5′-ectonucleotidase mediates multiple-drug resistance in glioblastoma multiforme cells†
The authors declare that have not conflict of interest.
Abstract
Glioblastoma multiforme (GBM) cells are characterised by their extreme chemoresistance. The activity of multiple-drug resistance (MDR) transporters that extrude antitumor drugs from cells plays the most important role in this phenomenon. To date, the mechanism controlling the expression and activity of MDR transporters is poorly understood. Activity of the enzyme ecto-5′-nucleotidase (CD73) in tumor cells, which hydrolyses AMP to adenosine, has been linked to immunosuppression and prometastatic effects in breast cancer and to the proliferation of glioma cells. In this study, we identify a high expression of CD73 in surgically resected samples of human GBM. In primary cultures of GBM, inhibition of CD73 activity or knocking down its expression by siRNA reversed the MDR phenotype and cell viability was decreased up to 60% on exposure to the antitumoral drug vincristine. This GBM chemosensitization was caused by a decrease in the expression and activity of the multiple drug associated protein 1 (Mrp1), the most important transporter conferring multiple drug resistance in these cells. Using pharmacological modulators, we have recognized the adenosine A3 receptor subtype in mediation of the chemoresistant phenotype in these cells. In conclusion, we have determined that the activity of CD73 to trigger adenosine signaling sustains chemoresistant phenotype in GBM cells. J. Cell. Physiol. 228: 602–608, 2013. © 2012 Wiley Periodicals, Inc.
Glioblastoma multiforme (GBM, WHO glioma grade IV) is the most common nervous system primary tumor with one of the worst prognosis of all cancers. It is the second cause of death in pediatric cancers, after leukaemia (Jemal et al., 2010). GBM remains one of the most fatal and least successfully treated solid tumors because it has a high capacity for proliferation and is extremely chemoresistant (Chang et al., 2006). The WHO recommends that these patients be managed with multimodal therapies including surgical resection where feasible, plus radiotherapy and chemotherapy with temozolomide. This is currently the only drug that has been shown, in clinical trials, to offer a modest improvement in patient survival, from 12.1 to 14.6 months (Stupp et al., 2005). However, with GBM being highly infiltrative, cancer often invades into normal brain tissues preventing surgical resection which inevitably leads to recurrence. Besides this, to date, chemotherapy has not proved to be efficient in preventing recurrence, nor does it have a considerable impact on patient survival (Clarke et al., 2010). For this reason, the clinical management of patients with GBM remains essentially palliative.
It is thus clear that major advances in the chemotherapeutic treatment of high-grade brain tumors will require a comprehensive understanding of the mechanisms of both specific- and multiple-drug resistance. The underlying mechanism of the multidrug-resistant (MDR) phenotype is the expression of one or more proteins belonging to the ABC transporter gene family (ATP-binding cassette) in tumor cells (Sharom, 2008). These proteins function as pumps for the removal, from cells, of different metabolites and xenobiotics, including antitumor drugs (Ozben, 2006). Glial cells normally express low levels of some drug-resistant transporters (Loscher and Potschka, 2005). While, studies using cytofluorometric analysis and immunohistochemistry of glioma samples reveal a high expression of the MDR proteins Mrp1, Mrp3, and Mrp5 in high-grade glioma (Benyahia et al., 2004; Matsumoto et al., 2004; Calatozzolo et al., 2005; Declèves et al., 2006). In a similar manner, studies in glioblastoma cell lines corroborate the predominant content of MRP subfamily members, particularly Mrp1 encoded by ABCC1 gene (Declèves et al., 2002). Therefore, it has been proposed that reversal of multidrug resistance in GBM might be achieved by an inhibition of Mrp1 activity (Peigñan et al., 2011).
Another noteworthy characteristic of glioblastoma specimens was that expression of the enzyme ecto-5′-nucleotidase (CD73) was found to be densely distributed at cell membranes (Ludwig et al., 1999). The activity of CD73 contributes to an increase in the extracellular availability of adenosine, by hydrolysis of AMP. Then, modulation of cell functions can occur through activation of some member of the adenosine receptors family (Fredholm et al., 2011). Expression of CD73 has recently been associated with a prometastatic phenotype in melanoma and breast cancer (Lee et al., 2003; Sadej et al., 2006; Leth-Larsen et al., 2009; Stagg et al., 2010). Further, it has been demonstrated that tumor-derived CD73 activity is involved in the mechanism of tumor immune escape (Wang et al., 2008; Stagg et al., 2011) and in the proliferation of glioma cells (Bavaresco et al., 2008).
In this study, we aim to demonstrate that CD73 activity is linked with refractoriness of GBM cells to antitumor agents. Particularly, its role on regulate the expression and/or activity of Mrp1. These findings will support the evaluation of new therapeutic strategies for the treatment of GBM, targeting CD73 in favor of chemotherapy.
Materials and Methods
Brain tumours
Tissue samples were obtained from surgical resection procedures in brain tumor patients at the Department of Neurosurgery, Institute of Neurosurgery Asenjo, Santiago, Chile. All procedures were carried out with the approval of the Bioethics Committee of the Universidad Austral de Chile and Servicio de Salud Metropolitano Oriente. Fractions of each sample were fixed for immunohistochemistry in 4% formalin, and processed for primary culture. Subsequent procedures were performed once the presence of glioblastoma multiforme had been confirmed by histological analyses. The World Health Organization (WHO) has established that for classification of gliomas, the following findings must be considered (i) atypical nuclei, (ii) mitosis, (iii) endothelial proliferation, and (iv) necrosis. For glioblastoma multiforme, at least three of these characteristics must be present, being the most important the presence of marked vascular proliferation and necrosis.
Cell cultures
The human glioblastoma multiforme T98G cell line and the SVGp12 cell line derived from human foetal glial cells were acquired from ATCC Company (ATCC CRL-1690 and CRL-8621). The cells were grown in DMEM/F12 medium supplemented with 10% foetal bovine serum, 1% penicillin/streptomycin, at 37°C in an atmosphere humidified with 5% CO2. The resected tissues were washed twice with D-Hanks' solution in aseptic conditions for preparation of GBM primary cultures. After removing visible blood vessel and necrotic tissues, glioma tissues were processed for primary culture as described previously (Peigñan et al., 2011). The cells were grown in DMEM supplemented with 20% foetal bovine serum, and 100 units/ml of penicillin/streptomycin and were incubated at 37°C in a humidified atmosphere and 5% CO2. The purity of cells and their identity were assessed by glial fibrillary acidic protein (GFAP)-antigen staining and expression of the tumoural proliferation marker Ki-67/MIB-1 (Peigñan et al., 2011).
Reverse transcription and PCR
Total cellular RNA was isolated using Trizol® Isolation Reagent (Invitrogen, Grand Island, NY). First strand cDNA was synthesised from 1 µg of total RNA using 50 U of MMLV Reverse Transcriptase (Stratagene) and oligo (dT)18 according to manufacturers' recommendations. PCR amplifications were carried out in a Personal Thermal Cycler (Eppendorf) using 1 µl of cDNA, 1×PCR buffer, 1.5 mM Mg2+, 0.4 mM dNTP's, 1.25 U Taq DNA polymerase (Invitrogen), and 0.5 µM of gene-specific oligonucleotide (Quezada et al., 2008). PCR amplifications were performed for 30 cycles, each of which consisted of denaturing at 92°C for 60 sec, annealing to 55°C for 45 sec and extension to 72°C for 30 sec. The PCR products were separated using 2% agarose gel electrophoresis. Ethidium bromide (EtBr) fluorescence intensities were quantified by densitometry analysis using the Molecular Analyst Software (BioRad). The relative abundance of transcripts from each gene was estimated by comparison with β-actin ratios.
Protein extracts and Western blots
Total protein extracts were obtained by lysis in 150 µl of extraction buffer (63.5 mM Tris/HCl pH 6.8, 10% glicerol, 2% SDS, 1 mM PMSF, 2 µM aprotinin, 1 µg/µl leupeptin and pepstatin). Then samples were sonicated three times for 5 sec and the protein concentrations quantified using Bicinchoninic Acid (BCA) Protein Assay (BioRad). For Western blots 50 µg of proteins extracts were fractionated by SDS-PAGE and transferred to PVDF membranes (Perkin–Elmer). Membranes were blocked in 1×PBS/0.05%tween/1%BSA and incubated with the primary antibodies (anti-Mrp1 (sc-18835), anti-CD73 (sc-14682) and anti- A3AR (sc-13938) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), A1AR (A-268) from Sigma, (Saint Louis, MO) A2AAR (ab101678) and A2BAR (ab40002) from Abcam, Cambridge). The immunodetection was developed using a secondary antibody-HRP conjugated and the chemiluminescence system ECL Western blotting (Amersham Pharmacia Biotech, Durham, NC).
MTT cell viability assay
1.0 × 104 cells per well were cultured in 96-well plates for 24 h and then exposed to vincristine (100 nM) alone or in combination with a CD73 inhibitor for 24 h. Cells were then incubated with 5 mg/ml MTT reagent (thiazole blue tetrazolium) in culture medium for 1 h. Formazan crystals were dissolved in 100 µl DMSO. Absorbance at 550 nm was measured using a microplate reader. Absorbance values are expressed as a percentage relative to control cells without treatment.
Immunohistochemistry
Brain tissues were fixed in formalin, paraffin-embedded and 5 µm sections were mounted on xylanized slides. For immunodetection, the sections were sequentially deparaffined and rehydrated, then incubated with 10 mM sodium citrate (pH 6.0) for 30 min, hydrogen peroxide (70% methanol, 3%) for 5 min and then blocked by immersing in 1×PBS containing 1% bovine serum albumin, 0.3% Triton X-100 and 5% fat-free milk for 30 min at room temperature. Sections were incubated with primary antibodies (anti-Mrp1 (sc-18835), anti-CD73 (sc-14682), anti- A3AR (sc-13938), anti-Ki-67 sc-56320 and anti-GFAP sc-52333 from Santa Cruz, A1AR (A-268) from Sigma, A2AAR (ab101678) and A2BAR (ab40002) from ABCAM) in blocking solution overnight at 4°C. They were then washed three times with 1×PBS for 5 min and signals were detected using the LSAB + System-HRP system (DakoCytomation).
Immunofluorescence
Cells grown on circular coverslips at semi-confluence were washed with 0.1 M phosphate buffer (pH 7.4), fixed with Histochoice (Sigma), and permeabilised using 0.3% Triton X-100 in 1×PBS. Preparations were blocked with 1% BSA and incubated with monoclonal antibody anti-Mrp1 (sc-18835: Santa Cruz Biotechnology). Secondary antibodies tagged with Alexa fluor 488 dye or Alexa fluor 594, were also used (Molecular Probes). The distribution of Mrp1 was visualized using a fluorescence microscope (Leica DM2500).
Mrp1 functional assays
The cells (2 × 105) were exposed to an inhibitor of CD73 activity or pharmacological modulators of adenosine receptors in serum-free medium DMEM for 24 h at 37°C in 24-well plates. They were then washed and loaded with 500 nM CFDA for 15 min. They were subsequently washed three times with 1×PBS and incubated for 15 min in serum-free DMEM medium. Cells were washed three times with ice-cold 1×PBS and lysed in PBS containing 0.4% Triton X-100. The fluorescence in cell extracts was measured with a spectrofluorometer (Perkin–Elmer) by excitation at 488 nm and emission collected at 530 nm (Echevarria-Lima et al., 2005). The accumulated fluorescence was corrected to total protein content of lysed cells.
RNA interference
siRNA specific to human CD73 was expressed using the pSilencer adeno 1.0-CMV System (Ambion) targeting the mRNA sequence 5′-AACCTGGAGACAGAGTAGTCA-3′. Glioblastoma cells were seeded in 6-well plates (80% confluence). Once attached to the plate, cells were transfected with CD73 siRNA plasmid (0.5 µg) using the transfection reagent Lipofectamine 2000. Expression levels of CD73 and Mrp1 activity were assayed 48 h after transfection. Regularly, the inhibition of the CD73 protein content was 85% (see Supplementary Data).
ATP and adenosine quantification
Cells were incubated in 1 ml Tyrode's buffer for 1 h at 37°C. A volume of 200 µl of incubation medium was mixed with 100 µl of citrate buffer at pH 6.0. Adenosine, AMP, ADP and ATP contents were quantified with 2-chloroacetaldehyde derivatizations, HPLC fractionation in a Chromolith Performance RP-18 column (Merck) and by fluorescent detections. Quantification procedures included adenosine and nucleotides standards. The concentrations were normalized to the total protein concentration in each test. For CD73 activity, cells (2 × 105) were exposed to AMP (25–1000 nM) for 15 min in Tyrode's buffer at 37°C and at controlled oxygen levels. The final content of adenosine was quantified as described above.
Pharmacological agents
The AOPCP (50 µM, adenosine 5′-alpha beta-methylenediphosphate) was used as CD73 inhibitor (Sigma, Saint Louis, MO). NECA (10 µM) is a general adenosine receptor agonist (Tocris Biosciences, Minneapolis, MN). The following selective antagonists from Tocris Bioscience were used: DPCPX (30 µM) for A1AR, ZM241385 (10 nM) for A2AAR, MRS1754 (50 nM) for A2BAR and MRS1220 (10 µM) is selective for A3AR (Fredholm et al., 2011).
Statistics
Analyses were carried out on raw data using the Peritz' F multiple comparison among means test. Student's t-test was applied for unpaired data and P < 0.01 was considered to be statistically significant.
Results
CD73 and Mrp1 are co-expressed in tumors and GBM cells
In accordance with known data for the abundance of transporters in cell cultures, the immunosignal of Mrp1 was clearly abundant in the tumor zone and was slightly detectable in the peritumour tissue in all six GBM samples analysed (Fig. 1A; Peigñan et al., 2011; Quezada et al., 2011). In a similar manner, the CD73 signal colocalised with the glial cell marker GFAP and the cell proliferation marker Ki-67 (Fig. 1A). Consistently, we have found these markers overexpressed in glioblastoma. When the AMP hydrolyzing activity was assayed in the standardized primary culture of GBM cells, we quantified a notably difference compared to barely activity in SVGp12 cells (Fig. 1B). The expression of CD73 was confirmed in primary cultures of GBM cells and in the T98G cell line. CD73 mRNA content was higher than that observed in the normal SVGp12 glial cell line (Fig. 2A) and the protein content was notoriously most abundant in the tumoral cells (Fig. 2B). Similarly, Mrp1 expression was more abundant in tumoural cells compared to SVGp12 (Fig. 2C), in agreement with functional data showing that only SVGp12 cells exhibited a strong decrease in the viability when exposed to antitumoural drugs substrates of Mrp1 (Garrido et al., 2011). In accordance with the detection of different CD73 expression levels, the extracellular metabolism of adenine-containing metabolites is preferentially tilted toward adenosine production evidenced by higher ratio between adenosine and ATP levels, detected in the extracellular mediums, in tumour cells compared with those seen in the normal glial cell line (Fig. 2D). The mean values of adenosine contents were 257, 278, and 97 nM in primary cultured, T98G and SVGp12 cells, respectively.
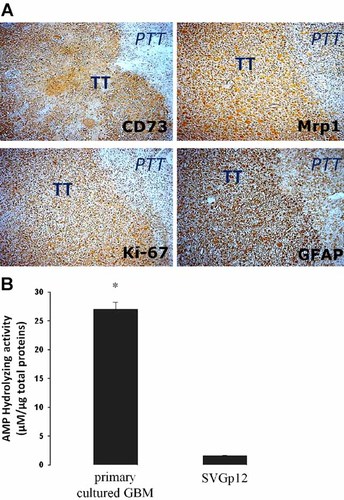
Localization of CD73 in glioblastoma multiforme. A: Biopsies from surgical resection of glioblastoma multiforme were processed for immunohistochemistry. The primary antibodies anti-CD73, anti-Mrp1, anti-GFAP, and anti-Ki-67 were used as indicated in each image. Immune detection was revealed using the peroxidise-DAB system. The samples were counterstained with haematoxylin. TT, tumoral tissue. PTT, peritumoral tissue. Original magnifications 200×. B: The AMP hydrolyzing activity of CD73 were evaluated in primary cultured GBM and SVGp12 glial cells. The cells were exposed to 400 µM of AMP for 15 min and quantification of adenosine in the extracellular medium was performed using HPLC. Values were normalized to total proteins. *, P < 0.01 versus SVGp12 (n = 5).
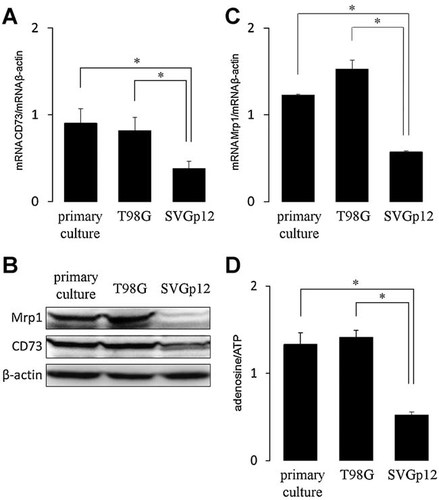
Expression of CD73 and Mrp1 in glioblastoma and glial cells. Total cellular RNA was isolated from primary cultured or T98G GBM cells and from SVGp12 non-tumoral glial cells. Transcript levels of CD73 (A) and Mrp1 (C) genes were measured by RT-PCR (see Materials and Methods Section). The graphs depict the means ± SD of ratios between amplification products of Mrp1 versus β-actin. *, P < 0.01 versus SVGp12 cells (n = 7). B: Protein level of CD73 and Mrp1 were assayed by Western blot in total extracts from cells. D: Extracellular contents of ATP and adenosine were quantified in the extracellular mediums of cultured cells by HPLC. The graph shows the means ± SD of ratios between adenosine versus ATP contents. *, P < 0.01 versus SVGp12 cells (n = 9).
The activity of CD73 is required to control Mrp1 in GBM cells
To further evaluate a correlation between the coexpression of ecto-5′-nucleotidase and Mrp1 in GBM cells, we studied the effect of blocking CD73 activity on the expression and activity of the MDR transporter. Treatment of GBM cells with AOPCP which blocks the AMPase activity mediated by CD73, reduced the mRNA and protein content of Mrp1 (Fig. 3A,B). Immunofluorescent detection of Mrp1 in primary cultured GBM cells was notably decreased by treatment with AOPCP (Fig. 3C). In a similar way, a siRNA designed to target CD73, reduced Mrp1 expression in GBM cells (Fig. 3). In contrast, treatment of GBM cells with NECA, the general agonist for adenosine receptors, increased the mRNA content of Mrp1 (Fig. 3A), suggesting that signalling induced by CD73-generated adenosine contributes to the MDR phenotype in GBM cells. In this context, treatment with AOPCP was also able to decrease the extrusion activity from cells as reflected by the intracellular accumulation of CFDA, a fluorescent substrate for Mrp1 (Fig. 4). However, NECA was able to increase the Mrp1 content only in primary cultured GBM cells and not further increase the high transporter content in T98G cells (Fig. 3B). Also, NECA was not able to increase the intrinsically elevated extrusion activity in the tumoural cells (Fig. 4). Probably, NECA was not able to modify the capability of cells to further extrusion activity through Mrp1, such as modification of glutathione levels or conjugation with it.
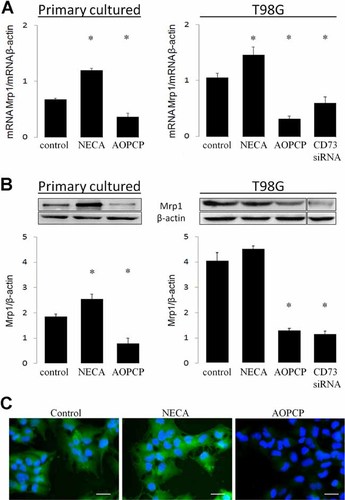
The inhibition of CD73 decreases the expression of Mrp1 in glioblastoma cells. Primary cultured or T98G GBM cells were exposed to the inhibitor of CD73 activity AOPCP (50 µM) or the general agonist of adenosine receptors NECA (10 µM) for 24 h. Also, T98G were transfected with a plasmid codifying an interfering RNA of CD73 expression. A: Total cellular RNA was isolated from cells and transcript levels of the Mrp1 gene were evaluated by RT-PCR. The graphs depict the ratios between the amplification products of Mrp1 versus β-actin. B: Western blot analysis of Mrp1 contents in treated cells. The graphs show the means ± SD of the ratios between protein content of Mrp1/β-actin. *, P < 0.01 versus control. (n = 7). C: Immunofluorescent detection of Mrp1 expression in primary cultured GBM cells treated for 24 h with AOPCP or NECA. Nuclear staining using DAPI is shown in blue. Magnification bar 20 µm.
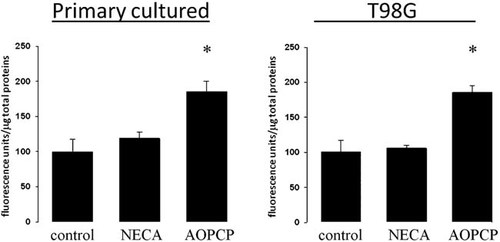
Role of CD73 on Mrp1 activity in human glioblastoma multiforme cells. Intracellular accumulation of CFDA, a fluorescent-specific Mrp1 substrate, was quantified in primary cultured GBM and T98G cells treated with AOPCP (50 µM) or NECA (10 µM) for 24 h. The graphs represent the means ± SD of the remaining intracellular fluorescence. The activity in controls was normalized to 100. *, P < 0.01 versus control (n = 6).
Signalling by adenosine A3 receptor subtype sustains Mrp1 activity in GBM cells
Using immunohistochemistry, we revealed that adenosine receptor subtypes A2A and A3 were clearly overloaded in the tumoural area of GBM (Fig. 5). The effect of adenosine receptor pharmacological modulators on the expression and activity of Mrp1 in GBM cells was also evaluated. Neither of treatments has effect on expression nor activity of Mrp1 in non-tumoural SVGp12 glial cells. In contrast, as shown in Figure 6, the mRNA content of Mrp1 and the extrusion of CFDA were decreased by treatment with the adenosine A3 receptor antagonist MRS1220, as seen before with AOPCP treatment.
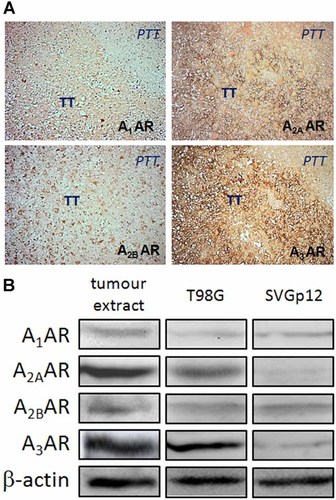
Expression of adenosine receptors in glioblastoma multiforme. A: Surgical resected samples of tumours were processed for immunohistochemistry. The primary antibodies for adenosine receptor (AR) subtypes were used as indicated in each image. Immune detection was revealed using the peroxidise-DAB system. TT, tumoural tissue. PTT, peritumoural tissue. Original magnifications 200×. B: Western blot analysis of the expression of adenosine receptor (AR) subtypes in total protein extracts from resected tumours, T98G and SVGp12 cells.
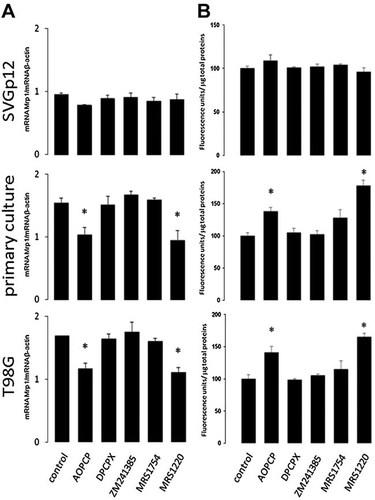
Role of adenosine receptors (AR) on Mrp1 expression and activity. Primary cultured GBM, T98G and SVGp12 cells were exposed for 24 h to AOPCP (50 µM) and the A1AR antagonist DPCPX (30 µM), A2AAR antagonist ZM241385 (10 nM), A2BAR antagonist MRS1754 (50 nM), or A3AR antagonist MRS1220 (10 µM). A: Total cellular RNA was isolated and the transcript level of Mrp1 gene was amplified by RT-PCR. The graphs depict the ratios between the amplification products of Mrp1 versus β-actin. B: The activity of Mrp1 was assayed by intracellular accumulation of fluorescent CFDA. The graphs represent the means ± SD of accumulated intracellular fluorescence units corrected for protein concentrations. Activity of controls was normalized to 100. *, P < 0.01 versus control (n = 7).
The GBM cells are chemosensitized by blocking of CD73
We know that GBM cells are highly resistant to antitumour drugs (Benyahia et al., 2004; Declèves et al., 2006; Peigñan et al., 2011). When SVGp12 cells were exposed to vincristine the cell viability was decreased for more than 50% (see Garrido et al., 2011). In contrast, the cell viability was not significantly affected in GBM cells (Fig. 7). However, primary cultured and T98G GBM cells were chemosensitised to the antitumor drug when CD73 expression was partially blocked with siRNA or when cells were treated with the inhibitor AOPCP (Fig. 7).
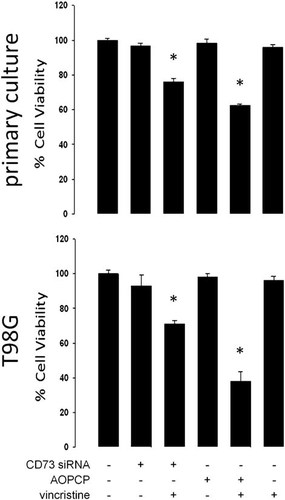
Chemosensitization of human glioblastoma multiforme cells by inhibition of CD73 activity. Primary cultured GBM or T98G cells were transfected with the siRNA-CD73 or treated with the inhibitor of CD73 activity AOPCP (50 µM). The cells were also exposed to the antitumoural drug vincristine (100 nM). Cell viability was quantified with the tetrazolium dye reduction assay (MTT) over a 24 h treatment period. The graphs represent the means ± SD. *, P < 0.01 versus control (n = 7).
Discussion
GBM cells are characterized by being extremely resistant to antitumour drugs (Lu and Shervington, 2008; Peigñan et al., 2011). Therefore, the available chemotherapy for treatment of this tumor type has, for decades, made only a very limited useful contribution. In the present study, we reveal CD73 to be a possible target for chemosensitisation of GBM cells because this enzyme may play a role as physiological regulator of the expression and activity of Mrp1. Further, we show that it is the adenosine A3 receptor that mediates this cellular response. To our knowledge, this is the first study that identifies an endogenous modulator of multiple drug resistance in GBM tumour cells. Further, it could have a high clinical impact in focus to better outcomes in the treatment of GBM.
Using immunohistochemical analyses of GBM biopsies and by studying cell lines or primary cultures of GBM cells, our research group and other authors have shown the following: (i) GBM tumour cells express increased levels of Mrp1, (ii) Mrp1 expression correlates positively with the degree of glioma malignancy, (iii) GBM cells are refractory to antiproliferative drugs mainly due to Mrp1 activity, and (iv) inhibition or blockage of Mrp1 expression considerably increases the cytotoxic and antiproliferative effect of the substrates vincristine, etoposide, and paclitaxel (Bronger et al., 2005; Calatozzolo et al., 2005; Matsumoto et al., 2005; de Faria et al., 2008; Garrido et al., 2011; Peigñan et al., 2011). In this context, reversion of the multiple drug resistance mediated by the interception of adenosine signaling may play a role in alleviating the extreme chemoresistance of GBM cells.
We have determined that extracellular metabolism is displaced towards adenosine synthesis in primary cultures of GBM cells, but this could not occur in the non-tumor SVGp12 glial cell line. ATP hydrolyzing activity was previously characterized in the C6 glioblastoma cell line of rat (Grobben et al., 1999). Bavaresco et al. (2008), also showed that glioma cells exhibit a significantly higher AMP dephosphorylation activity compared with normal astrocytes. In GBM biopsies, we detected a strong expression of CD73 in tumour tissue, which is in contrast to the slight expression seen in peritumour tissue. Expression of CD73 was first described in glioma cells (Ludwig et al., 1999) and in several types of cancer, including cancer of the breast, bladder, lung, pancreas, ovary, thyroid, oesophagus, prostate and in leukaemia and melanoma (Sadej et al., 2006; Stagg and Smyth, 2010; Stagg et al., 2010; Zhi et al., 2010). Recent research has also attributed CD73 activity as having a vital role in breast cancer progression (Zhou et al., 2007; Wang et al., 2008; Stagg et al., 2010). However, to date there is only one study that correlates the existence of chemoresistant cell lines (MDR positive) with a co-expression of elevated CD73 levels (Ujhazy et al., 1996). This might suggest a link between adenosine production and multiple drug resistance. Our own investigation of chemoresistance in primary cultured GBM cells shows that the presence of the CD73 inhibitor AOPCP, or blockage of CD73 expression with siRNA, also inhibits Mrp1 expression and leaves cells sensitive to vincristine. A recent study has described the use of a neutralizing monoclonal antibody against CD73 to block progression and pulmonar metastasis of breast cancer cells in an in vivo model (Stagg et al., 2010), suggesting a valuable tool for in vivo evaluation of the chemosensitization of GBM.
One of the criteria used for diagnosis of tumor cells in GBM biopsies, is visible evidence of necrotic areas showing intra-tumor hypoxia. It is recognized that hypoxia promotes tumor angiogenesis, cancer invasion and therapeutic resistance in GBM (Heddleston et al., 2009; Jensen, 2009). It is also well known that tissue hypoxia induces an increase in extracellular adenosine (Synnestvedt et al., 2002; Li et al., 2006). It would be interesting to be able to find a link between the particular intra-tumor environment, the activity of CD73 and adenosine signaling. Adenosine A3 receptors have a low affinity for the ligand (Fredholm et al., 2011) and thus cell effects should be triggered by an increased concentration of adenosine as occurs in hypoxic tissues.
Several studies seem to show that adenosine would have direct effects on tumor cells, favoring progression of gliomas. In general, this nucleoside may act as a proliferative factor (Morrone et al., 2003). Adenosine can induce glioma cell proliferation through an antiapoptotic effect that is inhibited by cotreatment with selective A3 AR antagonists (Taliani et al., 2010). Adenosine also modulates metalloproteinase-9 protein levels via A3 receptor activation and plays a role in augmenting cell invasion in the glioblastoma cell line U87MG (Gessi et al., 2010). Activation of the adenosine A3 receptor-Akt pathway has been previously described. It mediates inactivation of the proapoptotic protein Bad, thus protecting hypoxic cells from paclitaxel-induced apoptosis in human glioblastoma cells (Merighi et al., 2007a). The A3 receptor also increases expression of the hypoxia-inducible factor, HIF-1α, in response to hypoxia in cancer cells of the colon, in glioblastoma and in melanoma (Merighi et al., 2005, 2006, 2007b). These findings also highlight the role of the A3 receptor under conditions in which GBM cells survive a hypoxic environment.
Collectively, our data recognize a possible new target for treatment of GBM that involves the interception of adenosine production and therefore cellular signaling pathways that may mediate the chemoresistant phenotype of these cells. It suggests a novel alternative to the GBM therapy.




