and BKCa channels are activated by hypotonic-induced cell swelling as the result of intracellular Ca2+ increase, and their activity is required for the RVD process to take place. We also gathered circumstantial evidence that the influx of external Ca2+ through mechanosensitive channels, such as Piezo1, may play a key role in Ca2+ signaling underlying cell volume regulation.
2 MATERIALS AND METHODS
2.1 Cell culture
U87-MG (ICLC catalog code: HTL00013) GBM cell line was grown in high-glucose Dulbecco's minimum essential medium supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/mL penicillin G, 100 mg/mL streptomycin. The flasks were incubated at 37°C in a 5% CO2-humidified atmosphere. The medium was changed twice a week, and the cells were sub-cultured when confluent. For electrophysiological experiments, cells were seeded in Petri dishes at 40,000/50,000 cells/mL and used at 2 or 3 days after seeding. For cell volume measurements, cells were trypsinized the day of the experiment and then plated on dishes and allowed to settle for 30 min before conducting cell volume recordings. This procedure allowed the cells to obtain a spherical shape, ideal for extrapolating the cell volume changes from the relative area measurements (see “Cell volume measurements” paragraph for more details).
2.2 Electrophysiology
The whole-cell perforated configuration was used for electrophysiological recordings. Currents were amplified with a HEKA EPC-10 amplifier (List Medical), digitized with a 12-bit A/D converter (TL-1, DMA interface; Axon Instruments), and analyzed with the software Patch-Master package (version 2_60, ELEKTRONIK) and Microcal Origin 8.0. For on-line data collection, macroscopic currents were filtered at 3 kHz and sampled at 200 µs/point. The external standard Ringer's solution contained: NaCl 140 mM, KCl 5 mM, CaCl2 2 mM, MgCl2 2 mM, MOPS 5 mM, glucose 10 mM (pH 7.4). The external Ca2+-free solution contained: NaCl 140 mM, KCl 5 mM, MgCl2 4 mM, MOPS 5 mM, glucose 10 mM (pH 7.4). The internal solution contained: K2SO4 57.5 mM, KCl 55 mM, MgCl2 5 mM, MOPS 10 mM (pH 7.2). Electrical access to the cytoplasm was achieved by adding amphotericin B (200 μM) to the pipette solution. Access resistances ranging between 15 and 30 MΩ were achieved within 10–15 min from seal formation and were actively compensated to approximately 50%. Octanol (1 mM) was added to all external solutions to block the gap-junctions (Eskandari et al., 2002). All chemicals used were of analytical grade. Dimethyl sulfoxide (DMSO), amphotericin B, and gadolinium chloride (GdCl3) were purchased from Sigma. 4-[(2-Butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy] butanoic acid (DCPIB), TRAM-34, paxilline, and Yoda1 were purchased from Tocris Biosciences. GdCl3 was dissolved in water at the stock concentration of 100 mM and used at the final concentration of 10 μM. Amphotericin B, DCPIB, TRAM-34, paxilline, and Yoda1 were dissolved in DMSO at the stock concentration of 50, 10, 6, 10, and 10 mM, and used at the final concentration of 200, 10, 3, 1, and 10 μM, respectively. The maximal DMSO concentration in the recording solutions was 0.1%, which did not have effects on membrane currents (data not shown). The hypotonic solution was prepared by adding 30% distilled water to the extracellular Ringer's solution. All reagents were fresh daily solubilized at the concentrations stated, and bath applied with a gravity perfusion system. Experiments were carried out at room temperature (18–22°C).
2.3 Cell volume measurements
Cells were perfused with the identical extracellular Ringer's solution used for electrophysiological experiments. To study the hypotonic-induced cell swelling effect, a 30% hypotonic solution was applied. Relative projection area of cells was measured using a video-imaging technique, as previously described (Sforna et al., 2017, 2022). The cells were observed through a ×40 objective lens of a microscope connected to a video camera (Axiocam/Cm1, Zeiss). Images of the cells were saved as TIFF files and the area of each image was subsequently measured using ImageJ. Images were acquired every 2 min for the experiments shown in Figure 6a,b. Relative cell area (Arel) was calculated by dividing the area of the cell at each time point (At) upon exposure to hypotonic solution by the initial area (A0) taken under isotonic conditions (more precisely the average of the three time points recorded in the first 4 min). The extent of RVD was measured 62 min after the onset of the regulatory response, which started soon after the cell reached the peak of swelling under hypotonic solution, and was calculated as follows: %RVD = ((Arel,peak − Arel,62)/(Arel,peak)) × 100, where Arel,peak and Arel,62 are the relative areas at the peak of cell swelling and at the end of the experiment, respectively (Sforna et al., 2022).
2.4 Statistical analysis
All statistical analyses were performed using the Origin v.8 software. To evaluate the significance of a difference, we used either the one-sample or two-sample t-test, depending on whether we compared one normally distributed data to unity (Figures 1e and 5c) or two normally distributed populations, respectively. For multiple comparisons referred to a single control group (Figure 6c), we used a one-way analysis of variance test with post hoc Tukey's correction. For all experiments, p < 0.05 was considered statistically significant and data are shown as mean ± SEM.
3 RESULTS
3.1 Hypotonic stimulus activates both IKCa and BKCa currents in U87-MG cells
As a first step, we assessed whether the osmotic stress could activate K+ selective currents in U87-MG cells by performing electrophysiologic experiments using the patch-clamp technique in the whole-cell perforated configuration. In these experiments the extracellular solution was constantly supplemented with 10 µM DCPIB to block the ICl,swell. Under these conditions, the application of an extracellular 30% hypotonic solution was able to activate an outward current (Figure 1a,b) whose reversal potential was close to the equilibrium potential of K+ ions as calculated by the Nernst equation (Figure 1b). The hypotonic-activated K+ current evoked by voltage ramps, appeared to be composed of a voltage-independent and a voltage-dependent component, with features reminiscent of the highly expressed IKCa and BKCa currents in GBM cells (Figure 1b; Fioretti et al., 2006; Rosa et al., 2017). In accordance, the hypotonic-activated current was partially blocked by the BKCa inhibitor paxilline (1 µM) that completely removed the voltage-dependent component of the current (Figure 1a,b). Subsequent addition of the IKCa channel inhibitor TRAM-34 (3 µM) eliminated also the voltage-independent component, dropping the current to isotonic condition level. These results strongly suggested that in U87-MG cells the only K+ currents activated by the hypotonic stimulus are the IKCa and the BKCa currents.
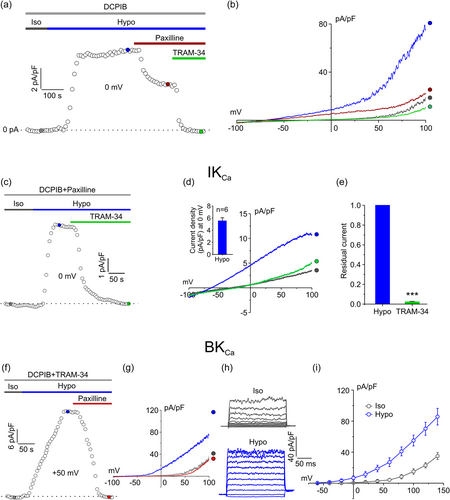
We next studied separately the two hypotonic-activated KCa currents, starting with the IKCa current. To isolate the IKCa current, the extracellular solution was supplemented with 10 µM DCPIB and 1 µM paxilline, inhibitors of VRAC and BKCa channels, respectively. Under these conditions, the hypotonic solution was able to activate an outward current at 0 mV, as shown by the representative time course in Figure 1c. The hypotonic-current showed the typical features of the IKCa current, such as voltage-independence and a reversal potential at −80 mV, (Figure 1d). In addition, the current was fully blocked by TRAM-34 (Figure 1c–e), as also confirmed by the quantitative analysis of the residual current in the presence of the inhibitor (Figure 1e). The TRAM-34-sensitive current elicited by the hypotonic stress, as measured at 0 mV of the applied potential, displayed an average density of 5.5 ± 0.5 pA/pF (Figure 1d, Inset).
To isolate the BKCa current, the extracellular solution was supplemented with 10 µM DCPIB and 3 µM TRAM-34. As shown by the time course of the current density in Figure 1f, constructed from the current ramps as shown in Figure 1g, taking the current at +50 mV, the application of a hypotonic solution elicited an outward current which exhibited the typical biophysical properties of the BKCa current (i.e., a strong voltage dependence and a relatively high current noise), including full block by 1 µM paxilline (Figure 1f,g). To quantify the hypotonic-induced activation of the BKCa current, we applied 200 ms voltage steps from −60 to +140 mV, from a holding potential of −40 mV, with increment steps of 20 mV (Figure 1h). Application of depolarizing steps under these conditions elicited an outward current associated with a high noise, typical of currents passing through the high-conductance BKCa. The quantitative analysis of the current-voltage relationship constructed from these voltage steps, showed that the BKCa current triggered by the osmotic stress was more than twice of that recorded under isotonic conditions (at +140 mV, the current density was 35.0 ± 4.6 vs. 85.7 ± 10.7 pA/pF under isotonic and hypotonic conditions, respectively).
3.2 Activation of IKCa and BKCa currents by hypotonicity requires the influx of extracellular Ca2+
Both IKCa and BKCa channels require an increase of intracellular Ca2+ to open. Many studies have shown that the hypotonic-induced cell swelling promotes cytosolic Ca2+ raises by triggering extracellular Ca2+ influx (Liu & Zhang, Men, et al., 2019; Park et al., 2002; Sheader et al., 2001a). Therefore, we hypothesized that the activation of both KCa channels upon exposure to osmotic stress might derive from cell swelling-induced Ca2+ entry. To verify this notion, we performed electrophysiological experiments as those shown in Figure 1 but initially using extracellular solutions devoid of Ca2+ ions. First, we probed the IKCa current, using the same pharmacological approach illustrated before (i.e., using extracellular solution constantly supplemented with 10 µM DCPIB and 1 µM paxilline). We began with a stabilization period of 5 min in 0-Ca2+ isotonic solution, after which cells were exposed to hypotonic solution, still in the absence of external Ca2+, to assess the activation of an outward current. After a stable period in 0-Ca2+ hypotonic solution, cells were probed with a hypotonic solution containing 2 mM Ca2+, which evoked a major increase of outward current, arguably due to the entry of external Ca2+, that was fully blocked by the IKCa current antagonist TRAM-34 (Figure 2a,b). Results from these experiments revealed that the hypotonic stimulus was able to activate a TRAM-34-sensitive current only upon the addition of external Ca2+ (Figure 2a,b). The quantitative analysis of the TRAM-sensitive current revealed that the hypotonic-activated current density was more than seven times greater when Ca2+ was added to the extracellular solution: 0.6 ± 0.2 versus 4.6 ± 0.3 pA/pF at 0 mV in the absence and presence of external Ca2+, respectively (Figure 2c).
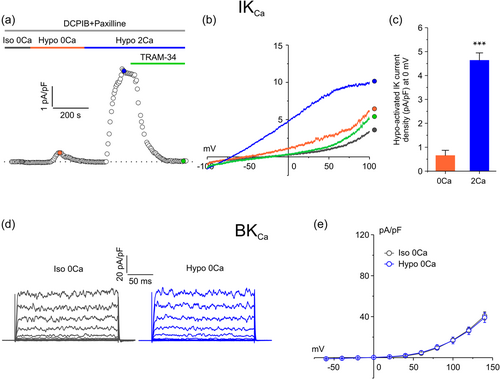
We then performed electrophysiological experiments aimed at measuring the BKCa current (with 10 µM DCPIB and 3 µM TRAM-34 constantly present in the extracellular solution). As observed for the IKCa current, removal of external Ca2+ prevented the activation of BKCa current by the hypotonic stimulus, as shown by both the representative current traces obtained in response to voltage steps and the average current-voltage relationship (Figure 2d,e), showing no difference in current density at any given voltage. Taken together, these results indicate that an influx of external Ca2+ is required for the hypotonic-induced activation of both IKCa and BKCa currents.
3.3 Ca2+ influx required for the activation of IKCa and BKCa channels goes through mechanosensitive Ca2+ channels
The influx of external Ca2+ during hypotonic stress may occur through mechanosensitive Ca2+ permeable channels activated by cell swelling. To test this notion, we applied hypotonic solutions containing gadolinium (Gd3+, 10 µM), a potent inhibitor of mechanosensitive channels (Hamill & McBride, 1996; Yang & Sachs, 1989), on cells already equilibrated for 5 min with Gd3+ (in isotonic solution) (Figure 3a). Also in this case, we recorded IKCa and BKCa currents separately. First, we probed the IKCa current using extracellular solution constantly supplemented with 10 µM DCPIB and 1 µM paxilline. As found with zero external Ca2+ (Figure 2a–c), in the presence of Gd3+ only a very small outward current induced by hypotonicity was observed, while a significant TRAM-sensitive current was elicited upon removal of the mechanosensitive channel blocker Gd3+ (Figure 3a,b). Specifically, the average current density recorded at 0 mV, taken from the representative current ramps (Figure 1b), was 0.6 ± 0.3 vs. 4.5 ± 0.7 pA/pF in the presence and absence of Gd3+, respectively (Figure 3c).
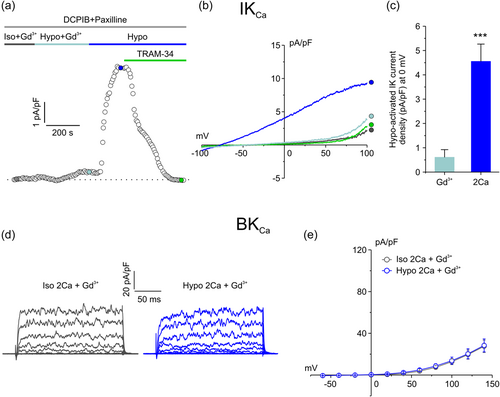
We then recorded the BKCa current by using an extracellular solution supplemented with both DCPIB and TRAM-34. Results from these experiments revealed that also the activation of the BKCa current was fully prevented or blocked when the entry of Ca2+ through mechanosensitive channels was not allowed (Figure 3d,e).
3.4 Piezo1 selective agonist Yoda1 activates a current virtually identical to that elicited by hypotonic-induced cell swelling
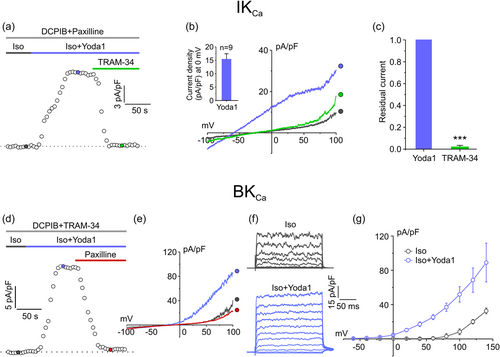
It has been recently reported that Piezo1, a Ca2+-permeable channel identified as the essential component of the mechanically activated current that mediates mechanotransduction in vertebrates (Coste et al., 2010), is heavily overexpressed in high grade GBM (Chen et al., 2018; Qu et al., 2020). We verified its involvement in the activation of both IKCa and BKCa currents by assessing whether the hypotonic stimulus was able to activate a Piezo1 current. We first characterize the biophysical properties of Piezo1 in our U87-MG cells, by applying the highly selective Piezo1 synthetic agonist Yoda1 (Syeda et al., 2015). Experiments were carried out by either applying 1 s voltage ramps from −100 to +100 mV or 200 ms voltage steps from −100 to +100 mV protocols. As Piezo1 is a nonselective cation channel which mediates the flux of Ca2+, Na+, and K+ ions, currents were measured at both +80 mV (to appreciate K+ flux) and −80 mV (to appreciate both Ca2+ and Na+ fluxes). Activation of KCa and VRAC currents were prevented by supplementing the extracellular solution with TRAM-34, paxilline, and DCPIB. As shown in the representative time course in Figure 4a, acute application of 10 μM Yoda1 under isotonic conditions elicited an outward and an inward current (as measured at +80 and −80 mV, respectively, from the current ramps shown in (a, inset)) that were both fully blocked by the acute application of 10 μM Gd3+. The representative current ramps in Figure 4a (inset) showed that the Yoda1-activated current displayed a strong outward rectification, somehow expected because of its significant voltage dependence (in addition to stretch activation) (Moroni et al., 2018). Rectification and voltage modulation of Yoda1-mediated Piezo1 current were also clearly shown during the application of a voltage-step protocol ranging from −100 to +100 mV (20 mV increment steps) and by the corresponding I–V relationship (Figure 4b,c).
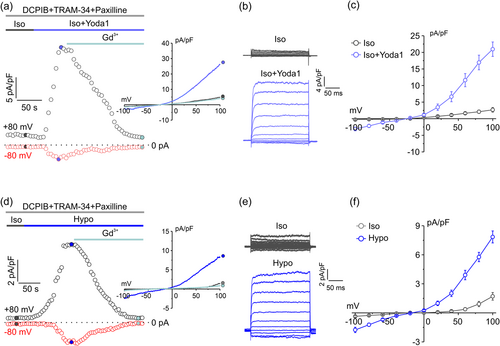
We then performed identical electrophysiological experiments, where cells were subjected to a 30% hypotonic solution instead of applying Yoda1 (Figure 4d–f). The extracellular solution was, as before, supplemented with TRAM-34, paxilline, and DCPIB. As can be seen from the current ramps, the step current traces and the related analysis, the biophysical and kinetic properties of the current elicited by the hypotonic solution were virtually identical to those of Yoda1-activated current, with a strong voltage-dependent outward rectification (Figure 4e,f). The hypotonic-induced currents were however significantly smaller than those elicited by Yoda1 (at 100 mV the hypotonic current and the Yoda1 current were 7.8 ± 0.6 pA/pF and 20.9 ± 2.1 pA/pF, respectively). Taken together, these results suggest that Piezo1 might be responsible for a large part of hypotonic induced Ca2+ influx in U87-MG cells.
3.5 Piezo1 channel agonist Yoda1 activates both IKCa and BKCa currents
To verify whether IKCa and BKCa channels may be activated as the result of Piezo1 opening, we performed experiments in which the specific Piezo1 activator Yoda1 was acutely applied under isotonic conditions. As clearly shown in Figure 5, acute application of Yoda1 under isotonic conditions was able to elicit currents exhibiting the typical biophysical and pharmacological features of both IKCa and BKCa currents (Figure 5a–g), indicating that both KCa channels can be activated by Ca2+ influx through Piezo1.
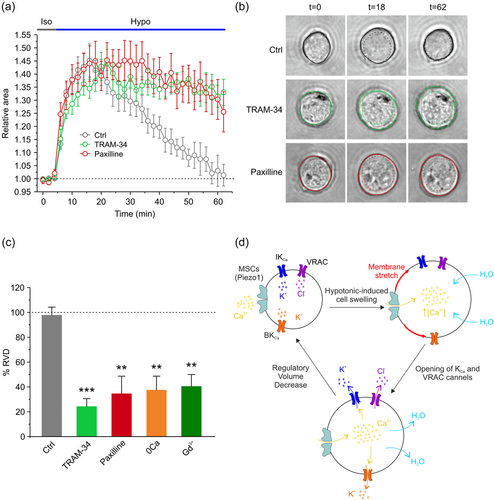
3.6 Both IKCa and BKCa channels are involved in RVD
Finally, we assessed the contribution of both IKCa and BKCa currents in the RVD process, by measuring changes in cell area in control conditions, and after either channel was pharmacologically blocked. We monitored cell volume changes with a phase-contrast microscope, and images were video recorded at 2 min intervals, over a long-time period (62 min). During the first 4 min of control recording, cells were exposed to isotonic solution, and then to 30% hypotonic solution for the remaining 58 min of the experiment. The average time course of the relative cell area showed that cell volume underwent a rapid and marked increase upon the application of the hypotonic solution. However, while in control conditions cells exhibited a marked ability to completely recover their original cell volume, the selective inhibition of IKCa or BKCa channels significantly impaired the re-establishment of the original cell volume (Figure 6a). The level of cell volume recovery was calculated as %RVD = ((Arel,peak − Arel,62)/(Arel,peak)) × 100, where Arel,peak and Arel,62 are the relative areas at the peak of cell swelling and at the end of the experiment, respectively (see Section 2). %RVD was 97.6%, 24.1%, and 34.4% for cells in control conditions, in the presence of 3 µM TRAM-34, and in the presence of 1 µM paxilline, respectively (Figure 6c). Importantly, the RVD was also markedly affected by the absence of external Ca2+ (37.3%) or the presence of the mechanosensitive blocker 10 µM Gd3+ (40.3%), strongly suggesting the involvement of mechanosensitive-mediated Ca2+ influx in the RVD process. Collectively, these data indicated that KCa channels play a key role in the regulation of cell volume, as their selective inhibition strongly hampers the RVD process.
4 DISCUSSION
4.1 Main findings
The present study examined the involvement of IKCa and BKCa channels in RVD, the cell response to hypotonic-induced cell swelling that ultimately results in the re-establishment of the original cell volume. We show that cell exposure to hypotonic solution in the constant presence of DCPIB activates a macroscopic current essentially sustained by IKCa and BKCa channels. The hypotonic-induced activation of IKCa and BKCa channels requires the influx of Ca2+ from the extracellular environment, as demonstrated by the result that removal of external Ca2+ prevents the activation of both channels. Moreover, the observation that the nonselective mechanosensitive channel blocker Gd3+ prevents the hypotonic-induced activation of both IKCa and BKCa channels indicates that the Ca2+ entry passes through mechanosensitive channels. Among the mechanosensitive channels that are activated by cell swelling, Piezo1 may represent an important component since: i) it is highly expressed in high grade gliomas; ii) Yoda1 can activate both IKCa and BKCa channels under isotonic conditions; iii) the Yoda1-activated current exhibits the biophysical and pharmacological properties of nonspecific mechanosensitive cation currents, such as a reversal potential close to 0 mV, strong outward rectification, and block by Gd3+. Worth of notice, these properties are fully shared by the current activated by the hypotonic stimulus in the presence of DCPIB, TRAM-34, and paxilline, congruent with Piezo1 as the underlying channel. On the other end, the observation that Yoda1, under isotonic conditions, can activate both IKCa and BKCa channels, shows that these channels are under the control of Piezo1 (although not necessarily exclusive).
We finally show that the recovery of the original cell volume (RVD) following exposure to hypotonic solution is significantly hampered by the selective inhibition of either IKCa or BKCa channel. As expected, a marked inhibition of RVD is also observed when the influx of Ca2+ from the extracellular space is prevented, either by removing the external Ca2+ or blocking its influx through mechanosensitive channels.
4.2 How the influx of Ca2+ determines the IKCa and BKCa channels activation
Although IKCa and BKCa channels are both activated by internal Ca2+, the impact that given cytoplasmic Ca2+ concentrations will have on their activation is strongly affected by two distinctive features of these channels: Ca2+ sensitivity and voltage dependence. IKCa channels are fully voltage independent but are activated by low concentrations of cytoplasmic Ca2+ (EC50 100–200 nM). By contrast, BKCa channels have a much lower Ca2+ sensitivity (EC50 1–5 µM, at 0 mV) but are sensitive to voltage. Their opening thus requires cytosolic Ca2+ increases in the micromolar range, even at moderate depolarizations. In the virtual absence of cytosolic Ca2+, BKCa channels can be activated only with extreme depolarizations, that can never be experienced by non-excitable cells such as GBM.
Several studies using fluorescent dyes have shown that hypotonic-induced cell swelling can raise the global cytoplasmic Ca2+ concentration in the range of 200-400 nM (Nitschke et al., 1993; Wong et al., 1990; Yellowley et al., 2002). While these Ca2+ concentrations would be sufficient to activate a large fraction of IKCa channels, they are way lower than those required for the activation of BKCa channels (in the range of physiological membrane potentials). This makes it difficult to explain how the hypotonic shock can activate BKCa channels, especially in intact cells, as in the RVD experiments, with negative (resting) voltage. Yet, our data clearly demonstrate that it does, as BKCa channels have been shown essential for the re-establishment of the original cell volume in the RVD process, and current mearurements indicate that they open at potentials close to the resting membrane potential (i.e., −30/−40 mV in our cell model) during the hypotonic shock.
These results compel us to consider the hypothesis that BKCa channels are confined in specialized compartments where they can sense a Ca2+ concentration, following a hypotonic shock, much higher than the Ca2+ bulk assessed with fluorescent probes. These compartments can be found near Ca2+ sources, such as mechanosensitive Ca2+-permeable channels in the plasma membrane where Ca2+ concentrations can increase up to several hundred micromolar. Several instances have been reported in the literature where BKCa channels associate, via a direct protein-protein interaction, with Ca2+ entry channels, as in rat brain where BKCa channels are linked to L-type voltage-gated Ca2+ channels (Berkefeld et al., 2006). In our specific case, we should consider also that BKCa channels could be located in specialized membrane patches in the plasma membrane, called lipid rafts, in close proximity with Ca2+-release channels of intracellular stores, as reported in glioma cells (Weaver et al., 2007). We did not investigate these aspects of channel coupling and Ca2+ sensing in the present study, which we count to address them in a future study, however.
4.3 Does the influx of Ca2+ needed for the activation of KCa channels goes through Piezo1 channels?
The mechanism of KCa channels activation upon cell swelling is poorly understood. A few reports claim that BKCa channels can be directly activated by membrane stretch during hypotonic-induced cell swelling (Allard et al., 2000; Christensen, 1987; Mallouk & Allard, 2000; Taniguchi & Guggino, 1989). Conversely, others reported that the BKCa channel activation was due to stretch-induced Ca2+ influx (Taniguchi & Imai, 1998). This study shows that the opening of BKCa channels upon osmotic shock requires cytosolic Ca2+ elevation and excludes their activation by increased membrane stretch per se. Rather, the hypotonic-induced activation of BKCa channels needs the opening of mechanosensitive channels, which they do respond to the increased membrane tension during cell swelling. Similarly, the activation of IKCa channels by the hypotonic stimulus, occurs only in the presence of extracellular Ca2+ influx and the resulting cytosolic Ca2+ elevation (Vázquez et al., 2001; Wang et al., 2003).
Most mechanosensitive channels are nonselective cation channels with high Ca2+ permeability, that convert mechanical force into Ca2+ influx. The results presented in Figures 2 and 3 show that the hypotonic-induced cell swelling activates IKCa and BKCa channels by inducing entry of Ca2+ through mechanosensitive channels. The involvement of mechanosensitive channels is suggested by the lack of hypotonic-induced KCa activation in presence of their nonselective blocker Gd3+. Clearly this pharmacological approach (i.e., the use of nonspecific blocker Gd3+) does not allow to identify the specific mechanosensitive channel(s) involved. Nor do their biophysical properties, as were inferred from the hypotonic-induced current recorded in presence of DCPIB, TRAM-34 and paxilline, as they are shared by many types of mechanosensitive channels (i.e., reversal potential at 0 mV, strong outward rectification).
Over the past years, several mechanosensitive channels have been considered as possible sensors of the stretching forces on plasma membrane during cell volume regulation. Members of the transient receptor potential (TRP) subfamilies, especially TRPV4 and TRPV7, have been shown to play important roles either in sensing or transducing mechanical stimuli acting on plasma membrane, but also in regulating cell volume (Arniges et al., 2004; Benfenati et al., 2011; Kim et al., 2017; Lanciotti et al., 2012; Liedtke, 2005; Numata et al., 2021; Toft-Bertelsen et al., 2017). The several discrepancies present in the conclusions reached by the different studies make the identification of the swelling-activated Ca2+ permeable channel, which serves as a route for Ca2+ influx, still elusive.
In 2010, a small family of evolutionarily conserved channels, encompassing Piezo1 and Piezo2, was identified as the essential component of the mechanically activated Ca2+-permeant channels that mediate mechanotransduction in vertebrates (Coste et al., 2010). Both channels are controlled by membrane stretch that results from hypotonic-induced cell volume increase, allowing the permeation of cations including Ca2+. Interestingly, it has been recently reported that Piezo1 overexpression correlates with the grade of GBM malignancy (Chen et al., 2018; Qu et al., 2020).
We recently reported that Piezo1 is activated by hypotonic stimuli and controls both cell volume and migration in HEK293 cells (Sforna et al., 2022), suggesting that Piezo1 works as trandrucer of mechanical forces into intracellular signals for volume regulation. These findings and the recognized properties of Piezo1 prompted us to verify if it represents the main mechanosensitive channel, responsible for the activation of IKCa and BKCa channels upon hypotonic-induced cell swelling in U87-MG cells. To this end we compared the hypotonic-activated current in presence of a cocktail of blockers for all the major known currents (DCPIB, TRAM-34, and paxilline), except for the mechanosensitive current and the current activated by the selective Piezo1 agonist Yoda1. Most interesting, the two currents shared important biophysical properties as voltage rectification and reversal potential, suggesting that they were sustained by the same channels. Unfortunately, other mechanosensitive channels share these properties, so we are not able to clarify the matter conclusively. However, combining the above observations with the result that activation of both IKCa and BKCa channels by the highly selective Piezo1 activator Yoda1 and the notion that Piezo1 expression is highly correlated with the grade of glioma malignancy (Chen et al., 2018; Qu et al., 2020), it is conceivable to infer that this channel may play a key role in mechanotransduction related to volume regulation in these cells. Dedicated studies using specific genetic approaches, lingering the absence of selective Piezo1 blockers, are needed to elucidate this unsolved issue.
4.4 The role of KCa channels in the hypotonic induced RVD
A key result of this study is that both IKCa and BKCa channels control RVD. Our interpretation is that these channels respond to both global (IKCa) and local (BKCa) intracellular Ca2+ elevation mediated by the opening of mechanosensitive channels (such as Piezo1) upon an increase of lateral membrane tension induced by cell swelling. The resulting K+ efflux through KCa channels, in conjunction with the Cl− efflux through VRAC, is essential not only for the extrusion of KCl necessary for the obliged loss of water, but also for the maintenance of membrane potential at values between the equilibrium potentials of Cl− (ECl = −20 mV) and K+ (Ek = −80 mV), which guarantees the driving force for the efflux of both ions throughout the RVD process. However, the reason why GBM cells use two Ca2+-activated K+ channels, remains unsolved. Although the involvement of KCa channels in RVD has been reported by many studies in different cell types, none of these works reported that KCa channels are concomitantly required for volume regulation. For instance, some authors showed that RVD is under the control of IKCa but not BKCa channels (Barfod et al., 2007; Vázquez et al., 2001; Wang et al., 2003). Others reported, instead, a predominant role of the BKCa channel (Fernández-Fernández et al., 2002; Lionetto et al., 2010a, 2010b; Sheader et al., 2001b). The discrepancies observed among these studies suggest that regulation of cell volume is a quite complex process and the type of channels involved strongly depend on the type of cell. We plan to investigate this issue in future works.
Another important aspect that emerges from our data and that deserves discussion is that the blockade of both KCa channels is not able to fully abolish the RVD process. The lack of a complete inhibition of RVD occurs also when VRAC channels are blocked with DCPIB (Serpe et al., 2022). Why blockade of both KCa (and VRAC) channels is not able to fully abolish the RVD process remains an unsolved question for which we can only try to give a plausible explanation. RVD is a quite complex process in which many membrane transporters (besides KCa and VRAC channels) participate. An example is the Cl/K cotransporter (KCC) which promotes a nonelectrogenic efflux of K+ and Cl− out of the cell whose activity depends on the amount of cell swelling. This transporter has a very important role in the regulation of cell volume in erythrocytes and together with other transporters could explain the residual percentage of RVD when KCa and VRAC channels are blocked.
4.5 Conclusion
We have shown that the influx of Ca2+ through mechanosensitive channels upon hypotonic-induced cell swelling is an essential step for opening both IKCa and BKCa channels, whose activation is crucial for the efflux of K+ ions and recovery of the original cell volume (i.e., RVD). Although the critical contribution of K+ and Cl− channels in cell volume regulation is well established, their role in GBM cell migration and invasion remains a more debated issue (Liu & Stauber, 2019; Rosa et al., 2017, 2018). However, our study performed on an established GBM cell model, could be of relevance for understanding the mechanisms underlying GBM tumor cell invasion through the narrow extracellular space of healthy brain parenchima, where cell volume regulation seems to be a key process.
To this regard we would like to speculate that the same mechanosensitive channel activation/Ca2+ influx/KCa activation pathway is triggered by more localized mechanical stimuli. This occurrence would greatly help explaining the GBM cells behavior and underlying mechanism they use to modify their shape and steer their movements to avoid obstacles that they can encounter in vivo, and pass through the narrow spaces of the brain parenchyma.
Several open questions remain unanswered, however. One regards the mechanosensitive channel(s) involved in the above pathway. Another is the mechanism through which the entry of Ca2+ induced by hypotonicity can activate two channels (IKCa and BKCa) that exhibit such a marked difference in Ca2+ sensitivity. Future studies will be designed to seek answers to these open questions.
AUTHOR CONTRIBUTIONS
Antonio Michelucci and Luigi Catacuzzeno: conceived and directed the study. Antonio Michelucci, Luigi Sforna, and Angela Di Battista: performed the experimental work and data analysis. Antonio Michelucci and Luigi Sforna: designed and performed patch clamp recordings, analyzed, and interpreted data. Antonio Michelucci and Angela Di Battista: performed cell volume assessment, analyzed, and interpreted data. Fabio Franciolini: participated in the discussions and edited the manuscript. Antonio Michelucci, Fabio Franciolini, and Luigi Catacuzzeno: wrote and edited the manuscript. Antonio Michelucci and Luigi Catacuzzeno: are co-corresponding authors.
ACKNOWLEDGMENTS
This study was supported by a grant to Luigi Catacuzzeno from Fondazione Cassa di Risparmio di Perugia (Project #J95F21000050007).
CONFLICT OF INTERESTS STATEMENT
The authors declare no conflicts of interest.




