Hypoxia Promotes the Inflammatory Response and Stemness Features in Visceral Fat Stem Cells From Obese Subjects
Abstract
Low-grade chronic inflammation is a salient feature of obesity and many associated disorders. This condition frequently occurs in central obesity and is connected to alterations of the visceral adipose tissue (AT) microenvironment. Understanding how obesity is related to inflammation may allow the development of therapeutics aimed at improving metabolic parameters in obese patients. To achieve this aim, we compared the features of two subpopulations of adipose-derived stem cells (ASC) isolated from both subcutaneous and visceral AT of obese patients with the features of two subpopulations of ASC from the same isolation sites of non-obese individuals. In particular, the behavior of ASC of obese versus non-obese subjects during hypoxia, which occurs in obese AT and is an inducer of the inflammatory response, was evaluated. Obesity deeply influenced ASC from visceral AT (obV-ASC); these cells appeared to exhibit clearly distinguishable morphology and ultrastructure as well as reduced proliferation, clonogenicity and expression of stemness, differentiation and inflammation-related genes. These cells also exhibited a deregulated response to hypoxia, which induced strong tissue-specific NF-kB activation and an NF-kB-mediated increase in inflammatory and fibrogenic responses. Moreover, obV-ASC, which showed a less stem-like phenotype, recovered stemness features after hypoxia. Our findings demonstrated the peculiar behavior of obV-ASC, their influence on the obese visceral AT microenvironment and the therapeutic potential of NF-kB inhibitors. These novel findings suggest that the deregulated hyper-responsiveness to hypoxic stimulus of ASC from visceral AT of obese subjects may contribute via paracrine mechanisms to low-grade chronic inflammation, which has been implicated in obesity-related morbidity. J. Cell. Physiol. 231: 668–679, 2016. © 2015 Wiley Periodicals, Inc.
Adipose tissue (AT) serves as a storage site for lipids but is also an endocrine organ that secretes biologically active molecules that participate in whole-body energy metabolism and systemic inflammation (Trayhurn, 2013). In particular, subcutaneous and visceral ATs show anatomical and functional differences, as subcutaneous AT is mainly involved in energy storage, while visceral AT shows interactions with cells of the immune system, probably playing an important role in immune response (Trzeciak-Ryczek et al., 2011). By dysregulating these functions, AT itself is involved in obesity-related disorders (Bays et al., 2008).
Low-grade chronic inflammation is widely recognized to be a salient feature of obesity and many of its accompanying pathologies (Hotamisligil, 2006). This condition frequently occurs in central obesity and is strictly related to the characteristics of the visceral AT microenvironment, which is characterized by a hypoxic status and altered relationships among resident cells, including adipose-derived stem cells (ASC), which are multipotent stem cells that possess similar properties to bone marrow mesenchymal stem cells (Zuk et al., 2002). Hypoxia is a known inducer of the inflammatory response through activation of hypoxia-inducible factor (HIF)-1α, which is the master regulator of the cellular response to hypoxia (Semenza, 2003), and nuclear factor-kappa B (NF-kB), a major mediator of inflammation that controls transcriptional programs to execute and regulate the inflammatory response (Ghosh and Hayden, 2008). Activation of NF-kB typically results in the expression of cytokines, adhesion molecules, cell surface receptors, and pro-inflammatory enzymes. NF-kB also induces the expression of suppressor of cytokine signaling 1 (SOCS1), which participates in degradation of the NF-kB complex, decreasing NF-kB transcriptional activity (Ghosh and Hayden, 2008). Termination of the inflammation restores a homeostatic state. On the contrary, chronic inflammation can result in local tissue remodeling (Wynn, 2007; Jayachandran et al., 2009). As also demonstrated in obese AT, the persistence of an inflammatory stimulus seems to be responsible for excessive expression of several genes encoding extracellular matrix components, which can determine a pathological state of the tissue, such as fibrosis, and can also be expressed in preadipocytes (Henegar et al., 2008; Keophiphath et al., 2009; Kwon et al., 2012). Preadipocytes are also inflammatory cells, as they express and release inflammation-related factors (Lacasa et al., 2007; O'Hara et al., 2012).
A number of studies strongly demonstrated that hypoxia occurs in obese AT mainly due to adipocyte hypertrophy in the absence of efficient neoangiogenesis (Trayhurn, 2013). Oxygen concentration is an important regulator of adipogenesis because hypoxia inhibits the differentiation of preadipocytes into adipocytes by attenuating the expression of both PPARγ and its coactivator PGC1A which regulate many adipocyte-specific genes (Yun et al., 2002; Kim et al., 2005; Lin et al., 2006). In addition, hypoxia plays a critical role in the maintenance of the undifferentiated phenotype of preadipocytes (Lin et al., 2006) also through activation of the transcription factor OCT4, which is essential for the maintenance of stem cell pluripotency (Covello et al., 2006).
We previously reported that subcutaneous and visceral ASC isolated from obese subjects, particularly the latter, showed impaired behavior, possibly due to the altered microenvironment of obese AT (De Girolamo et al., 2013). To further investigate this issue, here the molecular and functional features of two subpopulations of ASC isolated from subcutaneous and visceral AT of obese patients were compared with two subpopulations of ASC isolated from the same sites in non-obese individuals. In particular, we evaluated the behavior of these cells during hypoxia to determine whether ASC from the AT of obese patients may contribute to chronic inflammation, which has been implicated in obesity related-morbidity.
Materials and Methods
Patients enrolled
Human ASC were obtained from both subcutaneous and visceral depots of four non-obese subjects (two women and two men, age 52.2 ± 8.5 years, body mass index (BMI) at the time of surgery 25.5 ± 0.4 Kg/m2) who underwent elective laparoscopic cholecystectomy for symptomatic gallstone disease and nine obese individuals (seven women and two men, age 41.3 ± 2.9 years, BMI 43.4 ± 2.6 Kg/m2) who underwent laparoscopic sleeve gastrectomy as a primary definitive procedure. Patients younger than 18 and older than 66 years, who were affected by neoplastic and autoimmune diseases or infections or who were on chronic steroid treatment were considered ineligible. The study was approved by the ethical committee of the Sapienza University of Rome, Policlinico Umberto I, and was carried out in accordance with the Declaration of Helsinki. Informed consent was obtained before the surgical procedure from all of the patients.
ASC isolation and culture
Subcutaneous and visceral AT specimens were dissected from fibrous material and visible blood vessels and minced into small pieces. The samples were digested with 2 mg/ml collagenase type I (Sigma–Aldrich, St. Louis, MO) in 2% BSA for 70 min at 37°C. Cell suspensions were passed through a 40 μm nylon mesh filter to remove debris. The collected cells were plated in DMEM supplemented with 10% FBS, 50 U/ml penicillin, 50 µg/ml streptomycin and 2 mM l-glutamine (Sigma-Aldrich) and maintained at 37°C in a humidified atmosphere with 5% CO2. After 24 h, non-adherent cells were removed and fresh medium was added and changed twice a week (De Girolamo et al., 2013).
Cell morphology
The cell culture morphology was observed under an optical microscope (Leica Microsystems, Wetzlar, Germany) and the cell ultrastructure was observed using a transmission electron microscope (TEM). For the TEM analysis, the cells were washed twice with 0.1 M phosphate buffer at pH 7.3 and fixed in a 2% glutaraldehyde solution in the same buffer for 1 h. Then, the cells were gently scraped and post-fixed as a suspension in the same fixative. After several rinses with the buffer, the cells were additionally fixed in osmium tetroxide and processed in accordance with a standard schedule for embedding in Epon resin. Semi-thin plastic sections were stained with Azur II and basic fuchsin. Ultrathin sections were stained with uranyl acetate and lead hydroxide. A Philips CM-10 TEM was used for observation and photographic analysis.
Cell proliferation
The cells were plated and grown until subconfluence. At that time, the cells were harvested, counted using a Neubauer counting chamber and re-plated in fresh medium at the starting density. The evaluation was repeated from passage 2 to passage 5 in order to draw a growth curve. Because the four types of ASC grew with different kinetics and the interval between two consecutive passages could vary, at each passage the data were expressed as doubling time.
Fibroblast-colony forming unit assay (CFU-F)
The clonogenic ability of all of the ASC populations was evaluated at passages 2 through 5. The cells were plated in six-well plates at limiting dilution (15–500 cells/well) and cultured for 14 days. The cells were fixed with methanol and stained with crystal violet (Sigma–Aldrich). The CFU-F frequency was determined by scoring individual colonies composed of at least 50 cells and was expressed as a percentage relative to the number of seeded cells (De Girolamo et al., 2013).
Quantitative PCR analysis
Total RNA was extracted from the ASC using Trizol reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed to single-strand cDNA using random primers and Superscript II (Invitrogen). Gene expression levels in the four types of ASC both in control cells and after exposure to hypoxia were evaluated using TaqMan Array Microfluidic Cards designed with a purposely chosen panel of genes and the ABI Prism 7900HT Fast Real-Time PCR System Detector (Applied Biosystems, Foster City, CA) according to the manufacturer's default cycling conditions. To evaluate the effect of parthenolide on mRNA expression, the ABI Prism 7000 Sequence Detection System was used. The cDNA template was amplified in a reaction mixture containing TaqMan Universal PCR master mix and a primers/probe mixture according to the manufacturer's default cycling conditions. For each amplification reaction, a standard curve was generated using serial dilutions of reverse-transcribed RNA extracted from obV-ASC exposed to hypoxia, which were run concurrently with the test samples (Salvatori et al., 2012). All of the amplification reactions were performed in triplicate. Human 18S ribosomal RNA was used as an endogenous reference gene.
Flow cytometry
To evaluate the expression of specific mesenchymal stem cell surface markers, cytofluorimetric analysis using a FACSCalibur System (BD Biosciences, San Jose, NJ) was performed (De Girolamo et al., 2008).
Cell culture in hypoxic conditions
The day prior to treatment, ASC were plated at subconfluence. The following day, the culture medium was removed from the plates and replaced with O/N hypoxia-preconditioned medium. The cells were then incubated in a hypoxia chamber (Billups-Rothenberg, Del Mar, CA) flushed with an atmosphere containing 1% oxygen, 5% CO2 and balanced with nitrogen for 2, 4, 8, 16, or 24 h according to the experimental design.
To analyze the effects of parthenolide on the hypoxia-induced effects, the ASC were pre-treated for 2 h with the inhibitor, and then the culture medium was removed and replaced with O/N hypoxia-preconditioned medium and supplemented again with parthenolide. The cells were grown for an additional 2 h (to evaluate nuclear translocation) or 8 h (for gene expression analysis) in the hypoxic environment. The most effective concentration of parthenolide among those tested (5–20 μM) was 5 μM; therefore, this dose was used in the experiments described.
Western blot analysis
To evaluate the expression of target proteins after ASC exposure to oxygen deprivation, both nuclear extracts and whole cell lysates were prepared using the Nuclear Extract Kit (Active Motif, Carlsbad, CA) according to the manufacturer's instructions. Equal amounts of proteins (10 μg nuclear extracts or 20 μg total cell lysates) were subjected to 8% SDS–PAGE and electrophoretically transferred to PVDF membranes. The membranes were probed with monoclonal antibodies anti-HIF-1α, anti-p65, anti-COX2 (BD Transduction Laboratories, New Jersey, NJ), anti-P2X7R, anti-VEGF, anti-EGFR (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-β-actin (Sigma–Aldrich) followed by incubation in the presence of the appropriate secondary antibody conjugated to peroxidase (Bio-Rad, Hercules, CA). The signal was detected by an enhanced chemiluminescence detection system.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v5.01 (GraphPad Software, San Diego, CA). Evaluation of the effect of treatments with respect to untreated cells was carried out using the paired t-test. One-way ANOVA with Bonferroni post-test for multiple comparisons was used to compare ASC populations. For the statistical analysis of proliferative and clonogenic capacity, two-way ANOVA with Bonferroni post-test was performed to account for time and cell type. The level of significance was set at < 0.05.
Results
Clinical characteristics of patients
To investigate their potential role in obesity, we compared ASC isolated from both subcutaneous and visceral AT of four non-obese subjects and nine obese individuals (mean BMI of 25.5 and 43.4, respectively). All of the non-obese donors had normal levels of blood parameters. The obese subjects showed normal (7/9) or impaired (2/9) fasting blood glucose levels and normal plasma HbA1C levels. Moreover, 4/9 had mild atherogenic dyslipidemia and 9/9 showed a slight increase of C-reactive protein (CRP) blood levels (mean ± SEM = 1 mg/dl ± 0.29, normal values < 0.5 mg/dl).
ASC isolated from obese patients, especially obV-ASC, present peculiar biological and molecular features
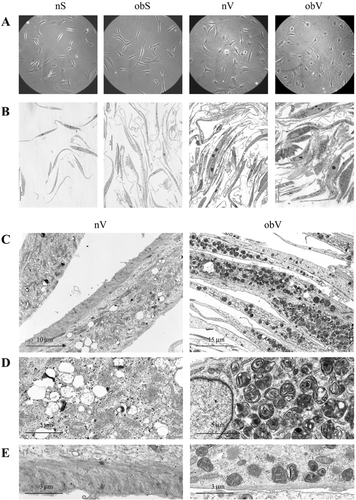
As displayed in Figure 1A, ASC isolated from subcutaneous AT of non-obese (nS-ASC) and obese subjects (obS-ASC) had similar morphologies, appearing as spindle-shaped (mesenchymal-like) cells. Differently, ASC isolated from visceral AT of obese individuals (obV-ASC) appeared as very large cells with non-homogeneous cytoplasm when compared to cells from visceral AT of non-obese individuals (nV-ASC), which presented an intermediate phenotype, although it was more similar to that of the subcutaneous ASC. Further analysis of cell morphology performed on semi-thin plastic sections highlighted that the cytoplasm of the obV-ASC contained a large number of metachromatic dense granules (Fig. 1B). Interestingly, at TEM these granules appeared as osmiophilic bodies containing layered myelin-like structures (Fig. 1C–E). The same granules were also present in nV-ASC, although to a much lesser extent (Fig. 1B and C). Analysis of cell ultrastructure also unveiled that the cytoplasm of the nV-ASC presented clear vesicles representing droplets of non-osmiophilic saturated lipids (Fig. 1C and D), marked cell membrane-associated cytoskeleton at the basal pole (Fig. 1C and E) and β-particles of glycogen, which are typical of mesenchymal cells (Fig. 1D and E). Of particular interest was the observation of the scarce presence of cytoskeleton at the basal pole of obV-ASC (Fig. 1E), which was consistent with their flat appearance. However, elongated and well-preserved mitochondria of the orthodox form were visible in these cells as well as β-particles of glycogen (Fig. 1E). Features similar to those of the nV-ASC were exhibited by both types of subcutaneous ASC (data not shown).
The proliferation rate and clonogenic capacity of all of the types of ASC were evaluated up to 5 passages in culture after the cells were isolated from the tissue. The obS-ASC showed a trend toward lower proliferative activity and significantly lower (P < 0.0001) clonogenic capacity than the nS-ASC (Fig. 2A, B, and C, left panels). However, the obV-ASC exhibited a constant trend toward reduced growth and clonogenicity compared to the nV-ASC, although the differences were not statistically significant due to high variability among the patients (Fig. 2A, B, and C, right panels).

In agreement with the findings described above, analysis of several stemness markers suggested that both the obS- and obV-ASC tended to have lower levels of expression than the nS- and nV-ASC, respectively (Fig. 2D).
The microenvironment of the AT of obese individuals has reduced oxygen availability which can affect the phenotype of ASC through regulation of the expression of oxygen-dependent genes. With this in mind, basal expression of key genes able to regulate the cellular response to hypoxia, such as HIF-1α, the NF-kB subunits RELA (coding for p65) and NFKB1 (coding for p50), and the inhibitor of κ B kinase β (IKBKB), as well as the expression of pro-inflammatory genes, adhesion molecules, cytokines and growth factors activated during hypoxic inflammation were evaluated. In addition, the basal levels of several markers of ASC differentiation potential were also measured. Consistent with previous observations, Figure 3A and B show that the two populations of subcutaneous ASC exhibited gene expression profiles that were similar overall, although the obS-ASC showed non-significantly lower levels of many genes. Compared to the nV-ASC, the obV-ASC had a peculiarly higher level of VCAM, whereas they showed lower expression of the other genes analyzed. It should be noted that compared to subcutaneous cells of the same individuals, the nV-ASC showed a trend toward higher expression of many inflammation-related genes as well as of the differentiation marker PGC1A which can also have a role in the inflammatory response. On the other hand, compared to the subcutaneous ASC of obese subjects, the obV-ASC showed higher expression of only some genes related to the inflammatory response, such as IL-6, P2X7R, VCAM, and PGC1A.
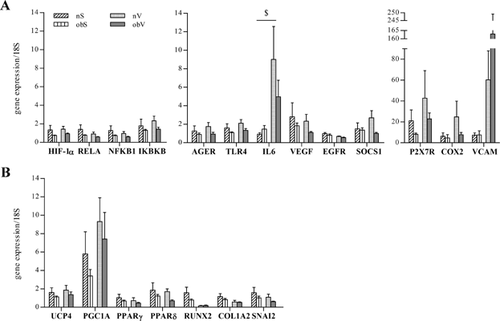
Taken together, these results suggest that obese ASC, especially obV-ASC, have lower levels of all of the parameters analyzed, which is consistent with a less stem-like phenotype. Moreover, obV-ASC display a peculiar inflammatory profile.
Exposure to hypoxia induces strong nuclear translocation of NF-kB only in obV-ASC
The evidence of different phenotypes suggests that the four subpopulations of ASC may respond differently to microenvironmental stimuli. To evaluate the effect of hypoxia, nuclear translocation of HIF-1α was analyzed at different times (ranging from 2 to 24 h). Both populations of ASC isolated from obese patients showed a similar strong presence of HIF-1α in the nucleus, which peaked after 4 h of hypoxia exposure and was comparable to the expression observed in the two populations of ASC isolated from non-obese donors (Fig. 4A). The HIF-1α translocation started just 2 h after oxygen deprivation in the nS- and nV-ASC, whereas in the ASC from obese individuals it was delayed and clearly detected starting from 4 h. In addition, the effect of hypoxia on the NF-kB pathway was evaluated through analysis of the nuclear translocation of the p65 NF-kB subunit at different times after oxygen deprivation (2–24 h), as displayed in Figure 4A and B. The ASC isolated from both subcutaneous tissues, irrespective of the pathological state of the donor, did not show increased p65 nuclear translocation. When comparing the two types of visceral ASC, a slight but significant increase in p65 activation was observed in the nV-ASC after 8 h of oxygen deprivation, whereas the obV-ASC displayed strong biphasic nuclear accumulation of p65 that was maximal after 2 h and 24 h.
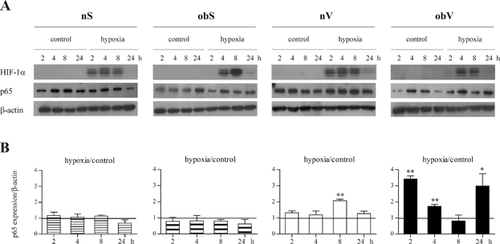
These results highlight that unlike HIF-1α, NF-kB displays a tissue-specific response to hypoxia that is clearly evident in visceral AT of obese subjects.
Oxygen deprivation induces the expression of inflammation-related molecules in obV-ASC
Expression levels of the master genes activated by hypoxia, namely HIF-1α and the NF-kB complex, as well as the expression of several NF-kB target genes were investigated at different times after oxygen deprivation (ranging from 1 to 48 h, data not shown). Because maximal activation was achieved after 8 h, all of the subsequent experiments concerning the analysis of gene expression were performed at that time. Figure 5A shows that after 8 h of exposure to a hypoxic stimulus, HIF-1α, NFKB1, and IKBKB mRNA levels were significantly decreased in the nS- and obS-ASC compared to the basal value (indicated by a horizontal line in the graph). Differently, the mRNA expression did not change in the obV-ASC and slightly decreased in the nV-ASC (Fig. 5C). After oxygen deprivation, the expression of RELA mRNA coincided with the p65 protein activation previously shown in Figure 4. In fact, no change in gene transcription was evident in both types of subcutaneous ASC (Fig. 5A), whereas the RELA level increased slightly in the nV-ASC and significantly in the obV-ASC (Fig. 5C).
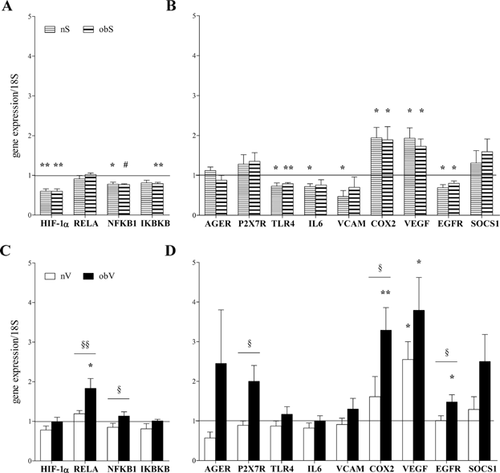
The analysis of NF-kB target genes after hypoxia exposure showed a comparable response in both types of subcutaneous ASC (Fig. 5B). In particular, the mRNA levels were often significantly down-regulated (TLR4, IL-6, VCAM, and EGFR), sometimes did not change (AGER, P2X7R, and SOCS1) and were rarely up-regulated (COX2 and VEGF). On the contrary, the ASC isolated from visceral AT of the obese and non-obese subjects displayed very different responses to oxygen deprivation (Fig. 5D). In fact, the nV-ASC did not show modulated gene expression (with the exception of VEGF), whereas in the obV-ASC hypoxia induced the maximum increase in expression in the majority of the genes analyzed (AGER, P2X7R, COX2, VEGF, EGFR, and SOCS1).
Analysis of the protein expression of several inflammation-related molecules showed comparable results to the evaluation of mRNA levels. Among the different times analyzed (8–24 h), 16 h of hypoxia exposure induced maximal protein expression in only the obV-ASC (Fig. 6A), where a strong and significant increase in the level of all of the molecules analyzed was observed (Fig. 6B).
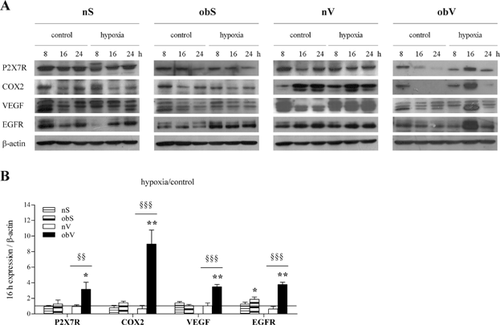
Taken together, these data highlight that obesity causes a strong increase in the hypoxia-induced inflammatory response only in obV-ASC.
Hypoxia induces stemness features in obV-ASC
In a previous study, we reported that obesity impaired the plasticity of ASC, as obV-ASC were not able to differentiate towards adipogenic and osteogenic lineages (De Girolamo et al., 2013). To further investigate this aspect of the biology of ASC, we evaluated the effect of a hypoxic environment on the expression of markers of adipogenic (UCP4, PGC1A, PPARγ, and PPARδ) and osteogenic (RUNX2) differentiation. Eight hours of hypoxia exposure significantly decreased the mRNA expression of all of the genes in both the nS- and obS-ASC (Fig. 7A). In contrast, a different response was observed when comparing the ASC isolated from visceral adipose tissues. In fact, the obV-ASC did not display significant changes, whereas the nV-ASC presented peculiar behavior, ranging from significant inhibition to the absence of modulation of the genes analyzed (Fig. 7A). Evaluation of the mRNA levels of markers of fibrosis showed that the hypoxic stimulus significantly induced the expression of the COL1A2 and SNAI2 genes only in the obV-ASC (Fig. 7B). Parallel analysis of the effect of hypoxia on the expression of stem cell markers indicated that the level of OCT4 significantly increased in the ASC isolated both from the non-obese and obese subjects, although mostly in the latter, whereas KLF4 expression was significantly up-regulated only in the obV-ASC (Fig. 7C). Interestingly, the very low expression of the two stemness markers in the obV-ASC in normoxia (obV C; Fig. 7D) rose up to levels comparable to those of the normoxic nV-ASC (nV C) after hypoxia exposure (obV H).
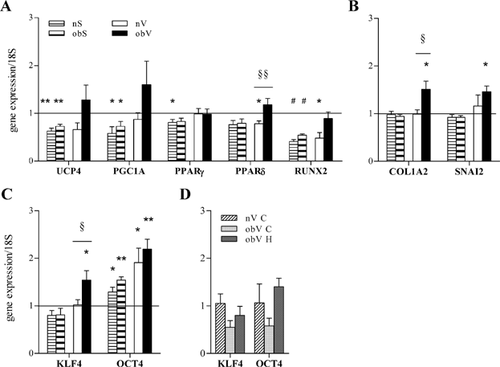
Taken together, these results make the interesting suggestion that the differentiation potential of obV-ASC is peculiarly influenced by hypoxia, as the adipogenic and osteogenic differentiation genes do not change, the expression of markers of fibrosis is induced and the stemness markers show the maximum increase.
Inhibition of NF-kB blocks the expression of inflammation and fibrosis markers and reduces the level of VEGF and OCT4 in obV-ASC
The effects of the NF-kB inhibitor parthenolide were evaluated in the obV-ASC, which showed a maximal increase in the expression of inflammation-related, fibrosis and stemness genes during oxygen deprivation, as described above. As shown in Figure 8A, the hypoxia-induced nuclear translocation of p65 was significantly down-regulated by 5 μM parthenolide, whereas a non-significant reduction of HIF-1α translocation was observed. Moreover, the hypoxia-induced mRNA expression of NF-kB transcriptional targets, such as the molecules of the inflammatory response P2X7R, COX2, EGFR, and SOCS1, the marker of fibrosis COL1A2 and the stemness gene KLF4 was completely inhibited in the presence of parthenolide (Fig. 8B). Interestingly, the increase in VEGF and OCT4 expression induced by oxygen deprivation was only partially affected by exposure to the NF-kB inhibitor.
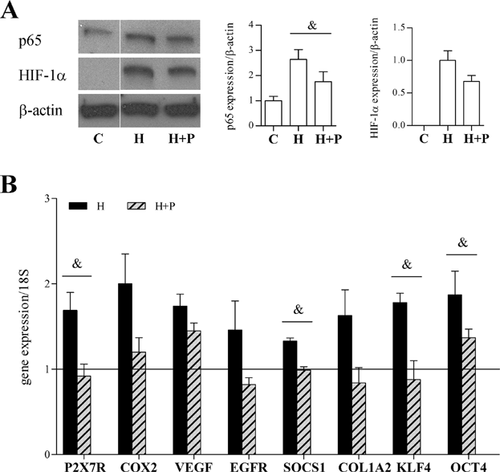
These data confirm the role of NF-kB as a mediator of the effects induced by hypoxia in obV-ASC.
Discussion
This study investigated the relevance of ASC in obesity with particular attention to obesity-related inflammation. It was clearly evident that obesity influenced several features of ASC. Interestingly, the analysis of cell morphology and ultrastructure showed that the obV-ASC had clearly distinguishable shapes and a peculiar accumulation of pseudo-membranous myelin-like structures that were likely composed of complex lipids, suggesting impaired lipid metabolism. Perturbations in the efficiency of lipid storage and trafficking are central to the development of metabolic diseases. In this regard, the osmiophilic density, metachromatic staining, and myelin-like structure of the dense granules found in the obV-ASC were very similar to glycolipid bodies observed in sphingolipid storage disorders, such as Fabry's disease (Fischer et al., 2006). The evaluation of the molecular and biological behavior of the ASC showed that obesity, particularly central obesity, was associated with lower expression levels of genes related to stemness, the inflammatory response and differentiation capacity as well as with lower proliferative and clonogenic abilities.
Probably due to the interplay between visceral AT and the immune system (Trzeciak-Ryczek et al., 2011) maximal expression of some inflammation-related genes was found in visceral ASC compared to subcutaneous ASC. However, the observation that the nV- and obV-ASC had different expression levels of these genes suggests that the inflammatory profile of the obV-ASC, compared to that of the nV-ASC which have a physiological phenotype, could be influenced by prolonged exposure to the altered and hypoxia-remodeled microenvironment of AT in visceral obesity. In fact, the obV-ASC showed increased expression only of certain genes that could be involved in chronic inflammation (Abe et al., 1996; Aga et al., 2002; Kaplanski et al., 2003; Olesen et al., 2012). Moreover, the very low expression of EGFR and VEGF in the obV-ASC seemed consistent with the reduced proliferation potential of these cells and with the hypoxic state due to the lack of efficient neoangiogenesis in the obese visceral AT. Interesting information could be obtained by further investigation of the role of these genes in visceral obesity.
Together, the findings described above clearly suggest that obV-ASC might have different capabilities. Consistent with these data, our previous report showed that the plasticity of ASC isolated from obese patients was impaired, as obS-ASC were scarcely able and obV-ASC completely unable to differentiate toward both osteogenic and adipogenic lineages (De Girolamo et al., 2013). On these bases, we hypothesized that the obV-ASC had undergone an early commitment possibly due to the altered microenvironment typical of obesity that could prevent their differentiation.
To better understand the effects of the microenvironment on the behavior of ASC derived from obese individuals, we performed further investigations in hypoxic conditions, analyzing the expression of several sensors and mediators of the inflammatory response. In fact, in obesity, AT becomes hypoxic as adipocyte size and tissue mass expand, which contributes to the development of the inflammation that leads to the initiation and progression of the major obesity-associated diseases (Hotamisligil, 2006; Trayhurn, 2013). The two subpopulations of ASC isolated from subcutaneous AT and the two types of ASC from visceral AT of the obese and non-obese subjects responded to oxygen deprivation through comparable activation of the HIF-1α protein. However, the analysis of HIF-1α mRNA transcription showed that the hypoxia-induced down-regulation observed in both types of subcutaneous ASC, which has also been described in other cell models as negative feedback induced by the HIF-1α protein itself (Uchida et al., 2004; Chamboredon et al., 2011), was strongly deregulated in the visceral ASC, particularly in the obV-ASC. Altered regulation of the gene expression of other components of the NF-kB complex, comparable to that of HIF-1α, was also evident. In the same context, it should be noted that the deregulation of HIF-1α mRNA expression was consistent with the activation of the NF-kB subunit p65, which was evident only in visceral AT, mostly in the obV-ASC, at both the mRNA and protein levels. In this regard, our findings clearly showed that the tissue-specific hypoxia-induced activation of NF-kB in the obV-ASC correlated with the deregulated up-regulation of genes activated during the inflammatory response. In fact, the obV-ASC showed hyper-responsiveness to oxygen deprivation, indicating a lack of control of the inflammatory response in these cells compared to the nV-ASC, which were essentially unresponsive to hypoxia. Differently, the nS- and obS-ASC, which showed similar basal expression levels of inflammation-related genes, also responded to the hypoxic stimulus with comparable behavior consisting of the down-regulation of gene expression or an absence of changes, with the sole exception of COX2 and VEGF, which were up-regulated because they are transcriptional targets of both HIF-1α and NF-kB (Forsythe et al., 1996; Schmedtje et al., 1997; Shibata et al., 2002; Kiriakidis et al., 2003; Kaidi et al., 2006).
Consistent with their increased inflammatory response, the obV-ASC also showed up-regulated expression of markers of fibrosis. Actually, the AT of obese subjects is characterized by an excessive amount of interstitial fibrosis, and extracellular matrix components could play a major role in connecting local inflammatory phenomena to the alteration of AT metabolic functions and tissue deterioration (Henegar et al., 2008). It has been reported that microenvironment stimuli, such as macrophage-secreted factors, promote a profibrotic phenotype in human ASC, in which nuclear accumulation of p65 is responsible for increased expression of fibrogenic proteins, which in turn are able to activate NF-kB, thereby contributing to amplification of the inflammatory loop (Keophiphath et al., 2009). In this context, our data strongly indicate that in obV-ASC hypoxia-induced pro-inflammatory and fibrogenic responses could dramatically contribute to microenvironmental alterations and the systemic low-grade inflammation typical of obesity-related morbidity. In this respect, the findings that all of the obese donors of obV-ASC enrolled in this study showed a mild increase in serum CRP levels, as frequently observed in obesity (Lubrano et al., 2012), are very suggestive.
Oxygenation level is an important aspect of the microenvironment of the niches that influences stem cell behavior by affecting quiescence and differentiation (Arai and Suda, 2008). Although the data in the literature conflict, it is known that hypoxia modulates mesenchymal stromal cell differentiation. In agreement with reports showing the effect of hypoxia in human stromal cells from bone marrow (D'Ippolito et al., 2006; Holzwarth et al., 2010; Park et al., 2013), our results showed that the expression of adipogenic and osteogenic differentiation genes could be inhibited in ASC during oxygen deprivation. However, an effect of obesity on the differentiation of visceral ASC was observed. In fact, the lack of any change in the expression of adipogenic and osteogenic markers in the obV-ASC confirmed that these cells were not able to modulate these two pathways, neither in response to hypoxia nor to specific differentiation stimuli (De Girolamo et al., 2013). Consistent with other reports (D'Ippolito et al., 2006; Holzwarth et al., 2010; Park et al., 2013), our findings clearly indicated that hypoxia maintained both types of subcutaneous ASC in an undifferentiated state, as demonstrated by increased levels of the OCT4 stemness gene and reduced expression of differentiation markers. However, when comparing the two visceral ASC populations, we observed that the obV-ASC, which we hypothesized to be pre-committed, showed greater recovery of the stem-like phenotype, as demonstrated by the strong increase in the stemness markers KLF4 and OCT4. In this respect, it is important to note that after hypoxia exposure, both KLF4 and OCT4 expression increased to levels comparable to those of the normoxic nV-ASC, indicating that appropriate stimuli can restore stemness features in obV-ASC. These results are in agreement with the findings that stemness can be conferred on differentiated cells through re-expression of the canonical stemness genes OCT4, c-MYC, KLF4, and SOX2 (Takahashi and Yamanaka, 2006), providing a potential mechanism whereby hypoxia can regulate stem cell function. The re-acquisition of some stem-like molecular characteristics would also be consistent with the expression of fibrosis genes observed in the obV-ASC after oxygen deprivation.
The findings that HIF-1α activation was similar in the ASC from the obese and non-obese subjects, whereas only the obV-ASC showed marked NF-kB activation, which is in agreement with the tissue-specific increased expression of inflammation-related and fibrosis genes, strongly suggest that NF-kB is a crucial player in mediating the behavior of ASC during hypoxia. This hypothesis was further supported by the impaired gene expression observed in the obV-ASC in the presence of the NF-kB inhibitor. Moreover, in agreement with reports showing that HIF-1α expression is mediated by NF-kB, in our cell system HIF-1α activation was also inhibited by parthenolide. In fact, although HIF-1α and NF-kB can act independently in regulating the inflammatory response, they also have a profound level of cross-talk (Walmsley et al., 2005; Belaiba et al., 2007; Rius et al., 2008; Van Uden et al., 2008). Of particular interest is the observation that in the obV-ASC treatment with parthenolide completely blocked the hypoxia-induced increase in the expression of inflammation-related and fibrosis genes but only partially inhibited OCT4 expression and scarcely affected VEGF expression. These data support the therapeutic potential of NF-kB inhibitors, which could positively change the way in which obV-ASC affect the AT microenvironment. In fact, they could prevent the deregulated pro-inflammatory response of the obV-ASC but could only scarcely affect the recovery of stemness and angiogenic activity in the obV-ASC.
In conclusion, this study provides novel information concerning the origin of inflammation during obesity, unveiling a role for ASC in the physiological as well as pathological behavior of subcutaneous and visceral AT. In particular, each type of ASC contributes to AT responses according to marked differences in the functional and molecular parameters that characterize their phenotype and that are determined by the metabolic status of the donor in addition to the anatomical location of the AT from which the ASC were taken. Our findings suggest that the deregulated hyper-responsiveness to hypoxic stimuli observed in the obV-ASC may contribute via paracrine mechanisms to low-grade chronic inflammation, which has been implicated in the interplay between obesity and metabolic disorders. On the other hand, in the obV-ASC, hypoxia tended to restore some stemness features typical of the nV-ASC. The evidence of the involvement of the NF-kB pathway in the response of obV-ASC in a hypoxic environment supports an innovative therapeutic approach for obesity.
Acknowledgments
This study was partially supported by the Italian Ministry of Health (Ricerca Finalizzata, Progetto Ordinario, RF-IOG-656853). The authors thank Francesca Caporuscio for her precious help.