Retracted: Long noncoding RNA CHRF exacerbates IL-6-induced inflammatory damages by downregulating microRNA-146a in ATDC5 cells
Abstract
Osteoarthritis (OA) is a frequent chronic musculoskeletal disorder which lacks applicably effective therapeutic strategy. In this study, we attempted to investigate whether long noncoding RNA (lncRNA) cardiac hypertrophy-related factor (CHRF) participated in mediating interleukin-6 (IL-6)-induced in vitro inflammatory damages as well as the regulatory mechanisms. ATDC5 cells were stimulated with IL-6, and then cellular damages were evaluated on the basis of cell viability by CCK-8, apoptotic cells by observation with flow cytometry, apoptosis-associated proteins by western blot analysis, and accumulation of inflammatory factors by quantitative reverse transcription polymerase chain reaction (RT-qPCR), enzyme-linked immunosorbent assay (ELISA), and western blot analysis. Then, effects of lncRNA CHRF on IL-6-treated cells were evaluated. We further explored the downstream factor of lncRNA CHRF and demonstrated whether lncRNA CHRF functioned through the downstream factor. Afterwards, crucial signaling cascades were anatomized. We found that IL-6 reduced cell viability, elevated apoptosis, induced upregulation of inflammatory factors, as well as upregulated lncRNA CHRF and down-regulated miR-146a expression. Then, we found lncRNA CHRF overexpression aggravated IL-6-induced alterations, and lncRNA CHRF knockdown showed the opposite effects. Furthermore, miR-146a was identified to be negatively regulated by lncRNA CHRF, and its overexpression abrogated the roles of lncRNA CHRF in IL-6-treated cells. IL-6-induced the accumulation of IκBα, p65, JAK1, and STAT3 at phosphorylated level was further facilitated by lncRNA CHRF whereas repressed by miR-146a. In conclusion, lncRNA CHRF aggravated the IL-6-induced inflammatory damages in ATDC5 cells. We further outlined a possible mechanism that through downregulating miR-146a, lncRNA CHRF evoked the activation of NF-κB and JAK1/STAT3 signaling cascades.
1 INTRODUCTION
Osteoarthritis (OA) is recognized as a frequent chronic musculoskeletal disorder that affects almost 30 million populations in the United States (Kandahari, Yang, Dighe, Pan, & Cui, 2015). Unlike the initial views of OA, which is a “wear and tear” disease causing loss of cartilage, the pathological changes of OA include loss and destruction of articular cartilage, formation of osteophytes, degradation of ligaments and menisci, thickening of the subchondral bone, and hypertrophy of the joint capsule (Loeser, Goldring, Scanzello, & Goldring, 2012). OA is a leading causative factor of disability, and the prevalence of OA is rising as the increases of aging population and obesity epidemic (Lawrence et al., 2008). As there is no applicably effective therapeutic strategy for OA treatments, it is still a great challenge to the medical community and the regulatory mechanism underlying OA progression is waiting to be well studied.
As a joint degenerative disease, the characteristic of OA is the progressive loss of articular cartilage. Chondrocytes equilibrate synthesis and degradation of extracellular matrix macromolecules (Wilusz, Zauscher, & Guilak, 2013). Once OA is initiated, damage-associated molecular patterns (DAMPs) is released to stimulate toll-like receptors (TLRs) on chondrocytes, and finally results in inflammatory reaction (Kandahari et al., 2015; Schelbergen et al., 2012; Sohn et al., 2012). A former literature has documented that OA is a complex disease associated with inflammation (Berenbaum, 2013). Interleukin-6 (IL-6) is normally accepted as a multifunctional cytokine which involves in cartilage damage because of inflammation (Wojdasiewicz, Poniatowski, & Szukiewicz, 2014). It participates in anabolic and catabolic regulation of cartilage physiology and thereby brings damage to the cartilage (Haseeb, Ansari, & Haqqi, 2017; Ryu et al., 2011). Therefore, chondrocytes with IL-6-induced inflammatory injury can be used as an in vitro cell model system for screening potential strategies for OA.
Long noncoding RNAs (lncRNAs) are widely identified as a class of noncoding RNAs which are more than 200 nucleotides (nt) (Feng, Xu, Brown, Kaplan, & Nestler, 2018). Emerging evidence has reported that lncRNAs are clearly associated with numerous pathological processes, including OA (Park, Lee, Chun, & Jin, 2017; Zhang et al., 2016). An lncRNA cardiac hypertrophy-related factor (CHRF) whose length is 1,843 nt is located in the first intron of deleted in colorectal carcinoma (Dcc) of mouse. A previous study has proved that lncRNA CHRF could inhibit microRNA (miR)-489 expression and thereby regulates hypertrophy of heart (Wang et al., 2014). What's more, miR-489 has been reported to suppress inflammation in a mouse model of silicosis (Wu et al., 2016). Hence, we hypothesized that lncRNA CHRF might regulate IL-6-induced inflammatory response in chondrocytes. However, the related literatures are limited currently.
Herein, we established in vitro cell model to mimic OA with mouse ATDC5 cells which have a potential of differentiating into chondrocytes under IL-6 stimulation. The expression of lncRNA CHRF after IL-6 stimulation as well as the effects of lncRNA CHRF on IL-6-induced inflammatory injury was explored. Moreover, an innovative downstream miR of lncRNA CHRF and associated signaling pathways were simultaneously investigated.
2 MATERIALS AND METHODS
2.1 Cell culture and stimulation
ATDC5 cells were obtained from RIKEN BioResource Research Center (RIKEN BRC, Koyadai, Tsukuba, Japan). The cells were grown in Dulbecco's modified Eagle's medium/Ham's F-12 (DMEM/F-12; Hyclone, Logan, UT) which contained 5% (v/v) fetal bovine serum (FBS; Hyclone) and penicillin–streptomycin at a final concentration of 1% (Sigma-Aldrich, St. Louis, MO). The cells were cultured in a humid incubator consisting of 5% CO2 and 95% air at 37°C. The cells between the fifth and tenth passages after thawing were exploited. Every 3 days until the confluence was achieved, the culture medium was refreshed. As for inflammatory damages, IL-6 (Sigma-Aldrich) was applied. In short, the cells were incubated in DMEM/F-12 containing IL-6 at a concentration of 10–30 ng/ml for 24 hr at 37°C.
2.2 Cell transfection
To silence lncRNA CHRF, short-hairpin RNA (sh-RNA) was designed and synthesized by GenePharma (Shanghai, China). sh-CHRF directly against lncRNA CHRF was inserted into the pGPU6/Neo plasmid (GenePharma) with a negative control (sh-NC). To enforce the upregulation of lncRNA CHRF, the full-length CHRF sequences were constructed into pcDNA3.1 plasmid (Invitrogen, Carlsbad, CA; pc-CHRF) with an empty vector as negative control (pcDNA3.1). The constructed plasmids were subsequently transfected into ATDC5 cells with Lipofectamine 3000 with reference to user's manual provided by Invitrogen. The cells were inoculated in culture supplemented with 0.5 mg/ml G418 (Sigma-Aldrich) and the stably transfected cells were then obtained. As for miR-146a overexpression, miR-146a mimic was obtained from Genepharma and transduced into cells by Lipofectamine 3000 (Invitrogen) according to suggested protocol with mimic-NC as a negative control.
2.3 Cell viability assay
After transfection with or without IL-6 stimulation, cell viability assay was performed with a commercial kit, cell counting kit-8 (CCK-8) obtained from Dojindo Molecular Technologies (Kumamoto, Japan). Shortly, the cells were plated into 96-well plates at a density of 5 × 103 cells/well, followed by being incubated at 37°C. After treatments, CCK-8 agent (10 μl) was added, and then the culture was maintained in the incubator for another 1 hr. Relative cell viability was assessed by a Microplate Reader (Bio-Rad, Hercules, CA) and the absorbance was examined under 450 nm.
2.4 Apoptosis evaluation
After transfection with or without IL-6 stimulation, apoptotic cells were observed using a flow cytometry after stained by fluorescein isothiocynate (FITC)-conjugated Annexin V/propidium iodide (PI). In short, the cells were collected and washed with phosphate buffered saline (PBS; Sigma-Aldrich). Then, the cells were re-suspended in binding buffer before being stained using FITC-Annexin V/PI (Invitrogen) as recommended by the supplier. Stained cells were subjected to CytoFLEX Flow Cytometer (Beckman Coulter, IN). Data analysis was proceeded with FlowJo software (Tree Star, San Carlos, CA).
2.5 Enzyme-linked immunosorbent assay (ELISA) for inflammatory cytokines
To detect the concentration of inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) and monocyte chemotactic protein 1 (MCP-1), enzyme-linked immunosorbent assay (ELISA) kits were exploited in our study. In short, the cells were seeded into 24-well plates in a density of 1 × 105 cells per ml. After transfection and treatment with or without IL-6, the culture supernatant was collected for measurement of the concentration of TNF-α and MCP-1 using Mouse TNF-alpha Quantikine ELISA Kit (SMTA00B) and Mouse/Rat CCL2/JE/MCP-1 Quantikine ELISA Kit (SMJE00) (both obtained from R&D Systems, Abingdon, UK) following the manufacturer's description.
2.6 Reverse transcription-quantitative PCR (RT-qPCR)
Trizol reagent (Invitrogen) was used for isolation of total RNA from ATDC5 according to user's manual. LncRNA CHRF were assessed using the One Step SYBR® PrimeScript™ PLUS RT-RNA PCR Kit (TaKaRa Biotechnology, Kyoto, Japan). As for miR-146a, Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and Taqman Universal Master Mix II (Applied Biosystems) were applied for reverse transcription and real-time polymerase chain reaction (PCR), respectively, following supplier's protocol. For estimation of mRNA levels of inflammatory cytokines, the PrimeScript™ RT Master Mix (TaKaRa Biotechnology) was used to perform reverse transcription to obtain cDNA, and the following real-time PCR was carried out using the SYBR® Advantage® qPCR Premix (TaKaRa Biotechnology). Relative expression was analyzed using the 2−ΔΔCt method according to a previous report (Livak & Schmittgen, 2001). GAPDH acted as the internal control to normalize lncRNA CHRF level and inflammatory cytokine mRNA levels. U6 acted as a housekeeping gene for miR-146a.
2.7 Western blot analysis
After transfection and stimulation with IL-6, proteins were extracted from the cells using the radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China), and then the concentration of total proteins were detected using bicinchoninic acid assay (BCA)™ Protein Assay Kit (Pierce, Appleton, WI). Then, the proteins were separated and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, MA). Afterwards, those membranes carrying protein blots were probed by primary antibodies and then incubated with secondary antibody tagged with horseradish peroxidase (HRP) (goat anti-rabbit, ab205718) (Abcam, Cambridge, UK). Primary antibodies was shown as follows, pro caspase-3 (ab90437), cleaved caspase-3 (ab49822), TNF-α (ab6671), MCP-1 (ab25124), inhibitor of nuclear factor κB α (IκBα; ab32518), phospho (p)-IκBα (ab133462), p65 (ab32536), p-p65 (ab86299), β-actin (ab8229, all Abcam), pro caspase-9 (#9504), cleaved caspase-9 (#9509), Janus-activated kinase-1 (JAK1; #3332), p-JAK1 (#3331), signal transducer and activator of transcription-3 (STAT3; #4904) or p-STAT3 (#9134, Cell Signaling Technology, Beverly, MA). Finally, the proteins were visualized with the enhanced chemiluminescence kit (GE Healthcare Bio-Sciences, Pittsburgh, PA). Protein bands were determined by the ImageJ software (National Institutes of Health, Bethesda, MA) based on the intensity.
2.8 Statistical analysis
All experiments were performed at least in triplicate with three repeats. Obtained data were proceeded with Graphpad Prism 5 software (GraphPad, San Diego, CA). Results were depicted as the mean ± standard error of the mean (SEM). Variation analysis was calculated using the one-way analysis of variance (ANOVA), unpaired two-tailed t-test or multiple t-tests. The p-values less than 0.05 were considered to statistically present a notable result.
3 RESULTS
3.1 IL-6 caused inflammatory damages in ATDC5 cells
IL-6 was used to elicit inflammatory injury in ATDC5 cells. Under stimulation with IL-6 in an increasing dose, cell viability was significantly lowered by IL-6 at a concentration of 20 (p < 0.05), 30 (p < 0.01), 40 (p < 0.01), and 50 (p < 0.001) ng/mL in relative to the cells without IL-6 treatments (Figure 1a). When the concentration of IL-6 was 30 ng/ml, cell viability was declined to approximately 50%. Hence, 30 ng/ml IL-6 was applied to stimulate ATDC5 cells in subsequent experiments. Moreover, the results showed that apoptotic cells in the IL-6 group were dramatically (p < 0.001) more than that in the control group (Figure 1b). Consistently, the cleavage of caspase-3 and caspase-9 was obviously elevated by IL-6 stimulation (Figure 1c). Moreover, TNF-α and MCP-1 were also obviously increased after IL-6 stimulation both at mRNA and protein levels relative to the control group (p < 0.01 or p < 0.001, Figure 1d–f). Our findings stated that inflammatory lesions of ATDC5 cells were efficiently induced by IL-6 stimulation.
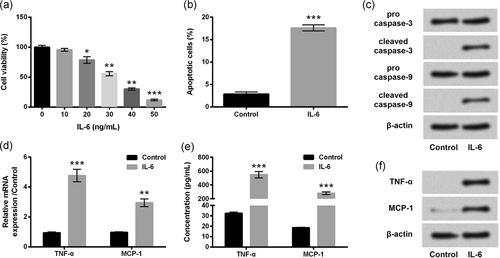
dIL-6 evoked inflammatory damages in ATDC5 cells. ATDC5 cells were incubated with IL-6 at the indicated concentrations, and cells without IL-6 treatment acted as control. (a) Cell viability was assessed by the CCK-8 assay. ATDC5 cells were treated with 30 ng/ml IL-6, and nontreated cells acted as control. (b) Apoptotic cells were measured by flow cytometry assay after stained. (c) Proteins involved in apoptosis were assessed by western blot. mRNA and protein levels of TNF-α and MCP-1 were testified by RT-qPCR (d) and ELISA (e)/western blot analysis (f), respectively. Data were presented as the mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. ELISA: enzyme-linked immunosorbent assay; RT-qPCR: quantitative reverse transcription polymerase chain reaction
3.2 IL-6 upregulated lncRNA CHRF in ATDC5 cells
Meanwhile, lncRNA CHRF level in ATDC5 cells was assessed after stimulation with IL-6. As shown in Figure 2, lncRNA CHRF level was markedly (p < 0.05 or p < 0.01) elevated by IL-6 at a concentration of 20–30 ng/ml, as compared with the cells without IL-6 treatments or with IL-6 stimulation at a low concentration (10 ng/ml; p > 0.05). Our observation indicated lncRNA CHRF could be upregulated in ATD5 cells stimulated with IL-6.
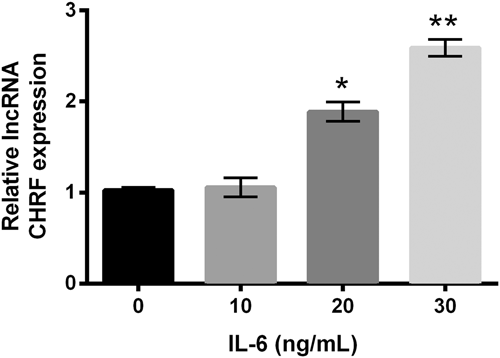
IL-6 upregulated lncRNA CHRF expression in ATDC5 cells. ATDC5 cells were incubated with IL-6 at different concentrations, and cells without IL-6 treatment acted as control. lncRNA CHRF was examined by RT-qPCR. Data were depicted as the mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01. CHRF: cardiac hypertrophy-related factor; lncRNA: long noncoding RNA; RT-qPCR: quantitative reverse transcription polymerase chain reaction
3.3 Overexpression of lncRNA CHRF aggravated IL-6-evoked inflammatory damages in ATDC5 cells
Next, we further investigate the effects of dysregulated lncRNA CHRF on inflammatory injury of ATDC5 cells induced by IL-6. After stable transfection, lncRNA CHRF level in ATDC5 cells which were transfected with pc-CHRF was distinctly (p < 0.001) higher than that in pcDNA3.1-transfected cells (Figure 3a). By contrast, lncRNA CHRF level in sh-CHRF-transfected ATDC5 cells was notably (p < 0.01) lower than that in sh-NC-transfected cells (Figure 3a). RT-qPCR results proved that lncRNA CHRF expression was successfully altered after cell transfection. In addition, our results showed that IL-6 caused alterations, including decline of cell viability (Figure 3b), exaggeration of apoptotic cells (Figure 3c), fortification of cleaved caspases (Figure 3d), accumulation of inflammatory cytokines (TNF-α and MCP-1; Figure 3e–g), were all significantly (p < 0.05 or p < 0.01) enhanced by lncRNA CHRF overexpression compared with those in IL-6 + pcDNA3.1 group, whereas lncRNA CHRF knockdown showed the notable opposite effects (p < 0.05 or p < 0.01) relative to the IL-6 + sh-NC group. All results proved that lncRNA CHRF could aggravate IL-6-caused inflammatory lesions in ATDC5 cells.
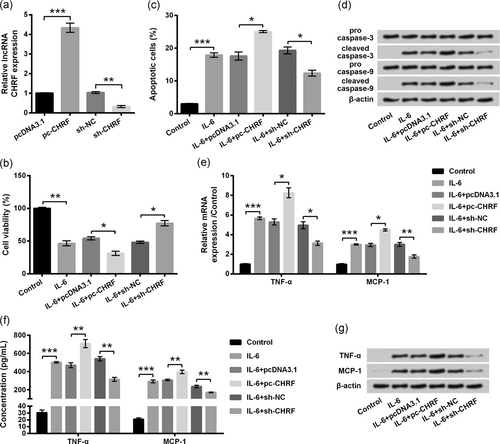
IL-6-caused inflammatory damages of ATDC5 cells were aggravated by lncRNA CHRF overexpression. ATDC5 cells were transfected with the indicated plasmids. (a) lncRNA CHRF was evaluated by RT-qPCR. Then, transfected and untransfected cells were treated with IL-6, and nontreated cells acted as control. (b) CCK-8 assay was carried out for examination of cell viability. (c) Percentage of apoptotic cells was measured by flow cytometry assay. (d) Proteins involved in apoptotic progress were assessed by western blot analysis. mRNA and protein of TNF-α and MCP-1 were testified by RT-qPCR (e) and ELISA (f)/western blot analysis (g), respectively. Data were presented as the mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. CHRF: cardiac hypertrophy-related factor; ELISA: enzyme-linked immunosorbent assay; lncRNA: long noncoding RNA; RT-qPCR: quantitative reverse transcription polymerase chain reaction
3.4 miR-146a level was negatively modulated by IL-6 and lncRNA CHRF
Meanwhile, we found miR-146a was suppressed by IL-6 in a dose-associated manner. miR-146a was remarkably repressed by IL-6 at concentrations of 20 (p < 0.05) and 30 ng/ml (p < 0.05) relative to that of the control whereas not significantly repressed by IL-6 at a low concentration (10 ng/ml) (p > 0.05) (Figure 4a). Next, the miR-146a level in ATDC5 cells was measured. As evidenced in Figure 4b, the miR-146a level was markedly decreased by replenishing lncRNA CHRF (p < 0.05), whereas was significantly increased by silencing lncRNA CHRF (p < 0.05) relative to the respective controls. Abovementioned findings indicated that IL-6 and lncRNA CHRF could negatively regulate miR-146a expression in ATDC5 cells.
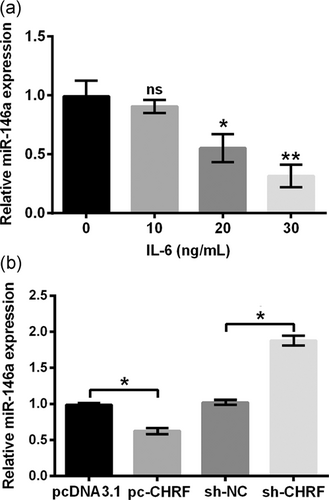
IL-6 and LncRNA CHRF negatively mediated miR-146a level in ATDC5 cells. (a) ATDC5 cells were incubated with IL-6 at the indicated concentrations, and cells without IL-6 treatment acted as control. miR-146a was detected by the RT-qPCR. (b) lncRNA CHRF level in ATDC5 cells transfected with the indicated plasmids was evaluated by RT-qPCR. Data were suggested as the mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01. CHRF: cardiac hypertrophy-related factor; lncRNA: long noncoding RNA; RT-qPCR: quantitative reverse transcription polymerase chain reaction
3.5 LncRNA CHRF affected IL-6-treated ATDC5 cells through downregulating miR-146a
Subsequently, we further investigated whether lncRNA CHRF affected IL-6-treated ATDC5 cells via regulating miR-146a. In Figure 5a, miR-146a levels were dramatically enhanced by transfection with miR-146a mimic relative to the mimic-NC-transfected cells (p < 0.001), indicating that miR-146a level was efficiently enhanced in ATDC5 cells after transfection. Thereafter, we found effects of lncRNA CHRF overexpression on IL-6-treated cells could be visibly abrogated by miR-146a overexpression, as proved by notably (p < 0.01) increased cell viability (Figure 5b; p < 0.01), reduced apoptotic cells (Figure 5c), downregulation of cleaved caspases (Figure 5d), decreased mRNA levels (Figure 5e; p < 0.01 or p < 0.001), protein levels (Figure 5f,g; p < 0.01) of inflammatory cytokines, relative to the IL-6 + pc-CHRF + mimic-NC group. Results suggested that miR-146a overexpression could abrogate the effects of lncRNA CHRF overexpression on IL-6-treated ATDC5 cells.
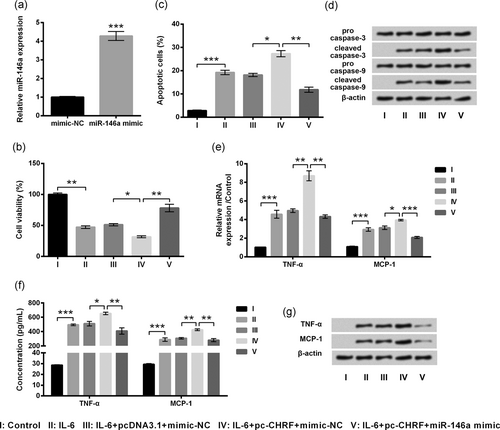
Effects of lncRNA CHRF on IL-6-treated ATDC5 cells were reversed by miR-146a overexpression. ATDC5 cells were transduced with mimic-NC or miR-146a mimic. (a) miR-146a was evaluated by the RT-qPCR. Then, cotransfected and untransfected cells were treated with IL-6, and nontreated cells acted as control. (b) Cell viability was determined by the CCK-8 assay. (c) Apoptotic cells were observed by flow cytometry. (d) Proteins implicated in apoptosis were assessed by western blot analysis. mRNA and protein levels of proinflammatory cytokines were testified by the RT-qPCR (e) and ELISA (f)/western blot analysis (g), respectively. Data were presented as the mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. CHRF: cardiac hypertrophy-related factor; ELISA, enzyme-linked immunosorbent assay; lncRNA: long noncoding RNA; RT-qPCR: quantitative reverse transcription polymerase chain reaction
3.6 LncRNA CHRF activated the NF-κB and JAK1/STAT3 signaling cascades via repressing miR-146a
Finally, we testified whether signaling cascades were implicated in the regulation of lncRNA CHRF in IL-6-treated ATDC5 cells. In Figure 6a,b, phosphorylated forms of IκBα, p65, JAK1, and STAT3 were remarkably aggrandized by IL-6 stimulation (p < 0.001), and were moreover exaggerated by lncRNA CHRF overexpression (p < 0.01). Of note, the effects of lncRNA CHRF overexpression on the phosphorylation of IκBα (p < 0.001), p65 (p < 0.001), JAK1 (p < 0.05), and STAT3 (p < 0.05) were markedly bated by miR-146a. Our data indicated that lncRNA CHRF might trigger the activation of NF-κB and JAK1/STAT3 pathways through repressing miR-146a expression in IL-6-treated ATDC5 cells.
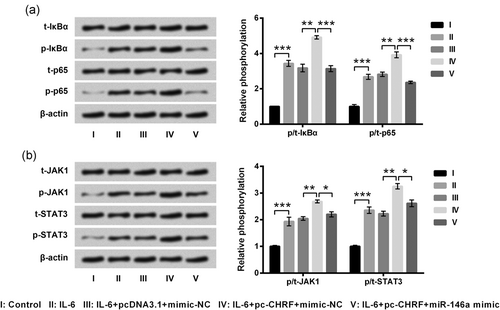
LncRNA CHRF activated the NF-κB and JAK1/STAT3 signaling cascades through suppressing miR-146a in ATDC5 cells. Cotransfected and untransfected ATDC5 cells were treated by IL-6, and nontreated cells were acted as control. Protein expression of key kinases in the NF-κB pathway (a) and JAK1/STAT3 pathway (b) was assessed by western blot analysis. Data were presented as the mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. CHRF: cardiac hypertrophy-related factor; lncRNA: long noncoding RNA
4 DISCUSSION
Despite degradation of articular cartilage, inflammation has been identified as another character of OA. In the present study, we established in vitro inflammatory cell model system in ATDC5 cells using IL-6 stimulation. Interestingly, IL-6 stimulation not only dampened cell viability, facilitated apoptosis and triggered inflammatory cytokines release but also upregulated lncRNA CHRF and down-regulated miR-146a. Afterwards, we found lncRNA CHRF aggravated IL-6 induced inflammatory injury and its knockdown showed the opposite effects. The following experiments showed miR-146a was negatively associated with lncRNA CHRF expression, and miR-146a overexpression abolished the effects of lncRNA CHRF on IL-6-stimulated ATDC5 cells. Moreover, we found that the NF-κB and JAK1/STAT3 signaling cascades were activated by lncRNA CHRF which downregulated miR-146a.
Chondrocytes, the sole type of articular cartilage, can regulate extracellular matrix (ECM) production and maintaining, and thereby play critical roles in maintaining structural and functional integrity of articular cartilage (Goldring & Otero, 2011). During OA progression, dysregulated chondrocytes are considered to disrupt the homeostasis of joint. TNF-α and MCP-1, significantly elevated by the TLR activation, are implicated in OA pathogenesis (Scanzello, 2017). Therefore, cell viability, apoptosis, and inflammatory cytokines were measured for evaluation whether inflammatory damage system was efficiently constructed. Moreover, lncRNA CHRF was also quantified in IL-6-treated ATDC5 cells for testifying whether lncRNA CHRF was involved in OA progression. All results suggested that IL-6 stimulation induced inflammatory insults in ATDC5 cells by activating the caspase-dependent pathway, along with upregulating lncRNA CHRF.
The regulatory function of lncRNA CHRF had been reported previously. For example, lncRNA CHRF silence represses silica-induced pulmonary fibrosis via upregulating miR-489 (Wu et al., 2016). Metastasis of colorectal cancer is promoted by lncRNA CHRF due to the miR-489 loss (Tao, Han, Zhang, Ma, & Sun, 2017). In addition, lncRNA CHRF can modulate cardiac hypertrophy through miR-489 (Wang et al., 2014). However, the role of lncRNA CHRF in IL-6-treated ATDC5 cells is poorly understood. Our findings suggested that lncRNA CHRF overexpression augmented the IL-6-induced inflammation lesions, as indicated by the reduced cell viability, elevated apoptosis, and enhanced levels of TNF-α and MCP-1. What's more, lncRNA CHRF knockdown showed the opposite influence on IL-6-treated cells. Results in the present study were notably consistent with a preceding study, in which lncRNA CHRF overexpression promoted myocardial cell apoptosis, and the valsartan's cardiac protective effects in vivo were reversed by lncRNA CHRF (Chen et al., 2018).
Despite miR-489, miR-93 was also reported as the downstream factors of lncRNA CHRF by Wo, Guo, Li, Yang, and Wo (2018) showing that lncRNA CHRF facilitated cardiac hypertrophy via miR-93 (Wo, Guo, Li, Yang, & Wo, 2018). Hence, we supposed that there might be other targets of lncRNA CHRF. miR-146a has been identified to be involved in OA cartilage pathogenesis (Yamasaki et al., 2009). A previous study has showed that aging- and trauma-induced OA can be alleviated by miR-146a (Guan et al., 2018). Another study also reported the therapeutic potential of miR-146a in OA, by the means of promoting proliferation and inhibiting apoptosis in OA chondrocytes (West & McDermott, 2017). Zeng et al. (2013) declared that miR-146a upregulation inhibits inflammation in acute lung injury. Therefore, we explored the association between lncRNA CHRF and miR-146a. Results presented that miR-146a expression was negatively mediated by lncRNA CHRF, and the effects of lncRNA CHRF overexpression on IL-6-treated ATDC5 cells was abolished by miR-146a. That is, lncRNA CHRF mediated cellular progresses by downregulating miR-146a expression. The roles of miR-146a in inflammatory insults in ATDC5 cells were consistent with literatures described above.
The NF-κB pathway is pivotal for regulation of cell survival, proliferation, apoptosis, and inflammation (Li, Withoff, & Verma, 2005; Meffert & Baltimore, 2005). The NF-κB pathway is activated in OA chondrocytes, and it is essential for induction of multiple inflammation-associated factors (Marcu, Otero, Olivotto, Borzi, & Goldring, 2010; Saito & Tanaka, 2017). IL-6 targets IL-6 receptor, leading to activation of the JAK1/STAT3 pathway (Cao et al., 2015). Song et al. have proved that IL-6 neutralizing antibodies can inhibit the JAK1/STAT3 pathway (Song, Rawal, Nemeth, & Haura, 2011). Therefore, the activation of the NF-κB and JAK1/STAT3 cascades was finally assessed in lncRNA CHRF and miR-146 overexpressed ATDC5 cells. Our findings suggested that these two cascades were triggered by IL-6 treatments and were further activated by lncRNA CHRF overexpression. Besides, miR-146a overexpression could reverse the lncRNA CHRF overexpression-induced activation, indicating that lncRNA CHRF might activate the NF-κB and JAK1/STAT3 signaling transduction cascades through repressing miR-146a expression. Those results were partially consistent with previous studies. Ye and Steinle (2016) have confirmed miR-146a alleviated inflammation through blocking NF-κB pathway in primary human retinal microvascular endothelial cells. Stickel et al. (2017) have proved that miR-146a can cause JAK1/2 inhibition, resulting in inhibition of the JAK/STAT pathway.
5 CONCLUSIONS
Collectively, we reported for the first time that lncRNA CHRF aggravated IL-6-evoked inflammatory damages in ATDC5 cells. miR-146a might be a downstream factor of lncRNA CHRF, and its downregulation induced by lncRNA CHRF might be an explanation for the proinflammatory effects of lncRNA CHRF. What's more, NF-κB and JAK1/STAT3 signaling cascades were activated by lncRNA CHRF via miR-146a in IL-6-stimulated ATDC5 cells. Our study enriched the function of lncRNA CHRF and provided potential therapeutic targets for OA.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.
AUTHOR'S CONTRIBUTION
CY and XS: Conceived and designed the experiments; CY, DS, ZL, and GW: Performed the experiments and analyzed the data; CY and DS: Wrote the manuscript; XS: Revised the manuscript.
FUNDING
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author with reasonable request.