A network system for vitellogenin synthesis in the mussel Mytilus galloprovincialis (L.)
Abstract
The aim of this study is to assess, by RT-PCR, in situ hybridization, electron microscopy, and immunohistochemistry, the site/s of vitellogenin (VTG) synthesis in the mussel Mytilus galloprovincialis. Our investigations demonstrate that, among the analyzed tissues, the synthesis of VTG occurs only in the female gonad, that is, within the oocyte and follicle and connective cells. Such a synthesis is just evident in early vitellogenic oocytes, whose cytoplasm is characterized by numerous RER cisternae and an extended Golgi complex surrounded by nascent yolk platelets. The synthesis of VTG goes on in vitellogenic oocytes assuming a pear form, and progressively reduces once the oocyte shows the pear or polygonal form, typical of those oocytes that have concluded the growth. The expression of VTG occurs also within follicle (auxiliary) and connective cells. In particular, it is noteworthy that follicle cells are characterized by numerous RER cisternae and an active Golgi complex surrounded by numerous vesicles and vacuoles containing electron dense material. The same material is also present along their plasma membrane, within the intercellular space between oocyte and follicle cells, and finally within invaginations of the oocyte surface, thus suggesting a VTG transfer to the oocyte via endocytosis. Differently, no VTG synthesis was observed within digestive gland. All together the findings here reported strongly suggest that in M. galloprovincialis, inside the gonad, the VTG synthesis occurs in the oocyte (autosynthesis) and in the follicle and adipogranular cells (heterosynthesis). J. Cell. Physiol. 228: 547–555, 2013. © 2012 Wiley Periodicals, Inc.
Vitellogenin (VTG) represents the most relevant molecule among those incorporated during the oocyte growth of all oviparous and ovoviviparous vertebrates, insects, worms, and other invertebrates. In vertebrates, the VTG is synthesized in the liver under hormonal regulation, and transferred to the growing oocytes via the bloodstream. Once internalized, VTG is processed by cathepsins into different polypeptides, the vitellins, that are stored inside large vacuoles, the yolk platelets (Wallace, 1985; Polzonetti-Magni et al., 2004).
Among invertebrates, VTG is synthesized in different district as the gut in nematodes (Hagedorn and Kunkel, 1979), fat body in insects and hepatopancreas in crustaceans (Whali, 1988). In molluscs the VTG synthesis, that is under the central nervous system and estrogen (E2) control (Gagné et al., 2002; Osada et al., 2003, 2004), takes place in different districts. In Mytilus edulis, based on morphological investigations, De Jong-Brink et al. (1983) and Pipe (1985) suggested that VTG was synthesized within the oocyte (autosynthesis). Autosynthesis and heterosynthesis were suggested in Crassostrea virginica; indeed, in such species (Eckelbarger and Davis, 1996) it was reported that VTG could be transferred within the oocyte from the vesicular connective tissue, a compartment surrounding the ovarian follicles (acini), in which gamete development occurs. More recently, in the scallop Patinopecten yessoensis (Osada et al., 2003, 2004) and in the abalone Haliotis discus hannai (Matsumoto et al., 2008), using immunohistochemical and molecular investigations, it was demonstrated that the VTG is synthesized within the follicle (auxiliary) cells, and therefore outside the oocyte. An heterosynthesis merges also from the assay of alkali-labile phosphate (ALP), an indirect technique utilized to determine the presence of VTG-like proteins (Blaise et al., 1999; Pampanin et al., 2005; Ricciardi et al., 2008). Indeed after ALP, the presence of vitelline-like proteins was also reported in the hemolymph of several molluscs treated with estrogen (Blaise et al., 1999; Gagné et al., 2001) or exposed to environmental contaminants (endocrine-disrupting chemicals) known to induce changes and alterations of the endocrine functions (Blaise et al., 1999; Gagné et al., 2002; Verslycke et al., 2002; Aarab et al., 2004, 2006; Marin and Matozzo, 2004; Quinn et al., 2004; Versonnen et al., 2004; Matozzo and Marin, 2005; Pampanin et al., 2005; Ortiz-Zarragoitia and Cajaraville, 2006; Zorita et al., 2006; Matozzo et al., 2008).
The aim of the present study was to elucidate the possible sites of VTG synthesis in samples of Mytilus galloprovincialis collected in the Naples Gulf. The investigations were performed on ovarian follicles containing developing oocytes from initial to final stages of the oocyte growth, that is, in stages 0–3, according to the classification of gonad developmental stages proposed by Duinker et al. (2008) (stage 0 previtellogenesis, stage 1 early vitellogenesis, stage 2 late vitellogenesis, and stage 3 full grown oocyte). Furthermore, investigations were also extended to the digestive gland, as such structure distributes nutrients to reproductive system during the oocyte growth (Gosling, 1992; Beninger et al., 2003; Dimitriadis et al., 2004).
Materials and Methods
Animals
One hundred female specimens in different phases of the reproductive cycle (stage 0, stage 1, stage 2, and stage 3 according to Duinker et al., 2008) of the mussel M. galloprovincialis were collected in the Gulf of Naples; the ovaries and digestive glands were excised using sterile RNase-free dissecting equipment. The ovaries and the digestive glands, once removed, were frozen at −80°C or fixed for 24 h in Bouin's solution and then dehydrated with graded ethanol and embedded in paraffin wax. Five micrometer thick sections were stained with Mallory's trichrome stain (Mazzi, 1977) or placed on Superfrost Plus or Polylisine glass slides (Menzel-Glaser, Braunschweig, Germany) for in situ hybridization and immunohistochemical experiments. Furthermore, part of the ovaries were used for electron microscopy.
RT-PCR
Total RNA was extracted with tri-reagent (Sigma–Aldrich, St. Louis, MO) from the gonads and digestive glands and was evaluated by 1% agarose gel electrophoresis. The RNA was then reverse-transcribed by using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA); the reaction was performed using oligo dT12–18 (Invitrogen), according to the manufacturer's protocol.
In order to amplify cDNA of VTG, we referred to the primer sequence reported by Puinean et al. (2006). The PCR thermal setting was as follows: 15 min at 95°C; 40 cycles of 30 sec at 95°C, 1 min at 58°C, 30 sec at 72°C; 7 min at 72°C. The 18S ribosomal subunit of Mytilus was used as internal control of cDNA. The PCR products were analyzed on 2% agarose gel and visualized by ethidium bromide staining.
The amplified fragment was gel eluted using the Pure Link Quick gel extraction kit (Invitrogen) according to the manufacturer's protocol, quantified, sequenced using the BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystem, Foster City, CA) and run on the ABI PRISM 310 Genetic Analyzer. The obtained nucleotide sequence was searched for similarity using BLASTn and aligned using ClustalW2.
In situ hybridization
Partial sequence of M. edulis VTG (GenBank accession number AY679116.1) cloned in pCR 2.1 TOPO vector (Invitrogen), kindly gifted by Dr. J. M. Rotchell (University of Hull, UK), was used for subcloning experiments in pGEM®-3Z vector and was linearized to perform in vitro transcription reaction. Digoxigenin (DIG)-labeled sense and antisense RNA probes from Mytilus Vtg were synthesize using RNA labeling kit (Roche, Applied Science, Penzberg, Germany), according to manufacturer's instructions. In situ hybridization was carried out on gonad and digestive gland sections, postfixed in 4% paraformaldehyde in PBS, washed in PBS and treated with proteinase K (Roche) (10 µg/ml in 20 mM Tris/HCl pH 7.0, containing 1 mM EDTA pH 7.2) at 37°C. After re-fixing with 4% paraformaldehyde, sections were washed in PBS, incubated in 0.1 M Tris/glycine buffer and then hybridized overnight at 50°C in a moist chamber in 40% formamide, dextran sulfate 20%, salmon sperm DNA 100 µg/ml, 1× Denhardt's solution, 5× SSC, 100 µg/ml tRNA (Sigma–Aldrich), and 1 ng/µl of sense or antisense Vtg digoxigenin-labeled ribo-probes. The sections were treated with 0.5× SSC, 20% formamide for 1 h at 60°C and subsequently post-hybridized with: (1) NTE (10 mM Tris–HCl pH 7.0, 0.5 M NaCl, 5 mM EDTA) at 37°C for 15 min; (2) NTE containing RNAse A (10 µg/ml) at 37°C for 30 min; (3) NTE at 37°C for 15 min; (4) 0.5× SSC, 20% formamide at 60°C for 30 min; (5) 2% Roche blocking solution in 100 mM maleic acid, 150 mM NaCl, pH 7.5 for 30 13 min. Sections were incubated overnight at 4°C in anti-digoxigenin alkaline phosphatase conjugated antibody (Roche), diluted 1:2,000 in 2% Roche blocking solution in 100 mM maleic acid, 150 mM NaCl, pH 7.5, 10% normal sheep serum (Sigma–Aldrich). Following washing in 0.1% Tween-20 and 0.5 mg/ml levamisole (Sigma–Aldrich), sections were incubated at room temperature in the developing solution, BM purple color substrate (Roche) in 2% Tween-20 and 1 mM levamisole. The reaction was stopped by rinsing the slides in 1 mM EDTA in PBS, sections were counterstained with Nuclear Fast Red (Vector, Burlingame, CA), mounted in Aquovitrex (Carlo Erba Reagenti, Rodano, Italy), and observed on a Zeiss Axioskop microscope and images were acquired by KS300 software (Zeiss, Oberkochen, Germany).
Transmission electron microscopy
For electron microscopy, samples of female gonads were immersed in Karnowsky's fixative (4% paraformaldehyde, 5% glutaraldeyde in phosphate buffer 0.1 M) for 2 h at room temperature. Tissues were then washed overnight in fresh buffer. Post-fixation was carried out in 2% osmium tetroxide in phosphate buffer for 1 h at 4°C. Following dehydration with a graded series of ethanol, the samples were transferred through two changes of propylene oxide, 5 min each at room temperature, infiltrated in 1:1 (v:v) propylene oxide:Epon, and finally embedded in Epon. Semi-thin and ultra-thin sections were cut on Reichert-Jung Supernova ultramicrotome. Semithin sections were stained with toluidine blue; ultrathin sections were stained with uranyl acetate and lead citrate, and examined under a CM 12 TEM. Electron microscope investigations were carried out at the “C.I.S.M.E” of the University of Naples Federico II.
Immunohistochemistry
Sections were dewaxed in xylene and rehydrated in graded ethanol concentration. Slides were then immersed in 0.1 M citrate buffer pH 6.0 in a microwave oven (2 × 10 min) for antigen retrieval. Endogenous peroxidases were inactivated with 2% H2O2 in methanol and non-specific background was reduced with the incubation in normal goat serum (Pierce, Rockford, IL). Sections were then incubated over night at 4°C with the primary antibody diluted 1:3,000 in normal goat serum. The primary antibody is an anti-vitellogenin of H. discus hannai, kindly gifted by Dr. Masahiko Awaji (National Research Institute of Aquaculture, Minami-Ise, Mie 516-0193, Japan). The reaction was revealed with a biotin-conjugated goat anti-rabbit secondary antibody and an avidin–biotin–peroxidase complex (ABC immunoperoxidase Kit; Pierce), using DAB as chromogen. Finally, sections were counterstained with Mayer's hemalum. Negative controls were carried out by omitting primary antibodies. Immunohistochemical signal was observed on a Zeiss Axioskop microscope; images were acquired with an AxioCam MRc5 camera and analyzed with AxioVision 4.7 software (Zeiss).
Results
RT-PCR
The amplification experiments produced a cDNA fragment of about 130 bp in length (Fig. 1). Results of BLASTn analysis of the obtained nucleotide sequence (NCBI Data Bank) revealed a high matching (97%) with known (GenBank accession number AY679116.1) Vtg sequence of M. edulis.
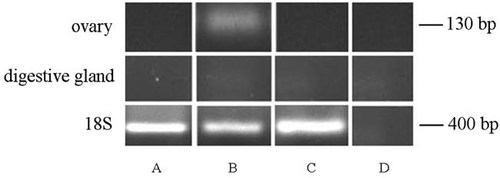
RT-PCR of Vtg in the ovary and digestive gland during the different phases of oocyte growth. The amplification product of about 130 bp is evident only in the gonad at the stages 1–2 (vitellogenesis) (B); no signal is evident in stage 0 (previtellogenesis) (A); and 3 (fully grown oocytes) (C) as well as in the digestive gland, independently from the organization of the ovarian follicle (A–C). Negative control was obtained by omitting cDNA (D). The 18S ribosomal RNA is the quality control of cDNA.
The expression of Vtg occurred only in specimens whose gonads contained follicles with oocytes in vitellogenesis (stages 1–2; Fig. 1B); differently, expression of Vtg was absent in follicles containing oocytes in stage 0, that is, in previtellogenesis (Fig. 1A), or in stage 3, that is, with fully grown oocytes (Fig. 1C). No PCR product was evident within digestive gland independently from the kind of ovarian follicles present in the ovary (Fig. 1A–C). Control PCR showed no band amplification (Fig. 1D).
Localization of VTG mRNA in ovarian follicles and in digestive gland
ISH investigations performed on ovarian follicles (Fig. 2A) showed a strong signal in the cytoplasm of oocytes and follicle cells, as well as in the cells of the connective tissue located among the ovarian follicles (Fig. 2B,C). The signal was just evident in early ovoid oocytes (stage 1; Fig. 3A,B,D); later it occurred in pear shaped vitellogenic oocytes (Fig. 3B) and finally in late vitellogenic oocytes that have acquired the typical pear shape (Fig. 3C,D; stage 2), where the signal was preferentially localized in the stalk till connected to the connective tissue trabecula (Fig. 3C,D). A strong signal was also evident within the follicle cells (Fig. 3A,B,D), that in such species are scant and did not encompass completely the oocyte (Fig. 3A′–D′). A significant signal was evident also at the level of connective tissue, in particular at the level of adipogranular cells intermingled among the acini during the stages 1–2 (Fig. 2B,C). By contrast, no signal was evident at the level of the hemocytes (Fig. 2B,C), as well as in full grown oocytes (Fig. 3E), in the connective tissue at stage 3 (Fig. 3E) and in the control sections performed using a sense probe (Fig. 3F).
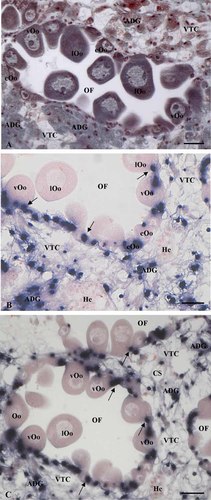
Localization of Vtg mRNA in mussel ovary by in situ hybridization. A: An ovarian follicle (OF), stained with Mallory trichrome stain, surrounded by adipogranular (ADG) and vesicular connective cells (VTC). Early (eOo), vitellogenic (vOo), and late vitellogenic (lOo) connected to the connective trabeculae bordering the ovarian follicle are present; (B,C) in situ hybridization: a strong signal, that appears as blue areas, is evident on female germ cells (eOo, vOo, lOo), follicle cells (arrows), and adipogranular connective cells (ADG). No signal is evident within hemocytes (He) and vesicular connective cells (VTC). Bars = 30 µm.
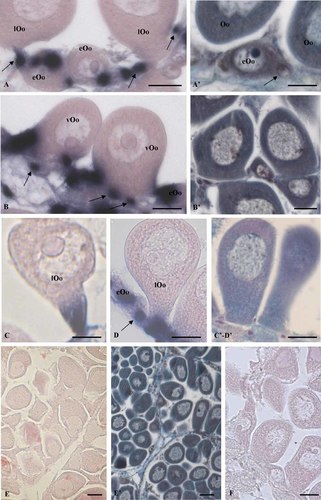
Localization of Vtg mRNA in mussel oocytes by in situ hybridization. The hybridization signal appears as blue areas (A–F). Oocytes stained with Mallory trichrome stain are also showed to put in evidence their organization at light microscope during the different phases of the growth, and the relationship with follicle cells (A′–E′). Strong positivity for Vtg mRNA is present in the cytoplasm of early vitellogenic oocytes (eOo) (A) and in the follicle cells (arrows) (A,B,D). When the oocyte growth goes on and the pear organization is progressively acquired (B,C,D), the hybridization signal is evident preferentially in the region of the oocyte facing the border of ovarian follicle (B) or in the stalk oocyte connected to the connective tissue trabeculae (C,D). No signal is observed in full grown oocyte (E) and in the control sections performed with the sense probe for VTG (F). Bars = 30 µm.
Finally, the digestive gland, that is constituted by numerous blind-ending tubules (Fig. 4A), showed no hybridization signal after ISH experiments performed with both digoxigenin (DIG)-labeled antisense (Fig. 4B,C) and sense riboprobes (Fig. 4D), independently from the organization of the ovarian follicles present within the female gonad (Fig. 4B,C).
Localization of Vtg mRNA in digestive gland by in situ hybridization (B–D). Digestive gland sections stained with Mallory trichrome stain are also showed to put in evidence the digestive gland organization (A). No signal was evident in digestive gland from mussels in stage 0 (B), stage 2 (C), and in the control sections performed using a sense probe for Vtg mRNA (D). Bars = 30 µm.
Transmission electron microscopy
Previtellogenic oocytes (stage 0), that adhered to the connective tissue trabeculae bordering the ovarian follicles, were round, lacking in secretory granules, and were bounded to a few follicular cells characterized by a few organelles (Fig. 5A). Later on (stage 1), the early vitellogenic oocytes showed an ovoid shape and were still anchored to the connective tissue trabeculae (Fig. 5B). Their ooplasm showed granules with electron dense core representing the nascent yolk platelets, as reported in other mussels (Pipe, 1985; Eckelbarger and Davis, 1996). Such granules were associated to Golgi complex and RER cisternae. As the oocyte growth goes on (Fig. 5C), the form of the oocyte was more ovoid (stage 1); the ooplasm was characterized by numerous RER cisternae and extended Golgi complexes surrounded by nascent yolk platelets (Fig. 5C). Material with the same electron density of yolk platelets was evident within cisternae of RER, vesicles, cisternae, and vacuoles of Golgi complexes (Fig. 5C and inset). The cytoplasm of follicle cells connected to the oocyte showed Golgi complex and RER cisternae (Fig. 5D and inset). No endocytotic activity was evident along the oocyte surface (Fig. 5C) and in the areas where the plasma membranes of follicle cells and the oocyte run parallel (Fig. 5D and inset). As the oocytes become peduncolated with the basal region connected to the wall of the ovarian follicle and the apical one projecting toward the lumen of the ovarian follicle (Fig. 6A,B; stage 2), the ooplasm showed a quite high number of RER cisternae, Golgi complexes, and yolk platelets connected to the apparatus of synthesis (Fig. 6A,B). In particular, Golgi complexes appeared engulfed by electron dense material evident at the level of cisternae, vesicles, as well as vacuoles located in trans Golgi face (Fig. 6C). It is interesting to note that several vacuoles showed an irregular surface due to the confluence of vesicles containing electron-dense material (Fig. 6B,C). Endocytotic activity was now evident along the oocyte plasma membrane, that runs parallel with that of follicle cell (Fig. 6D), as well as in basal region (Fig. 7A,B). In the latter one, in particular, numerous endocytotic pictures containing electron dense material were evident along the oocyte surface, that in this region was lacking in vitelline envelope (Fig. 7A,B). In late vitellogenic oocytes (early stage 3), the female germ cells showed the typical peduncolated shape (Fig. 8A). The follicle cells were still evident along the oocyte surface (Fig. 8A,B) and in basal region (Fig. 8D,E). The cytoplasm of the follicle cells located around the oocyte showed an extended Golgi complex and RER cisternae surrounded by vesicles and vacuoles containing electron dense material (Fig. 8B). The same material was also evident along the follicle cell surface inside and outside the cells (Fig. 8A,B). In the oocyte cortex of the peduncolated oocyte one could observe the presence of vesicles, next to vacuoles containing moderate electron density (Fig. 8C). By contrast, follicle cells located in the basal region showed a few organelles within their cytoplasm (Fig. 8D,E). The oocyte surface in such region was now characterized by a few endocytotic vesicles (Fig. 8E). Finally, it is interesting to note that the ooplasm present in basal region showed RER cisternae and Golgi complexes surrounded by yolk platelets (Fig. 8D). The remaining cytoplasm present in the apical region showed yolk platelets, lipid droplets, and a few Golgi complexes.

Developing oocytes at transmission electron microscope (stages 0–1). AL Previtellogenic oocyte connected to a follicle cell (stage 0). Note the presence of a few organelles in the cytoplasm of the follicle cell (FC) and the oocyte (Oo). Bar = 5 µm. B: An early vitellogenic oocyte (eOo) ovoid-shaped anchored to the connective tissue trabeculae (T) (stage 1). Note the presence of the yolk granules (Y) surrounded by RER cisternae. Bar = 5 µm. C) An ovoid early vitellogenic oocyte (eOo) connected to a follicle cell (FC) (stage 1). The cytoplasm of the oocyte is characterized by numerous Golgi complexes (GC) located next to the nucleus (N), RER cisternae, and yolk platelets (Y). No endocytotic vesicles are evident along the oocyte surface. Bar = 5 µm. Inset: Higher magnification at the level of Golgi complexes. Note the presence of nascent yolk platelets (arrowheads) in the trans Golgi face. Note also as the margin of yolk platelets is irregularly lined, suggesting that they are forming. Bar = 2 µm. D: Early ovoid oocyte (eOo) connected to a follicle cell (FC) (stage 1). The ooplasm is rich in RER and Golgi complexes. The same organelles are present within the follicle cell. Bar = 5 µm. Inset: Higher magnification of (D). Note the presence of an extended system of RER cisternae and the absence of endocytotic vesicles along the contact area between the follicle cell and the oocyte. Y = yolk platelets. Bar = 2 µm.
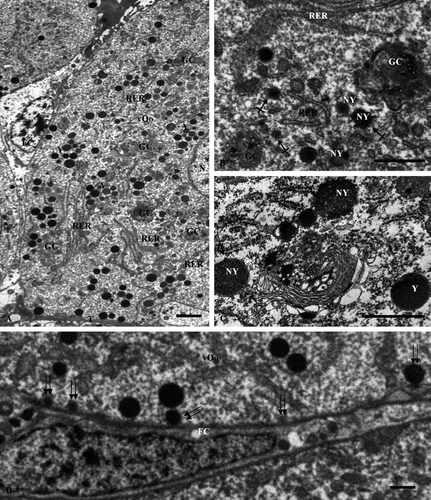
Vitellogenic oocyte at transmission electron microscope (stage 2). A: It is evident a vitellogenic oocyte (vOo) attached to the connective tissue trabeculae (T), that is assuming the typical pear shape. Note the well extended apparatus of protein synthesis represented by numerous RER cisternae and Golgi complexes (GC). Yolk platelets (Y) are evident all over the cytoplasm. A follicle cell (FC) is also evident. N = nucleus. Bar = 5µm. B: RER cisternae and Golgi complexes intermingled with numerous nascent yolk platelets (NY): the NY surface is irregularly lined for the confluence of small vesicles (arrows with bar) containing electron dense material. Bar = 5 µm. C: Golgi complex surrounded by numerous vacuoles containing electron dense material. Note the presence of both electron-dense material, similar to that present within yolk platelets, inside the cisternae of Golgi complex; several vacuoles different in size representing nascent yolk platelets (NY) are also evident. Bar = 5 µm. D: A follicle cell (FC) located next to a vitellogenic oocyte (vOo). Numerous endocitotic vesicles (double arrows) containing electron dense material are evident along the oocyte surface. The same electron dense material is evident within follicle cell. Bar = 2 µm.
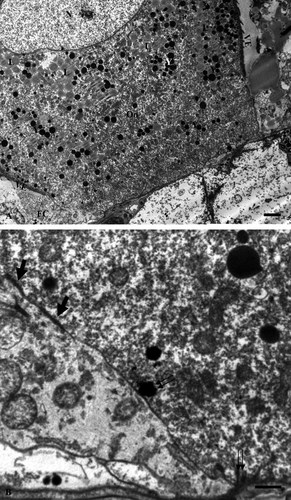
Vitellogenic oocyte at transmission electron microscope (stage 2). A: Low magnification of a vitellogenic oocyte (vOo) at the level of basal region showing numerous yolk platelets (Y) and liposomes (L). A follicle cell (FC) is also evident. Note also the typical absence of vitelline envelope (VE) in the basal region. B: Higher magnification of the basal region at the level of area lacking in vitelline envelope. Numerous endocytotic pictures (double arrows) are evident along the oocyte surface that is lacking in vitelline envelope. Note the presence of electron-dense material (large arrows) in the intercellular space between the oocyte and follicle cell surface FC = follicle cells; Y = Yolk platelets. Bars = 5 µm.
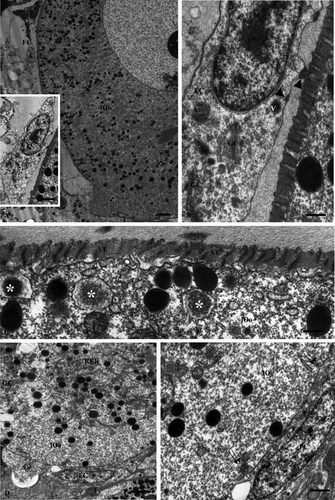
Late vitellogenic oocyte (early stage 3). A: Low magnification of a late vitellogenic oocyte (lOo) and a follicle cell (FC). The typical pear form of the oocyte is evident. The basal region is anchored to the trabeculae (T) of the connective tissue. The apical region is characterized by a lot of yolk platelets. Bar = 10 µm. Inset. Higher magnification of the follicle cell (FC). Bar = 5 µm. B: Further magnification of follicle cell in (A). The cytoplasm of follicle cell shows an extended apparatus of synthesis characterized by the presence RER cisternae, Golgi complexes (GC), and vacuoles (V) containing an electron dense material. The same material is evident next the plasma membrane inside and outside the cell (arrowheads). Bar = 2 µm. C: The oocyte cortex shows a moderate numbers of surface invaginations and vesicles suggesting an endocytotic activity. Note also the presence of different vacuoles irregularly lined (asterisks) containing a material with moderate electron density. VE = vitelline envelope. Bar = 2 µm. D: Basal region of a late vitellogenic oocyte in contact with the ovarian follicle wall (T). Note the presence of RER cisternae and Golgi complexes surrounded by numerous yolk platelets. Bar = 5 µm. E: Higher magnification of (D). Moderate endocytotic activity is evident at the level of the oocyte surface; note also that the follicle cell cytoplasm is poorly represented. Bar = 2.5 µm.
Immunohistochemistry
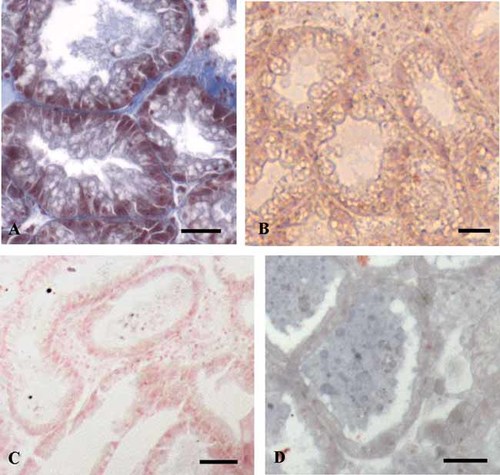
Immunohistochemistry revealed that VTG was widely distributed in both germ and somatic cells of ovary (Fig. 9A,B); differently no signal was evident within digestive gland (Fig. 9C), as well as in the control sections performed omitting the primary antibody (Fig. 9D). In particular, in the ovary, a positive reaction was observed within growing germ cells and in follicle and adipogranular cells (Fig. 9A,B.). No immunohistochemical signal was evident within vesicular connective cells (Fig. 9A), according to the in situ hybridization.
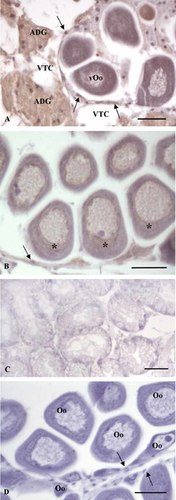
Distribution of vitellogenin in the ovary and digestive gland of Mytilus galloprovincialis. Ovary and digestive gland sections were incubated with anti-VTG antibody. A: An ovarian follicle containing vitellogenic oocytes (vOo) surrounded by connective cells. The immunolocalization signal, that appears as brown areas, occurs only in adipogranular cells (ADG), follicle cells (arrows), and in the vitellogenic oocytes; no immunohistochemical signal is evident in vesicular connective cells (VTC). Bar = 30 µm. B: An ovarian follicle containing full grown oocytes. A positive signal is evident all over the cytoplasm of the oocytes, in particular in the juxta-nuclear area (*). Arrow: A follicle cell positive to antibody anti-VTG. Bar = 30 µm. C: Digestive gland tubules. No immunohistochemical signal is evident. Bar = 40 µm. D: Control section performed omitting the primary antibody: no immunohistochemical signal is evident within oocytes (Oo) and follicle cells (arrows). Bar = 30 µm.
Discussion
The mussels, as well as the teleosts, are organisms used to determine the pollution stage in marine environment. In fact, these animals are sessile, filter large amounts of water every day and accumulate large amounts of toxic substances since they have little power to bioremediation. Hence in recent times they are considered sentinel species of the environmental pollution. To assess the presence of this toxic substances, mainly xeno-estrogens, the VTG, an estrogen dependent protein, represents one of the most widely sought biomarker (Blaise et al., 1999; Riffeser and Hock, 2002; Marin and Matozzo, 2004; Porte et al., 2006).
Unfortunately, the investigations so far carried out to characterize this protein in mussels and shellfish in general are still very scant (Matsumoto et al., 2003, 2008; Osada et al., 2003, 2004) and are entirely absent in M. galloprovincialis. On the other hand, this species could be used as sentinel to detect the state of pollution by xeno-estrogens in the Gulf of Naples, where such species is highly widespread.
We demonstrate that in M. galloprovincialis the expression of VTG mainly occurs within the gonad at the level of the oocyte, and the follicle and adipogranular cells. In the oocytes, in situ hybridization showed that the expression of VTG begins early and stops when the oocytes are ready to be released into the lumen of the ovarian follicle. TEM observations corroborate such a result as early vitellogenic oocytes, ovoid in shape, and vitellogenic oocytes, that are assuming a pear shape, show an extended synthesis apparatus constituted by numerous RER cisternae, extended Golgi complexes associated to nascent yolk platelets. These results suggest the presence of an autosynthesis of VTG, that takes place inside the oocyte. Besides the autosynthesis, we demonstrate the presence of an heterosynthesis, too, involving both follicle and adipogranular cells. Indeed, our investigation demonstrates that the follicular cells express mRNA for VTG suggesting that these cells contribute to VTG synthesis as previously reported in Patinopecten (Osada et al., 2003, 2004) and in Haliotis (Matsumoto et al., 2008). However in our opinion the contribution by follicle cells in VTG synthesis in M. galloprovincialis appears to be less relevant than that of the oocyte, specially when this assumes the typical pear shape, that is, during the 1–2 stages. In fact, although the highest number of endocytosis pictures observed during this phase suggest a transfer of VTG from the follicle cells to the oocyte, the number of follicle cells is so limited that their contribute to the oocyte growth might be less important.
We show also that a contribution to the VTG synthesis may come from the adipogranular cells that surround the ovarian follicles during the stages 1–2, as reported in C. virginica (Eckelbarger and Davis, 1996). Indeed in M. galloprovincialis, at the level of these cells a strong hybridization signal as well as a positive reaction to anti-VTG antibody was found. Therefore one can hypothesizes that the VTG, once synthesized, is transferred into the hemocoel that surrounds the ovarian follicles containing the growing oocytes, and/or the hemolymph and then the VTG is taken up by endocytosis by the oocytes, in particular at the level of the basal region, where the number of endocitotic pictures observed was very high in vitellogenic oocytes. At this regard, it must be remembered that in M. galloprovincialis some authors (Pampanin et al., 2005; Ricciardi et al., 2008) reported the presence of VTG into hemolymph, suggesting that VTG could be synthesized in extra-ovarian sites, that is, adipogranular cells. In any case, no contribution to the oocyte growth in M. galloprovincialis seems to be ruled out by the digestive gland. In fact, in our experimental conditions, we have found no positive ISH, RT-PCR, and immunohistochemical signal at the level of digestive gland. On the other hand, the presence of VTG mRNA detected by RT-PCR only in the gonad of M. galloprovincialis confirms that VTG is expressed in the gonad. Moreover, such an evidence is corroborate by the immunoistochemical observations that reported the presence of VTG within germ, adipogranular and follicle cells, but not within digestive cells.
In conclusion, our data demonstrate for the first time that in M. galloprovincialis the gonad is the site of VTG synthesis that occurs both into the oocyte (autosynthesis) and into the follicle and adipogranular cells (heterosynthesis).
Acknowledgements
We thank Dr. J. M. Rotchell (University of Hull HU6 7RX UK) for the gift of partial sequence of Mytilus edulis VTG and Dr. Masahiko Awaji (National Research Institute of Aquaculture, Minami-Ise, Mie 516-0193, Japan) for the gift of the anti-vitellin antibody. This research was supported by a grant of University of Naples Federico II (F.A.R.O 2011 to Dr. S. Aceto) and MIUR PRIN 2010-2011 to Prof. G. De Vico.




