An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells†
Conflict of Interest Statement: The authors have no conflict of interest to declare.
Abstract
Mesenchymal stem cells (MSCs) are multipotent adult stem cells capable of differentiating along the osteoblast, adipocyte, and chondrocyte lineages. Regulation of MSCs differentiation may be a useful tool for regenerative medicine and cell-based therapy. The discovery of small molecule that activates the osteogenic differentiation of MSCs could aid in the development of a new anabolic drug for osteoporosis treatment. We identified CW008, a derivative of pyrazole–pyridine, that stimulates osteoblast differentiation of human MSCs and increases bone formation in ovariectomized mice. CW008 promotes osteogenesis by activating cAMP/PKA/CREB signaling pathway and inhibiting leptin secretion. These results suggest that CW008 is an agonist of cAMP/PKA/CREB pathway in osteogenic differentiation and that application of CW008 may be useful for the treatment of bone-related diseases and for the study of bone biology. J. Cell. Physiol. 228: 617–626, 2013. © 2012 Wiley Periodicals, Inc.
Abbreviations:
hBMSCs, human bone marrow-derived mesenchymal stem cells; cAMP, cyclic 3′,5′-adenosine monophosphate; PKA, protein kinase A; CRE, cAMP response element; CREB, cAMP response element (CRE)-binding protein; PTH, parathyroid hormone; ALP, alkaline phosphatase; Runx2, runt-related transcription factor 2; OVX, ovariectomy; BMP2, bone morphogenic protein 2; ID2, inhibitor of DNA binding 2; LEP, leptin.
Bone continuously undergoes remodeling throughout life via a tightly regulated cycle involving bone-forming osteoblast and bone-resorbing osteoclast. An imbalance between osteoblast and osteoclast activity leads to the skeletal disorders such as osteoporosis, which is characterized by bone loss and an increased risk of fracture (Lane, 2006; Feng and McDonald, 2011). Many drugs have been developed for the prevention and treatment of osteoporosis, most of which are antiresorptive therapies that target for osteoclast activity (Yasothan and Kar, 2008). In contrast, the only anabolic drug that is currently available is parathyroid hormone (PTH) (Goltzman, 2002; Zaidi, 2007; Rachner et al., 2011). Anabolic drug targets osteoblast and stimulates bone formation rather than preventing further bone loss, thereby resulting in a faster increase in bone mass and strength. Accordingly, the identification of osteoblast-targeting small molecule is useful for the development of anabolic drugs and for studying the molecular mechanisms involved in osteoblast differentiation (Canalis et al., 2007).
Osteoblast is derived from mesenchymal stem cells (MSC), which give rise to adipocyte, chondrocyte, and myoblast (Pittenger et al., 1999; Bianco et al., 2001). Given the appropriate conditions, MSC differentiates toward the osteogenic lineage; this leads to the accumulation and mineralization of extracellular matrix containing hydroxyapatite (Jaiswal et al., 1997). Osteoblast differentiation is controlled by a number of transcription factors, growth hormones and cytokines. The master transcriptional regulator for osteoblast differentiation is runt-related transcription factor 2 (Runx2). Runx2 binds to DNA and serves as a platform for several cytokines and hormone such as bone morphogenic proteins (BMPs), transforming growth factor β1 (TGF-β1), fibroblast growth factors (FGFs) and PTH (Zaidi, 2007). In addition, wingless-type MMTV integration site family members (Wnts)/β-catenin signaling pathway and mitogen activated-protein kinase (MAPK) pathway are known to induce osteoblast differentiation (Jaiswal et al., 2000; Zhang et al., 2004; Hartikka et al., 2005; Zaidi, 2007). Multiple signaling pathways regulate the differentiation of MSC along the osteoblast lineage, but these pathways still remain largely undefined.
Protein kinase A (PKA) signaling is known to play a critical role in osteogenesis of MSC; however, the involvement of PKA is dependent upon the molecular and developmental context in which osteogenesis occurs (Yang et al., 2004; Siddappa et al., 2008). PKA is localized in the cytosol as an inactive enzyme and consists of two regulatory subunits and two catalytic subunits. PKA is activated when cyclic 3′,5′-adenosine monophosphate (cAMP) binds to each of the regulatory subunits. The catalytic subunits are then released, allowing them to translocate to the nucleus and activate gene expression by phosphorylating the cAMP response element (CRE)-binding protein (CREB) at Ser-133 (Sands and Palmer, 2008; Bidwell et al., 2010). The cAMP/PKA/CREB signaling pathway is known to stimulate the expression of genes such as BMP2 (Ionescu et al., 2004) and ID2 (Kurabayashi et al., 1995), which have been implicated in osteogenesis and bone formation (Siddappa et al., 2008) as well as osteogenic markers, including bone sialoprotein, osteocalcin, and type XXIV collagen (Huang et al., 2005; Matsuo et al., 2006). Also, cAMP is known to stimulate vascular calcification by enhancing the osteoblast-like differentiation of calcifying vascular cells (Tintut et al., 1998).
In this study, we screened a chemical library for osteogenesis-activating small molecules. We identified CW008, a derivative of pyrazole–pyridine that stimulates the differentiation of human bone marrow-derived mesenchymal stem cells (hBMSCs) into osteoblasts and enhances bone formation in vivo ovariectomized mice. And this osteogenesis-stimulating activity of CW008 is mediated by the cAMP/PKA/CREB signaling pathway. Taken together, these results suggest that CW008 is a new osteoblast activator and a potential new therapy for osteoporosis.
Materials and Methods
Chemical synthesis
All reactions sensitive to air and/or moisture were carried out in oven- or flame-dried apparatus, usually a 3-neck round-bottom flask equipped with a rubber septum inlet, under a dry nitrogen or argon atmosphere using magnetic stirring. 1H spectra were recorded at 300 MHz on a Bruker 300 NMR spectrometer. Chemical shifts (days) are reported in parts per million (ppm) with reference to tetramethylsilane or the solvent. HPLC analyses were obtained on an Waters 2695 separation module using the following conditions: INNO C18 HPLC column (5.0 µm, 250 mm L × 4.6 mm ID), 25°C column temperature, 1.0 ml/min flow rate, Waters 2996 photodiode array detector (210–600 nm), linear mobile phase gradient of 15–95% B over 20 min, holding 5 min at 95% B (mobile phase A: 0.1% TFA in water; mobile phase B: acetonitrile). Flash chromatography was carried out using silica gel 60 (230–400 mesh, Merck, Whitehouse Station, NJ). Reagent-grade chemicals were purchased from Sigma–Aldrich (St. Louis, MO) and used as received unless otherwise specified. Purity of the final compounds was >98% by HPLC. Synthetic procedures of CW008 are described in Supplementary Materials and Methods.
Cell culture
Human bone marrow-derived mesenchymal stem cells (hBMSCs) were purchased from Lonza (Walkersville, MD) and maintained in Dulbecco's Modified Eagle Medium (DMEM) (Lonza) supplemented with 20% fetal bovine serum (FBS, Gibco BRL, Grand Island, NY), 100 units/ml penicillin and 0.1 mg/ml streptomycin (Gibco BRL). For osteogenesis, hBMSCs were plated 104 cells/well in 96-well plates. After 2 days, hBMSCs were cultured under osteogenesis induction medium (OIM, 20% FBS, 10 mM β–glycerophosphate (USB Corp., Cleveland, OH), 50 µM ascorbate-2-phosphate (Sigma–Aldrich) and 100 nM dexamethasone (Sigma–Aldrich) in DMEM). OIM was changed every 3 days. Cells were grown at 37°C in a humidified atmosphere containing 5% CO2. hBMSCs for all experiments were used between cell passages 6–10.
Estimation of alkaline phosphatase (ALP) activity
Following culture, cells were lysed using protein lysis buffer (50 mM Tris–HCl (pH 7.5) and 0.1% TX-100). Cellular alkaline phosphatase activity was assayed colorimetrically by incubating cell lysates with the substrate p-nitrophenylphosphate (Sigma–Aldrich) in assay buffer (4 mM MgCl2 (pH 10.5) and 200 mM 2-amino-2-methyl-1-phosphate) at 37°C for 15 min. Absorbance was measured at 405 nm.
Alizarin red S staining
Mineral accumulation of differentiated osteoblasts was observed and photographed by using a Zeiss Axiovert 135 microscope plus Olympus DP71 CCD camera (Olympus Corporation, Japan).
To confirm the mineral deposition by differentiated osteoblasts, cells were washed twice with phosphate buffered saline (PBS), fixed in 4% paraformaldehyde for 20 min, washed twice with distilled water and then stained with a 1% alizarin red solution (Sigma–Aldrich) for 20 min. Cells were then washed three times with distilled water and examined for the presence of calcium deposits, which were identified by the presence of a red color. Images were obtained via scanning.
RNA extraction and real-time quantitative RT-PCR
Total RNA was extracted from hBMSCs using TRIzol reagent (Invitrogen, Grand Island, NY). cDNA was reverse-transcribed from 2 µg total cellular RNA using oligo (dT) primers and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Quantitative real-time PCR was performed using SYBR Green I Master mix (Roche, Indianapolis, IN) with a Roche LightCycler 480 Real-Time PCR system. PCR conditions consisted of a 10 min hot start at 95°C followed by 45 cycles of 15 sec at 95°C, 10 sec at 60°C and 30 sec at 72°C. Following primers were used: human alkaline phosphatase (ALP) 5′-taacatcagggacattgacg-3′ (sense), 5′-tgcttgtatctcggtttgaa-3′ (antisense); human runt-related transcription factor 2 (Runx2) 5′-cagcctgcagcccggcaaaa-3′ (sense), 5′-cgcaaccggggcactgcag-3′ (antisense); human bone morphogenic protein-2 (BMP2) 5′- ggcatcctctccacaaaaga-3′ (sense), 5′- gtggcagtaaaaggcgtgat-3′ (antisense); human inhibitor of DNA binding 2 (ID2) 5′- cgtcatcgactacatcttggac-3′ (sense), 5′-acacagtgctttgctgtcattt-3′ (antisense); human leptin (LEP) 5′-caaaaagtccaagatgacacca-3′ (sense), 5′- tgttggtagactgccagtgtct-3′ (antisense); the reference gene, human ribosomal protein large P0 (RPLP0) 5′-ggaatgtgggctttgtgttc-3′ (sense), 5′-tgcccctggagattttagtg-3′ (antisense). The expression levels of the mRNAs were normalized to the expression level of RPLP0 and compared.
In vivo osteoporosis model
Female 8-week-old mice were ovariectomized (OVX) under pentobarbital anesthesia and divided into five groups; normal, sham-operated control, OVX-vehicle, OVX-CW008 (15 mg/kg), and OVX-alendronate (5 mg/kg) (Alexander et al., 2001). Eight mice per group were examined. At 4 weeks after the surgery, the mice were subcutaneously injected with vehicle, CW008 or alendronate once a day. After 4 weeks, the mice were euthanized and the major organs were weighed. Bone formation was analyzed with a three-dimensional microcomputed (µCT) system (TOMONIX V90, RAY Co, Korea) as previously described (Ofek et al., 2006; Smoum et al., 2010). Animal study protocols were approved by the Institutional Animal Care and Use Committee at Pohang University of Science and Technology.
Immunoblotting
For immunoblotting, whole-cell lysates were prepared in lysis buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% TX-100, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 1 mM Na3VO4, 1 mM PMSF, and protease inhibitor cocktail (Sigma–Aldrich)). Lysates were then centrifuged at 14,000 rpm for 15 min (4°C). A total of 20 µg of protein was separated by **SDS-PAGE using 10% gels and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in Tween 20/Tris-buffered saline. For detection, the membranes were incubated with primary antibodies overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies were incubated with the membrane for 1 h. Signals were visualized by chemiluminescence (ECL system, Amersham Biosciences, Piscataway, NJ).
Plasmids and luciferase activity assay
To assess luciferase activity, a pCRE-luciferase reporter plasmid, which contains the SV40 30 intron and a polyadenylation signal, was purchased from Agilent Technologies (Santa Clara, CA). The pRL-SV40 construct, which was used Renilla luciferase expression vector, was purchased from Promega. hBMSCs were seeded into 24-well plates and cultured for 24 h before transfection. The cells were transfected with a DNA mixture containing the pCRE-luciferase reporter plasmid and the internal control plasmid pRL-SV-40 (25 ng) using the Lipofectamine transfection reagent (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection, the cells were treated with either forskolin or test compound and incubated for 6 h. The luciferase activity of the cell lysates was measured using the Dual-Luciferase® Reporter Assay System according to the manufacturer's instructions (Promega). Relative luciferase activity was normalized for transfection efficiency using the corresponding Renilla luciferase activity.
cAMP immunoassay
cAMP concentrations in hBMSCs were analyzed using the cAMP kit from R&D Systems (Minneapolis, MN) according to the manufacturer's instructions. In brief, hBMSCs (106 cells) were treated with vehicle, CW008 or forskolin for 6 h in osteogenic differentiation condition, and the cell lysates were used for the assay.
Detection of leptin secretion
Leptin concentrations in the conditioned medium were measured using the MILLIPLEX Human Bone Panel according to the manufacturer's instructions (Millipore, Billerica, MA).
General reagents
Phospho-CREB and CREB antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were obtained from KPL (Gaithersburg, MD).
Forskolin and H-89 were purchased from Calbiochem (San Diego, CA) and leptin was purchased from R&D Systems.
Statistical analysis
The data were analyzed using Student's t-tests. P < 0.05 and P < 0.01 were considered to be significant.
Results
CW008 stimulates osteogenesis of hBMSCs
To identify a small molecule that promotes osteoblast differentiation, we performed a stem cell-based screen of a chemical library of containing 192 kinase scaffold compounds. As a primary screening, we selected a small compound 1C2 that increased alkaline phosphatase (ALP) activity during early osteogenic differentiation. After then, more compounds were synthesized based on the hit compound of primary screening and we performed second screening, that is to identify small compounds to stimulate mineral accumulation in the extracellular matrix. The differentiation of hBMSCs into mature osteoblasts generally occurred after 7–12 days in the presence of osteogenic differentiation conditions in our system and osteoblast differentiation was confirmed by alizarin red S staining to detect the mineral accumulation and calcium deposits. As a result, we could select an active small compound, CW008, to promote differentiation of hBMSCs into osteoblasts (Fig. 1A and Supplementary Fig. 1). Treatment of hBMSCs with CW008 enhanced ALP activity at 2 and 4 days after osteogenesis induction (Fig. 1B). Under osteogenic differentiation conditions, CW008 induced dramatic mineralization of osteoblasts compared with vehicle treatment (Fig. 1C). Mineralization of differentiated osteoblasts was detected by alizarin red S staining at 12 days after osteogenesis induction (Fig. 1D). CW008 increased calcium deposits in the extracellular matrix in a dose-dependent manner. Remarkably, CW008 also induced the expression of osteogenic marker genes, including alkaline phosphatase (ALP) and Runx2, during hBMSC osteogenesis (Fig. 1E). These results show that identified small compound CW008 is a potent activator of osteogenesis. In contrast to the osteogenic effect of CW008, CW008 suppressed the adipocyte differentiation of hBMSCs in a dose-dependent manner (Supplementary Fig. 2). In response to CW008 treatment, accumulation of intracellular lipid droplets, a characteristic of mature adipocytes, was reduced and it was detected by oil red O staining.
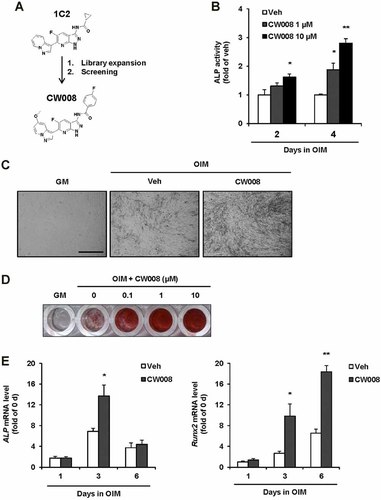
CW008 promotes osteogenesis of hBMSCs. A: Structures of identified small compounds. B: Effect of CW008 (1–10 µM) treatment on alkaline phosphatase (ALP) activity during osteogenesis. *P < 0.05 and **P < 0.01 versus the vehicle (Veh) treatment. C: hBMSCs were grown in the presence of growth medium (GM) or osteogenesis induction medium (OIM) for 12 days. Under osteogenic condition, CW008 (10 µM) enhanced osteogenesis of hBMSCs and mineral accumulation compared with the vehicle treatment. Morphological changes during osteogenesis were examined and photographed (50×) at 12 days after osteogenesis induction. Scale bars = 500 µm. D: Increased extracellular matrix mineralization by CW008 was also confirmed by alizarin red S staining at 12 days after osteogenesis induction. E: Expression levels of the osteogenic markers, ALP and Runx2, were examined by RT-PCR. *P < 0.05 and **P < 0.01 versus the Veh treatment at the same day. All data are presented as means ± SD.
CW008 enhances bone formation in vivo osteoporosis model
Next, we examined the effect of CW008 in ovariectomized (OVX) mice, which are widely used as a model of estrogen deficiency and bone loss (Jerome and Peterson, 2001; Yamane et al., 2009; Smoum et al., 2010). Mice were sham-operated or ovariectomized and left for 4 weeks to allow for the occurrence of bone loss. Trabecular bones exhibited marked bone loss after ovariectomy compared with those from sham-operated mice. Following bone loss, vehicle, CW008 (15 mg/kg), or alendronate (ALN, 5 mg/kg) was administrated daily for 4 weeks. Alendronate, which is currently used osteoporosis drug (Baron et al., 2011), was used as a control to compare the activity of CW008. Microcomputed tomography (µCT) analyses revealed that mice treated with CW008 showed a marked increase in bone mass compared with vehicle-treated mice (Fig. 2A). Similarly, the trabecular bone volume density (BV/TV) in the distal femoral metaphysis was increased by CW008 treatment to a degree comparable to that achieved by alendronate treatment (Fig. 2B). In addition, trabecular thickness (Tb.Th) and cortical thickness (Col.Th) in CW008-treated OVX mice were higher than those in vehicle-treated OVX mice (Fig. 2C,D). These results demonstrate that active compound CW008 increases bone formation in osteoporosis treatment model.
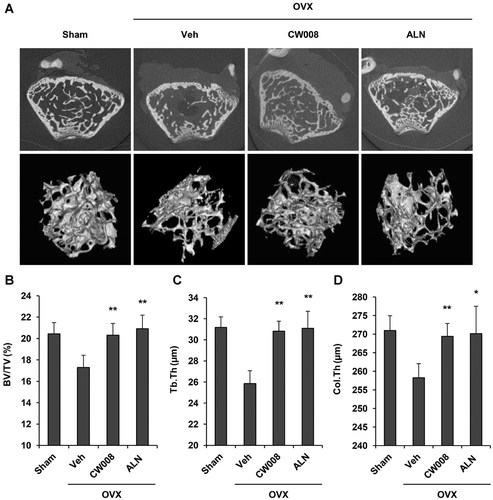
CW008 stimulates bone formation in OVX mice. Mice were injected daily with vehicle (Veh, DMSO), CW008 (15 mg/kg body weight/day) or alendronate (ALN, 5 mg/kg body weight/day) for 4 weeks after OVX (n = 8 mice/group). A: Representative µCT images of trabecular bone structures in the distal femoral metaphysic (upper) and 3-dimensional trabecular bone structure (lower). Sham, sham-operated mice; OVX, ovariectomy-operated mice. B–D: Histological analysis of trabecular bones of sham- and OVX-operated mice treated with Veh, CW008 or ALN for 4 weeks. BV/TV, bone volume over total volume; Tb.Th, trabecular thickness; Col.Th, cortical thickness. *P < 0.05 and **P < 0.01 versus the Veh-treated OVX mice. All data are presented as means ± S.D.
CW008 activates the cAMP/PKA/CREB signaling pathway
To define the molecular mechanism underlying CW008-stimulated osteogenic activity, we examined several known signaling pathways that have been implicated in osteogenic differentiation, including the cAMP/PKA, the Wnts/β-catenin and the MAPK pathway. hBMSCs were treated with vehicle or CW008 (1 µM) in osteogenic differentiation medium (Fig. 3A). During the first 12 h of differentiation, CW008 increased CREB phosphorylation compared with vehicle treatment. The level of CREB phosphorylation then returned to baseline at 24 h after treatment. The ability of CW008 to activate CREB phosphorylation was compared to that of the inactive compound CW 138 (1 µM) or forskolin, a PKA activator. Forskolin also induced CREB phosphorylation to a degree similar to CW008 treatment (Fig. 3B).
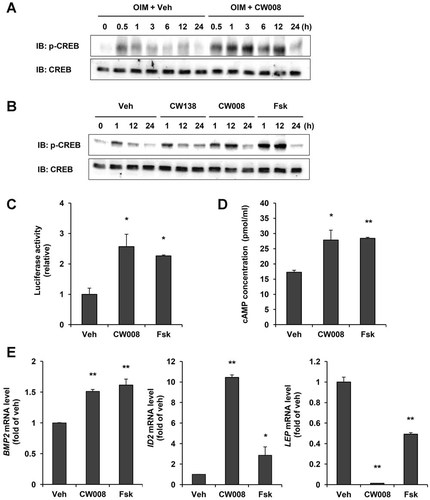
CW008 activates the cAMP/PKA/CREB signaling pathway. A: hBMSCs were treated with either vehicle (Veh) or CW008 (1 µM). CW008 activated CREB phosphorylation compared with Veh treatment in OIM. B: CW008 (1 µM) and forskolin (Fsk, 1 µM) both induced CREB phosphorylation as compared with either Veh or inactive compound CW138 (1 µM) treatment in the presence of OIM. C: hBMSCs were transfected with the CRE-Luc reporter gene. After 48 h, transfected cells were treated with Veh, CW008 or Fsk for 6 h in osteogenic differentiation condition. Firefly luciferase activity was assessed in cell extracts, using a dual-luciferase assay and normalized to Renillar luciferase activity to control for transfection efficiency. Data are presented as fold changes compared to Veh treatment. *P < 0.05 versus the Veh. D: cAMP production was increased at 6 h after CW008 or Fsk treatment in osteogenic differentiation condition. *P < 0.05 and **P < 0.01 versus the Veh. E: Gene expressions were examined in CW008-treated cells at 3 days after osteogenesis induction. *P < 0.05 and **P < 0.01 versus the Veh. All values are presented as means ± SD.
We next examined the effect of CW008 on CRE-dependent transcription via a CRE-directed luciferase reporter gene assay (Fig. 3C). hBMSCs were transfected with a CRE-Luciferase construct and treated with vehicle, CW008 (1 µM) or forskolin (1 µM) for 6 h in osteogenic differentiation medium. In response to CW008 treatment, CRE-luciferase activity was increased approximately 2.5-fold compared with cells treated with vehicle. Forskolin was used as a positive control.
To determine the correlation between CW008-mediated CRE-dependent transcription and CREB phosphorylation and intracellular increases in cAMP, we examined whether CW008 (1 µM) induced cAMP production in hBMSCs. We found that after 6 h of incubation, CW008 caused a slight increase in cAMP production. This increase in cAMP production was comparable to that caused by forskolin (1 µM) treatment (Fig. 3D).
As we observed that CW008 activated the cAMP/PKA/CREB signaling pathway, we examined whether CW008 regulated the expression of CRE-target genes. hBMSCs were treated with vehicle, CW008 (1 µM) or forskolin (1 µM) for 3 days in osteogenic differentiation medium. CW008 increased the expression of the known CRE-target genes BMP2 and ID2 compared to vehicle treatment (Fig. 3E). Forskolin also enhanced BMP2 and ID2 expression. Significantly, CW008-treated cells expressed higher levels of ID2 than forskolin-treated cells. We also examined changes in leptin (LEP) expression. Leptin is an adipocyte-derived hormone that has been known as an inhibitor of bone formation in vivo (Ducy et al., 2000; Elefteriou et al., 2004). PKA activity is known to regulate leptin secretion (Szkudelski et al., 2005), therefore, the effect of CW008 (1 µM) on LEP expression was examined (Fig. 3E). CW008-stimulated hBMSCs displayed reduced LEP expression compared to vehicle-treated cells. Forskolin also inhibited LEP expression. Significantly, LEP gene expression was almost undetectable following CW008 treatment. These results suggest that CW008 activates cAMP/PKA/CREB signaling pathway and regulates gene expressions of BMP2, ID2, and LEP.
Inhibition of PKA blocks CW008-stimulated osteogenesis
To determine whether CW008 exerts its osteogenic activity via the cAMP/PKA/CREB signaling pathway, we first confirmed that forskolin enhanced osteogenesis of hBMSCs (Fig. 4A).
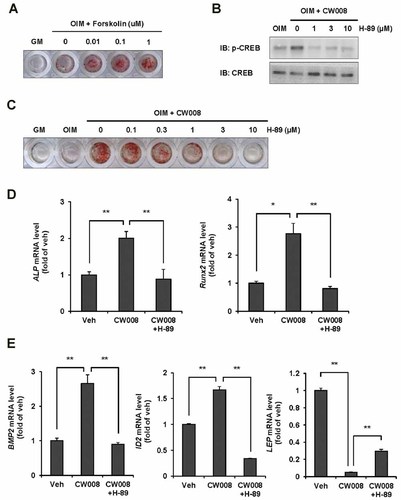
Inhibition of the cAMP/PKA/CREB pathway blocks CW008-stimulated osteogenesis. A: Forskolin promotes osteogenesis of hBMSCs. Alizarin red S staining was performed at 10 days after osteogenesis induction. B: hBMSCs were treated with CW008 (1 µM) in the absence or presence of H-89 for 6 h and CREB phosphorylation was examined by immunoblotting. C: Inhibition of CW008-stimulated osteogenesis by H-89 treatment was confirmed by alizarin red S staining at 7 days after osteogenesis induction. D: Osteogenic marker expression promoted by CW008 was abolished by H-89 (1 µM) co-treatment. Osteogenic marker expression was examined at 3 days after osteogenesis induction. *P < 0.05 and **P < 0.01 versus the vehicle (Veh) or CW008 treatment. E: CW008-activated upregulation of BMP2 and ID2 expression and downregulation of LEP expression were blocked in the presence of H-89 (1 µM). Gene expression was examined at 3 days after osteogenesis induction. **P < 0.01 versus the Veh or CW008 treatment. All data are presented as means ± SD.
Next, we examined the effect of H-89, which is an inhibitor of PKA activity, on CW008-mediated osteogenesis. In the presence of H-89 (1–10 µM), CW008-induced CREB phosphorylation was abolished and it was confirmed by immunoblotting (Fig. 4B). Additionally, H-89 treatment suppressed CW008-stimulated osteogenesis. In osteogenic differentiation condition, hBMSCs were treated with CW008 with or without H-89 for 7 days. Co-treatment with H-89 resulted in decreased mineral accumulation; in contrast to cells treated with only CW008, only baseline levels of differentiation were observed in H-89 co-treatment (Fig. 4C). Moreover, the increase in osteoblast marker expression by CW008 was abolished by H-89 (1 µM) co-treatment (Fig. 4D). Furthermore, CW008-mediated upregulation of BMP2 and ID2 expression was reduced in the presence of H-89. Conversely, CW008-mediated downregulation of LEP expression was increased in response to H-89 (Fig. 4E). Therefore, these results suggest that CW008-stimulated osteogenesis is mediated by the cAMP/PKA/CREB signaling pathway and that CW008-mediated upregulation of BMP2 and ID2 expression and downregulation of LEP expression are controlled via the cAMP/PKA/CREB signaling pathway.
CW008 promotes hBMSCs osteogenesis by suppressing leptin secretion
To further investigate the key mediator of CW008-stimulated osteogenesis, we focused on the suppression of LEP expression that was observed with CW008 treatment. Leptin secretion in conditioned medium (CM) from CW008-treated cells during osteogenic differentiation was markedly suppressed. In contrast to the CM from vehicle-treated cells, leptin was almost undetectable in the CM from CW008-treated cells at 3 and 6 days after osteogenesis induction (Fig. 5A). To confirm that PKA activation suppresses leptin secretion, H-89 (1 µM) was added to CW008-treated (1 µM) or forskolin-treated (1 µM) cells under osteogenic differentiation condition. After 6 days, the leptin concentration in the CM was examined (Fig. 5B). H-89 co-treatment remarkably abolished the suppressive effects of CW008 and forskolin on leptin secretion and restored leptin level in CM. Additionally, H-89 treatment increased leptin secretion in vehicle-treated cells.
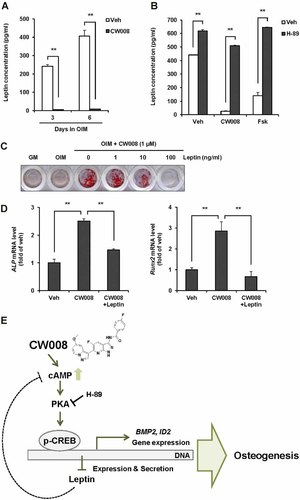
CW008 stimulates osteogenesis through leptin suppression. A: Leptin secretion during CW008-stimulated osteogenesis was measured using the MILLIPLEX Human Bone Panel. **P < 0.01 versus the vehicle (Veh). B: hBMSCs were treated for 6 days with Veh, CW008 (1 µM) or forskolin (Fsk, 1 µM) in the presence of OIM. H-89 (1 µM) co-treatment restored leptin concentration, both in CW008- and Fsk-treated cells. **P < 0.01 versus the Veh. C: hBMSCs were treated for 7 days with CW008 alone or with leptin under osteogenic differentiation condition. CW008-stimulated osteogenesis was inhibited by leptin co-treatment in a dose-dependent manner. D: Osteogenic marker expression was also suppressed by leptin (100 ng/ml) co-treatment at 3 days after osteogenesis induction. **P < 0.01 versus the Veh or CW008 treatment. All values are presented as means ± SD. E: Schematic diagram of CW008-stimulated osteogenesis.
To determine whether CW008-stimulated osteogenesis is mediated by the suppression of leptin secretion, hBMSCs were treated with CW008 in the presence or absence of leptin under osteogenic differentiation condition (Fig. 5C). After 7 days, osteogenic differentiation was confirmed by alizarin red S staining. Interestingly, leptin co-treatment reduced the CW008-mediated mineralization of osteoblasts in a dose-dependent manner. Furthermore, osteogenic marker expression activated by CW008 was also reduced in the presence of leptin (Fig. 5D). These results suggest that leptin plays a negative role in CW008-stimulated osteogenesis.
On the other hand, leptin has been known to promote hydrolysis of intracellular cAMP via phosphodiesterase (PDE) activation (Sahu, 2004; Hsu et al., 2006). We hypothesized that leptin might inhibit the osteogenesis activated by CW008 through stimulation of cAMP degradation. To demonstrate the hypothesis, 3-isobutyl-1-methylxanthine (IBMX, PDE inhibitor) was co-treated with CW008 and leptin in osteogenic differentiation condition and we could observe that IBMX recovered osteogenic activity of CW008 suppressed by leptin (Supplementary Fig. 3). These results show that activation of cAMP/PKA/CREB signaling pathway is essential and, at least part of, suppression of leptin secretion contribute to the osteogenic activity of CW008.
Discussion
In this study, we identified CW008 as a novel osteogenic activator by screening a small molecule library. CW008 stimulates differentiation of hBMSCs into osteoblasts and bone formation in OVX mice. This bone-forming activity of CW008 is mediated by the cAMP/PKA/CREB signaling pathway (Fig. 5E). CW008 activates CREB phosphorylation and regulates the gene expressions, upregulation of BMP2 and ID2 and downregulation of LEP. Our results show that leptin is a negative regulator of CW008-stimulated osteogenesis. Leptin secretion was remarkably reduced in CW008-treated cells and additional leptin inhibited CW008-stimulated osteoblast differentiation. Taken together, our results suggest a CW008-activated cAMP/PKA/CREB/leptin pathway in osteogenesis of hBMSCs.
cAMP acts as a second messenger that transduces the signals from a variety of cytokines and growth factors to the effector molecules. The CREB protein is the principal mediator in response to increased cAMP production following phosphorylation by PKA (Altarejos and Montminy, 2011). Intracellular cAMP levels can be elevated by G-protein-coupled receptor (GPCR) activation such as PTH receptor activation. PTH functions as a major mediator of bone remodeling by both decreasing osteoblasts apoptosis and increasing osteoblasts differentiation (Strewler et al., 1987; Jilka, 2007; Khosla et al., 2008). Continuous exposure to PTH stimulates osteogenic differentiation in immature osteoblasts, and the cAMP pathway functions as a key mediator of this PTH-induced differentiation (Isogai et al., 1996).
In our results, we identified CW008 as a stimulator of cAMP production and an activator of CRE-dependent transcription. CW008 increased BMP2 gene expression during osteogenesis. Protein BMP2 has been known to play critical roles in bone formation and recombinant BMP2 is used clinically for the treatment of long bone fracture (Khosla et al., 2008). Although we did not check whether CW008 stimulated BMP2 expression in protein level and its related signaling pathway, identification of small molecule CW008 as an activator of BMP2 expression would be useful for the therapeutic development.
In particular, our results show that CW008 considerably suppresses expression and secretion of leptin. Leptin is a hormone secreted by adipocytes and participates in the regulation of food intake and energy balance (Szkudelski, 2007). Leptin secretion can be influenced by several factors such as food consumption, fasting and insulin, and regulated by many intracellular molecules, including cAMP. An increase in intracellular cAMP levels decreases leptin secretion, whereas a decrease in cAMP exerts the opposite effect (Szkudelski, 2007). Additionally, leptin secretion induced by glucose, alanine, and leucine is attenuated by cAMP in PKA-dependent manner (Szkudelski et al., 2005) and forskolin, an adenylate cyclase activator, completely inhibits insulin-stimulated leptin secretion (Cammisotto and Bukowiecki, 2002). These reports support our finding that CW008 suppresses leptin secretion via cAMP/PKA signaling pathway.
In bone formation, there have been controversial reports about the role of leptin. Leptin functions as a negative regulator of bone formation in vivo (Ducy et al., 2000; Elefteriou et al., 2004). Leptin-deficient mice showed a higher bone mass compared with wild-type mice and increased rates of bone formation, whereas several reports suggest that leptin enhances osteogenesis in vitro (Thomas et al., 1999; Reseland et al., 2001). When we treated hBMSCs with leptin in osteogenic differentiation condition, there was no significant change (data not shown). But surprisingly, leptin co-treatment inhibited CW008-stimulated osteoblasts mineralization in a dose-dependent manner and suppressed CW008-mediated osteogenic marker expression. On the other hand, treatment of IBMX, PDE inhibitor, recovered the osteogenic activity of CW008 in the presence of leptin (Supplementary Fig. 3). Intracellular cAMP levels can be regulated by adenylyl cyclase and PDE (Daniel et al., 1998), which hydrolyze cAMP into 5′AMP. Several reports suggest that leptin promotes hydrolysis of intracellular cAMP via the Janus kinase 2 (JNK2)—phosphatidylinositol 3-kinase (PI3K)/Akt—PDE3—cAMP pathway (Sahu, 2004; Hsu et al., 2006). Accordingly, leptin might reduce CW008-activated osteogenesis via stimulating intracellular cAMP degradation (Fig. 5E).
In summary, we have identified a bioactive small molecule that increases bone formation in vitro and in vivo. As PTH is the only anabolic drug available for osteoporosis therapy, the discovery of small molecule that increases osteoblast differentiation and bone formation is very useful for the treatment of bone-related diseases. In addition to that, our results suggest identified small molecule acts as a cAMP inducer and suppressor of leptin secretion. It can be a useful tool for the dissection of the molecular mechanism underlying the osteogenesis, related to cAMP/leptin signaling pathway.
Acknowledgements
This work was supported by the Industrial Strategic technology development program (10027895) funded by the Ministry of Knowledge Economy (MKE, Korea) and supported by the Fusion Pioneer Project (PGB013) from the National Research Foundation of Korea.




