Artesunate induces apoptosis via a Bak-mediated caspase-independent intrinsic pathway in human lung adenocarcinoma cells
Abstract
This report is designed to explore the exact molecular mechanism by which artesunate (ART), a semisynthetic derivative of the herbal antimalaria drug artemisinin, induces apoptosis in human lung adenocarcinoma (ASTC-a-1 and A549) cell lines. ART treatment induced ROS-mediated apoptosis in a concentration- and time-dependent fashion accompanying the loss of mitochondrial potential and subsequent release of Smac and AIF indicative of intrinsic apoptosis pathway. Blockage of casapse-8 and -9 did not show any inhibitory effect on the ART-induced apoptosis, but which was remarkably prevented by silencing AIF. Of the utmost importance, ART treatment induced the activation of Bak but not Bax, and silencing Bak but not Bax remarkably inhibited ART-induced apoptosis and AIF release. Furthermore, although ART treatment did not induced a significant down-regulation of voltage-dependent anion channel 2 (VDAC2) expression and up-regulation of Bim expression, silencing VDAC2 potently enhanced the ART-induced Bak activation and apoptosis which were significantly prevented by silencing Bim. Collectively, our data firstly demonstrate that ART induces Bak-mediated caspase-independent intrinsic apoptosis in which Bim and VDAC2 as well as AIF play important roles in both ASTC-a-1 and A549 cell lines, indicating a potential therapeutic effect of ART for lung cancer. J. Cell. Physiol. 227: 3778–3786, 2012. © 2012 Wiley Periodicals, Inc.
Abbreviations:
ART, artesunate; CCK-8, cell counting kit-8; Cyt.c, cytochrome c; DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate; FCM, flow cytometry; FITC, fluorescein isothiocyanate; MOMP, mitochondrial outer membrane permeabilization; NAC, N-acetyl-cysteine; PI, propidium iodide; ROS, reactive oxygen species; shRNA, short hairpin RNA; STS, staurosporine; VDAC2, voltage-dependent anion channel 2.
The intrinsic pathway of apoptosis in vertebrates begins with the mitochondrial outer membrane permeabilization (MOMP), in which the activation of Bax/Bak is an important event (Wei et al., 2001). There are two models referring to their activation: direct and indirect activation model. In “direct model,” activators (i.e., Bim and Bid) directly bind to Bax or Bak and initiate their oligomerization. In “indirect model,” activated BH3-only proteins (i.e., Bad and Noxa) bind to antiapoptotic Bcl-2 proteins and neutralize their activity, leading to release of Bax and Bak and subsequent Bax and/or Bak homo-oligomerization into proteolipid pores (Chipuk and Green, 2008). Normally, Bak is inserted in the mitochondrial outer membrane (MOM), whereas Bax is predominantly cytosolic with a minor population loosely attached to the MOM (Westphal et al., 2011). Upon apoptosis induction, Bax undergoes a conformational change to expose its N-terminus which can insert into the MOM, subsequently translocates to the MOM, whereas Bak changes its conformation and directly leads to the MOMP (Westphal et al., 2011). Bax is reported to play a major or even entirely role in apoptosis upon stimuli and cell types (Lee et al., 2008; Zhang et al., 2009; Karlberg et al., 2010). However, Neise et al. (2008) reported that diverse apoptotic stimuli preferentially engaged the Bak pathway. Some reports showed that Bcl-xl, Mcl-1, Puma and Bim are involved in Bak-dependent cell death program (Cheng et al., 2001; Gélinas and White, 2005; Ren et al., 2010). In addition, mitochondrial voltage-dependent anion channel 2 (VDAC2) has been shown to interact with, and stabilize Bak as an inactive monomer in the MOM (Ren et al., 2009; Lazarou et al., 2010). Death signals activate “BH3-only” molecules such as tBid, Bim, or Bad, which displace VDAC2 from Bak, enabling homo-oligomerization of Bak and apoptosis (Cheng et al., 2003).
After MOMP, a number of apoptotic proteins, including cytochrome c (Cyt.c), AIF, EndoG and Smac, release from the mitochondrial intermembrane space into the cytosol. Cyt.c and Smac mainly mediated caspase-dependent apoptotic pathway by activating caspase-9 and -3 (Fandy et al., 2008; Autret and Martin, 2009), whereas AIF and EndoG-mediated caspase-independent apoptotic pathway (Gallego et al., 2004; Kim et al., 2008; Thayyullathil et al., 2008). Many reports demonstrate that AIF plays a central role in caspase-independent apoptosis (Liptay et al., 2002; Lorenzo and Susin, 2007; Seiler et al., 2008; Li et al., 2012). AIF is a 62-kDa flavoprotein with NADH oxidase activity that is anchored to the inner mitochondrial membrane (Hangen et al., 2010). After apoptotic stimulation, AIF is cleaved to be a soluble 57-kDa fragment that can release from the mitochondria and directly translocate to the nucleus, where it promotes chromatin condensation and large-scale DNA fragmentation (Lorenzo and Susin, 2007).
Artesunate (ART) is one of semisynthetic derivatives of artemisinin isolated from the traditional Chinese herb Artemisia annua L (qinghao, sweet wormwood; Xu et al., 2007). A number of publications have shown that ART possesses profound cytotoxicity against tumor cells in vitro and in vivo (Efferth et al., 2007; Du et al., 2010; Michaelis et al., 2010; Rasheed et al., 2010; Sertel et al., 2010). Rasheed et al. (2010) found that ART inhibited invasion and in vivo metastasis in non-small cell lung cancer (NSCLC) that accounts for approximately 80–85% of all cases of lung cancer. ART treatment can induce many gene expression imbalance in cancer cells, such as drug resistance genes, DNA damage response and repair genes, apoptosis-regulating genes and so on (Efferth et al., 2001, 2003a). Thioredoxin reductase and catalase are correlated to the cytotoxicity of ROS generated by ART (Efferth et al., 2003b). The level of γ-H2AX indication of DNA double-strand breaks increased after ART treatment in a dose-dependent manner, indicating ART-induced DNA breakage (Li et al., 2008). Transcription factors, such as C-Myc, Max, NFκB, and AP-1, may be transcriptional regulators for downstream genes determining the response of cancer cells towards ART (Sertel et al., 2010; Bachmeier et al., 2011). Mitochondrial apoptotic pathway involving ROS generation, Bcl-2 down-regulation, Bax activation, Cyt.c release, and caspase-3 activation have been reported to regulate ART-induced apoptosis in various cell lines (Efferth et al., 2007; Hamacher-Brady et al., 2011; Wang et al., 2011). Recent years, the semisynthetic derivatives of artemisinin, like ART and dihydroartemisinin, combined with ionizing radiation also showed a potential benefit in treatment strategies (Chen et al., 2009; Handrick et al., 2010; Zhao et al., 2011). However, the exact molecular mechanism of ART-induced apoptosis in human lung adenocarcinoma (ASTC-a-1 and A549) cell lines is still unclear.
The aim of this study is to dissect the details of apoptotic signaling induced by ART in both ASTC-a-1 and A549 cell lines. Our data firstly demonstrate that Bak but not Bax regulates MOMP and subsequent AIF release to dominate the ART-induced apoptosis.
Materials and Methods
Materials
ART (purity = 99.99%) was purchased from Bide Pharmaceutical Corporation (Guangzhou, China). Caspase-3 substrate (Ac-DEVD-AFC), caspase-8 substrate (Ac-IETD-AFC), and caspase-9 substrate (Ac-LEHD-AFC) were purchased from Alexis Biochemicals (Lausen, Switzerland). 2, 7-dichlorodihydrofluorescein diacetate (DCFH-DA) was purchased from Wako Ltd (Osaka, Japan). N-acetyl cysteine (NAC), Hoechst 33258, RNase A and propidium iodide (PI) were obtained from Sigma (St. Louis). Rabbit polyclonal anti-Bak, anti-Mcl-1 and mouse monoclonal anti-Bcl-xl were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit monoclonal anti-Smac/Diablo (clone Y12) antibody was from Millipore (Temecula). Mouse monoclonal anti-cytochrome c (7H8.2C12) antibody was purchased from Novus Biologicals (CO). Mouse monoclonal anti-Bak (Ab-2) and anti-Bax (Ab-6) were purchased from Calbiochem (San Diego, CA). Rabbit monoclonal anti-AIF antibody, anti-Bim antibody, anti-Cox IV (3E11) antibody, and mouse polyclonal anti-β-actin were all obtained from Cell Signaling Technology (Danvers). Rabbit polyclonal anti-VDAC2 (ab47104) was purchased from abcam (Cambridge, UK). Z-IETD-fmk (caspase-8 inhibitor), z-DQMD-fmk (caspase-3 inhibitor) and z-LEHD-fmk (caspase-9 specific inhibitor) were purchased from BioVision (San Francisco). Secondary goat anti-rabbit IgG conjugated to IRDye 800 antibody and secondary goat anti-mouse IgG conjugated to IRDye 680 antibody were supplied by Rockland Immunochemicals (Gilbertsville). Secondary goat anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC) antibody was purchased from Invitrogen (Carlsbad).
Cell culture and transfection
ASTC-a-1 and A549 cell lines obtained from the Department of Medicine, Jinan University (Guangzhou, China) were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco, Grand Island) supplemented with 10% fetal calf serum. Cell cultures were maintained at 37°C in a humidified 5% CO2 incubator. Plasmid GFP-cytochrome c (GFP-Cyt.c) was provided by Dr. G. J. Gores (Takikawa et al., 2001). GFP-Bax was provided by Prof. R. J. Youle (Nechushtan et al., 1999). DsRed-Mit was provided by Prof. Y. Gotoh (Tsuruta et al., 2002). Cells were transiently transfected with plasmids using Turbofect™ in vitro transfection reagent (Ferments, MD). ART was dissolved in DMSO (<1%, final concentration).
Short hairpin RNA (shRNA) transfection
For silencing experiments, ASTC-a-1 and A549 cells were seeded at 1 × 105 cells per well in 24-well plates and allowed to reach ∼50% confluence on the day of transfection. Cells were transiently transfected with 0.5–0.8 µg Bak, Bax, Mcl-1, AIF, Bim, Puma, VDAC2 shRNA, or negative control shNC (GenePharma, Shanghai, China), respectively, using Turbofect™ siRNA transfection reagent (Ferments) according to the manufacturer's transfection protocol. Twenty-four hours after transfection, cells were split into 96-well plates for detection of cell viability, and six-well plates for analysis of Bak and Bax conformational change using FCM. Efficiency of shRNA was measured by Western blotting analysis.
Assay of cell viability and apoptosis
Cell viability was assessed by Cell Counting Kit-8 (CCK-8, Dojindo, Japan) assay as described previously (Lu et al., 2010). All experiments were performed in quadruple occasions.
Cell apoptosis detection was examined by two approaches: Hoechst33258 staining and annexin V/PI dual staining. For Hoechst33258 staining, briefly, cells were grown on the coverslip of a chamber. After being washed with PBS once, cells were stained with 10 µg/ml Hoechst33258 for 20 min at 37°C in a humidified 5% CO2 incubator. The cells were then washed three times with PBS and visualized under a Zeiss fluorescent microscope (Axiovert 200 M). The images of Hoechst 33258 were recorded using a digital camera (Nikon, Tokyo, Japan) with 1,280 × 1,280 pixels resolution. Cell apoptosis detection was performed by flow cytometry (FCM, FACSCanto II, BD, NJ) analysis using Annexin V-FITC/PI apoptosis detection kit (Bender Medsystems, Vienna, Austria) as previously described (Lu et al., 2010) and 10,000 events were recorded for each FCM analysis. In addition, DNA content was also measured by FCM analysis using PI staining. The cells were harvested, washed twice with PBS, and then fixed in 70% ethanol at 4°C for 1 h and centrifuged. The pellets were treated with RNase (20 µg/ml) at room temperature for 30 min and then incubated with PI (50 µg/ml) for 30 min. PI fluorescence of nuclei was measured by FCM. Cells with a DNA content less than G0/G1 (hypodiploid) were defined as apoptotic cells (Hao et al., 2004).
Determination of intracellular ROS
Intracellular ROS production was determined by using DCFH-DA, which is cleaved intracellularly by non-specific esterase and becomes highly fluorescent 2, 7-dichlorofluorescein (DCF) upon oxidation by ROS. Cells were resuspended in 500 µl of PBS containing 5 µM DCFH-DA for 15 min in the dark and subsequently assayed by FCM.
Confocal microscopy imaging of Bax distribution inside living cells
Bax distribution was assessed using confocal fluorescence microscopy (LSM510; Zeiss, Jena, Germany). Cells transfected with GFP-Bax were treated with 100 µM ART for 36 h or 1 µM staurosporine (STS) for 6 h. All quantitative analysis of the fluorescence images was performed by Zeiss Rel3.2 image processing software (Zeiss). Excitation wavelength and detection filter settings for each of the fluorescent indicators were as follows. GFP was excited at 488 nm and fluorescence emission was recorded through a 500–550 nm band-pass filter. DsRed was excited at 543 nm with a helium–neon laser and emitted light was recorded through a 600–650 nm band-pass filter.
FCM analysis of the activation of Bak and Bax
Cells were grown in six-well dishes and treated with different conditions, then were harvested and fixed with 4% formaldehyde in PBS for 10 min at 37°C. Afterwards, the cells were treated with ice-cold 100% methanol for 10 min to permeabilize the cells. Fixed cells were blocked in PBS solution containing 1% bovine serum for 10 min at room temperature and then were incubated with either anti-Bak (Ab-2) or anti-Bax (6A7) (1:50) on room temperature for 60 min and then incubated with FITC-conjugated goat-anti-mouse IgG (1:200) for 30 min in the dark. After washing, the samples were analyzed by FCM. The results for each condition were calibrated by values for cells stained with mouse IgG as the primary antibody. Values for untreated controls were arbitrarily set to 100%. In parallel, cells for each condition were stained with antibodies to total Bak or Bax for comparison.
Quantitation of mitochondrial membrane potential (ΔΨm)
Rhodamine 123 (Rho 123; Sigma) was used to analyze ΔΨm by FCM as previously described (Lu et al., 2010). Briefly, cells were harvested and stained with 10 µM Rho 123 for 30 min at 37°C in the dark, and then washed with PBS twice and subsequently assayed by FCM. Results were expressed as the proportion of cells with low Rho123 fluorescence indicating the loss of ΔΨm.
Fluorometric assay for caspases activity
PBS-washed cell pellets were resuspended in extract buffer (25 mM HEPES (pH 7.4), 0.1% TritonX-l00, 10% glycerol, 5 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 10 mg/ml pepstatin, and 10 mg/ml leupeptin) and assay of activity of caspases was performed as previously described (Lu et al., 2010). Briefly, protein samples were incubated with the fluorogenic substrates Ac-IETD-AFC, Ac-LEHD-AFC, and Ac-DEVD-AFC of caspases, all at 100 µM final concentrations for 2 h at 37°C, and caspase activity was measured by monitoring the release of fluorogenic AFC using an auto microplate reader (infinite M200, Tecan, Austria) with 400 nm of excitation and 530 nm of emission. Caspase-like activity is reported as the ratio of the fluorescence output in treated samples relative to controls.
Western blotting analysis
Cells were lysed in lysis buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% Triton-100, 1 mM PMSF, and protease inhibitor cocktail set I). After removing insoluble material by centrifugation for 5 min at 12,000g, the protein concentration was estimated in the supernatant using the Bio-Rad protein assay (Bio-Rad, Munich, Germany) according to the manufacturer's protocol. Protein was separated by SDS–PAGE under reducing conditions before transferring onto nitrocellulose membranes (Millipore, Billerica). Blots were blocked in TBST buffer containing 5% non-fat dry milk for 1 h at room temperature. The membrane was incubated overnight at 4°C with the respective primary antibodies. After repeated washings with TBST, the membranes were incubated with the secondary antibody for 1 h at room temperature before continuing to wash with TBST. Detection was performed using the Odyssey Infrared Imaging System (LI-COR Biosciences, Nebraska). Equal loading was verified by antibodies against Cox IV, or β-actin.
Preparation of the mitochondrial/cytosol fraction and immunoblot analysis
For mitochondria isolation, cells were harvested and then fractionated using cytosol/mitochondria fractionation kit (Merch, Bad Soden, Germany) according to the supplier's recommendations. The cytosolic extract and mitochondria were then subjected to Western blotting analysis of Cyt.c, Smac/Diablo and AIF, respectively. The purity of fractions was tested by immunoblotting with specific antibodies for the cytosolic proteins β-actin or the mitochondrial proteins Cox IV.
Immunofluorescence staining
For immunofluorescence, cells were grown on coverslips and treated as indicated. Cells were collected and then fixed with 3.7% paraformaldehyde in PBS. Afterwards, the coverslips were treated with ice-cold methanol for 10 min to permeabilize the cells. Coverslips were then blocked in PBS solution containing 1% bovine serum for 1 h at room temperature, and then incubated with Mouse monoclonal anti-cytochrome c, rabbit monoclonal anti-AIF (1:200) overnight at 4°C. After three PBS rinses, coverslips were incubated for 2 h in the dark with a 1:100 dilution of goat anti-rabbit IgG conjugated to FITC. Samples were examined using confocal microscope (LSM510; Zeiss). The excitation wavelength was 488 nm, and the emission channel was 500–550 nm for FITC.
Statistical analysis
Results were expressed as mean ± SD of triplicates. Data were analyzed by repeated-measures ANOVA with parametric methods and LSD multiple comparison using the statistical software SPSS 10.0 (SPSS, Chicago). Two-tailed Student's t-test was also performed where appropriate. Throughout the work, P-values < 0.05 were considered to be statistically significant.
Result
ART induces ROS-mediated apoptosis
After exposure of cells to different concentration (0, 10, 30, 40, 50 µg/ml) of ART for 48 h, the cell viability was analyzed by CCK-8 assay. Our data showed that ART exerted a dose-dependent cytotoxicity (Fig. 1A). In what follows, 40 µg/ml of ART was selected for our experiments without indicated concentration. Moreover, a time-dependent cytotoxicity of ART was shown by 0, 12, 24, and 48 h treatment (Fig. 1B). We also found that ART elicited a rapid ROS generation by FCM analysis (Supplementary Fig. S1). Pretreatment with NAC, a ROS scavenger, for 1 h before ART treatment was found to largely attenuate the ART-induced cytotoxicity (Fig. 1C), implying that ROS played an important roles in ART-induced cytotoxicity.
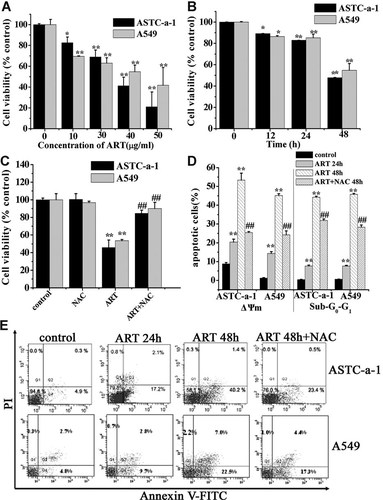
ART induces ROS-dependent apoptosis. A,B: ART-induced concentration- and time-dependent reduction of cell viability assessed by CCK-8 assay. Cells were incubated with indicated concentrations of ART for 48 h (A) or with 40 µg/ml ART for indicated time (B). *P < 0.05 and **P < 0.01, compared with control. C: ART induced ROS-mediated cell cytotoxicity determined by CCK-8 assay. Cells were treated with ART for 48 h in the presence or absence of NAC (5 µM). **P < 0.01, compared with control; ##P < 0.01, compared with ART treatment alone. D: Quantification of ART-induced breakdown of ΔΨm and DNA fragmentation assessed by FCM analysis. Cells were treated with ART for indicated time in the presence or absence of 5 µM NAC. **P < 0.01, compared with control; ##P < 0.01, compared with ART treatment alone. E: Flow cytometry (FCM) analysis of ART-induced apoptosis by fluorescence-activated sorting analysis of cells with AnnexinV-FITC/PI. Cells were treated with ART for 24 and 48 h, respectively.
To confirm whether the form of ART-induced cell death associate with apoptosis, we performed FCM analysis to investigate the changes of ΔΨm and DNA fragmentation of cells treated with ART for 24 and 48 h. Our data showed that ART induced significant loss of ΔΨm and DNA fragmentation, which were largely prevented by NAC pretreatment (Fig. 1D). In addition, anneixin V/PI staining was used to further characterize the form of ART-induced cell death, PS externalization, a hallmark of apoptotic cells, was detected. As shown in Figure 1E, ART treatment for 48 h induced notably PS externalization (Annexin V-positive), which was significantly attenuated by NAC pretreatment.
Collectively, these data demonstrate that ART induces ROS-mediated apoptosis.
AIF plays dominant role in ART-induced apoptosis
Western blotting analysis showed that ART-induced remarkable release of AIF and Smac but not Cyt.c from mitochondria (Fig. 2A), which was also further verified by immunofluorescence imaging (Fig. 2B and Supplementary Fig. S2). ART-treated cells presented significant translocations of AIF to nucleus at 36 h (Fig. 2B). To ascertain the role of AIF in ART-induced apoptosis, we used CCK-8 assay to assess the effect of silencing AIF by shAIF on ART-induced cytotoxicity. Western blotting analysis demonstrated that shAIF potently down-regulated the expression level of AIF (Supplementary Fig. S3A). Our data showed that silencing AIF largely attenuated ART-induced cell death compared with the cells treated with ART alone (Fig. 2C). These findings suggest that AIF plays an important role in ART-induced apoptosis.
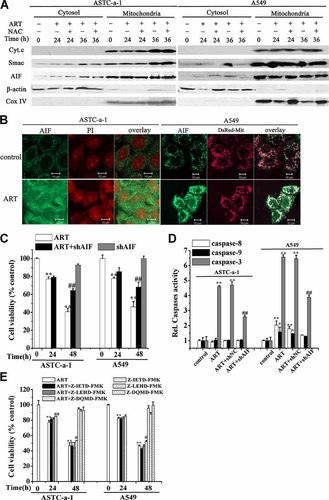
AIF plays a dominant role in ART-induced apoptosis. A: ART induced the release of AIF and Smac but not Cyt.c by Western blotting analysis. Cells were treated with ART in the presence or absence of NAC for 24 or 36 h, then the cytosolic and mitochondrial proteins were extracted using a mitochondria/cytosol fractionation kit and then analyzed by Western blotting analysis using antibodies against AIF, Cyt.c, Smac, CoxIV, and β-actin. As control for loading, β-actin was used in the cytosolic fraction and Cox IV in the heavy membrane fraction. B: Confocal fluorescence images of cells determined by immunofluorescence staining. Cells were treated with ART for 36 h and then subjected to immunofluorescence staining with a primary antibody against AIF followed by FITC conjugated secondary antibody. The cells were transfected with DsRed-Mit before ART treatment to fluorescently label mitochondria or stained with PI for monitoring the translocation of AIF to nucleus. Scale Bar: 10 µm. C: Silencing AIF largely attenuated ART-induced cell death assessed by CCK-8 assay. Cells were treated with ART for 24 and 48 h, respectively. The shAIF was used as a negative control. **P < 0.01, compared with control cells; ##P < 0.01 compared with ART treatment alone. D: Fluorescent substrate analysis of caspase-8, -9, and -3 activity for control and ART-treated cells in the absence or presence of silencing AIF. Cells were treated with ART for 48 h. ShNC was used as negative control. *P < 0.05 and **P < 0.01, compared with control; ##P < 0.01, compared with ART treatment alone. E: The effects of z-IETD-fmk, z-LEHD-fmk or z- DQMD-fmk on the ART-induced apoptosis assessed by CCK-8 assay. Cells were preincubated for 1 h with 10 µM of selective peptide inhibitors for caspase 8, 9, and 3 before treated with ART for 24 and 48 h, respectively. **P < 0.01, compared with control; #P < 0.05 and ##P < 0.01, compared with ART treatment alone. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
To determine the roles of caspase-8, -9, and -3 in ART-induced apoptosis, we used fluorometric substrate assay to detect the activity of caspase-8, -9, and -3. We found that ART did not induce a significant increase in the activity of caspase-8 and -9 in both ASTC-a-1 cells A549 cell lines, but a significant increase in caspase-3 activation level (Fig. 2D), and that silencing AIF completely inhibited the ART-evoked activation of both caspase-8 and -9 in A549 cells and largely but not completely prevented ART-induced caspase-3 activation in both cell lines (Fig. 2D). To further explore the roles of caspases in ART-induced apoptosis, cells were pretreated with selective oligopeptide inhibitors for caspase-8, -9, and -3 for 1 h followed by 24 and 48 h ART treatment, respectively. CCK-8 assay showed that compared with the cells treated with ART alone, inhibition of caspase-8 and -9 did not prevent the cytotoxicity of ART, and inhibition of caspase-3 very faintly inhibited the cytotoxicity of ART (Fig. 2E), suggesting that caspase-8 and -9 were not involved in the ART-induced cell death.
Collectively, these data demonstrate that ART induces apoptosis dominantly via a caspase-independent AIF pathway.
Bak dominates the ART-induced MOMP and apoptosis
To determine the roles of Bax/Bak in ART-induced apoptosis, we used Hoechst 33258 staining to assess the effect of silencing Bax or Bak on the ART-induced nuclear concentration and DNA fragmentation (Fig. 3A), the typical characteristics of apoptosis. Western blotting analysis showed that silencing Bax or Bak by shBax or shBak significantly down-regulated the expression level of Bax or Bak (Supplementary Fig. S3B). ART treatment for 48 h induced a remarkable nuclear concentration and DNA fragmentation that was remarkably prevented by shBak but not shBax, and the corresponding statistical results of cells showing nuclear condensation and DNA fragmentation under different conditions from at least 500 cells in three independent experiments were shown in Figure 3B, indicating the key role of Bak but not Bax in ART-induced apoptosis. Confocal imaging was used to detect whether ART-induced Bax translocation into mitochondria in single living cells co-expressing GFP-Bax and DsRed-Mit. Figure 3C showed the fluorescence images of cells at 36 h after ART treatment. STS-treated cells were used as positive control. In STS-treated cells, Bax translocated to mitochondria, but in ART-treated cells, Bax is still diffused in the whole cell the same as in healthy cells (Fig. 3C), suggesting that Bax was not activated during ART-induced apoptosis. In addition, silencing Bak but not Bax remarkably inhibited the ART-induced loss of ΔΨm (Fig. 3D) indicative of MOMP, and the AIF release from mitochondria (Fig. 3E), further demonstrating that Bak but not Bax played a key role in ART-induced apoptosis.
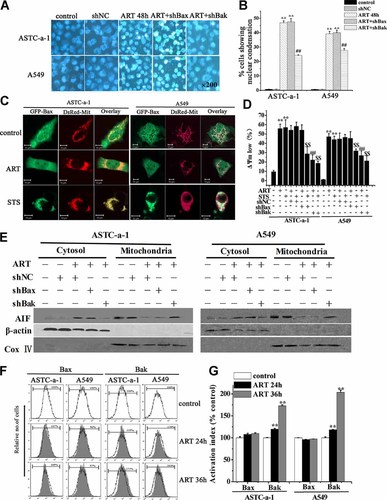
Bak, not Bax, is necessary for ART-induced apoptosis. A,B: Fluorescence images of cells stained with Hoechst 33258 in the presence or absence of silencing Bak or Bax by shBak or shBax (A) and the quantification of cells with nuclear condensation from at least 500 cells in three independent experiments (B). Nuclear morphology of apoptosis was examined by fluorescence microscope. Cells were treated with ART for 48 h. ShNC was used as negative control. Magnification × 200. **P < 0.01, compared with control and ##P < 0.01, compared with ART treatment alone. C: Spatial distribution of Bax in control, ART-, or STS-treated cells determined by confocal microscopy imaging. Cells were co-transfected with DsRed-Mit and GFP-Bax to fluorescently label mitochondria and Bax, respectively. After treatment with ART for 36 h, the fluorescence images were performed by laser fluorescence confocal microscopy. STS-treated cells were used as positive control. Scale Bar: 10 µm. D: Silencing Bak but not Bax largely inhibited ART-induced loss of ΔΨm determined by FCM analysis. Cells were treated with ART for 48 h. ShNC was used as negative control. **P < 0.01, compared with control; ##P < 0.01, compared with ART treatment alone; $$P < 0.01, compared with STS treatment alone. E: Silencing Bak but not Bax prevented ART-induced AIF release assessed by Western blotting. Cells were treated with ART for 36 h. β-actin and Cox IV were used as cytosolic fraction loading control and the heavy membrane fraction loading control, respectively. ShNC was used as negative control. F,G: ART-induced activation of Bak but not Bax analyzed by FCM analysis (F) and the statistical results from three independent experiments (G). Cells were treated with ART for the indicated times, subsequently fixed with paraformaldehyde and then incubated with 6A7 monoclonal anti-Bax antibody or with Ab-2 monoclonal anti-Bak antibody for 60 min. After incubation with FITC-conjugated second antibody for 30 min, the signals of activation of Bax and Bak proteins were measured by FCM analysis. The open and filled histograms were generated from control and ART-treated cells, respectively. **P < 0.01, compared with control cells. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
One of the evidence in Bax and Bak activation is an N-terminal conformational change and it can be detected by antibodies that specifically recognize the active conformers of Bax (6A7) or Bak (Ab-2) (Germain et al., 2008; Westphal et al., 2011). We used FCM analysis to investigate the percentage of cells with the active conformational change of Bax and Bak after ART treatment for 24 and 36 h, and found that ART induced a significant activation of Bak but not Bax (Fig. 3F) and the corresponding statistical results from three independent experiments were shown in Fig. 3G.
Bim and VDAC2 as well as to a lesser extent Mcl-1 mediate ART-induced Bak activation and apoptosis
It is reported that Mcl-1, Bcl-xl, Bim, Puma, and VDAC2 may be responsible for Bak-dependent apoptosis (Cheng et al., 2003; Willis et al., 2005; Ren et al., 2010). We used Western blotting analysis to detect the expression level of Mcl-1, Bcl-xl, Bim, and VDAC2 after ART treatment for 36 h, and found that ART treatment did not induce significant changes of the expression level of these proteins (Fig. 4A). Next, we examined the effects of silencing Mcl-1, Bcl-xl, Bim, and VDAC2, respectively, on the ART-induced apoptosis. The silencing effects of shMcl-1, shBim, and shVDAC2 were shown in Supplementary Figure 3C. As shown in Figure 4B, silencing Puma did not show any effect on the ART-induced cell death, but silencing Bim remarkably inhibited ART-induced apoptosis that was enhanced significantly by silencing Mcl-1 and more potently by silencing VDAC2, indicating that Bim, Mcl-1, and VDAC2 played important roles in ART-induced apoptosis. We further used FCM analysis to investigate the effect of silencing Mcl-1, Bim, and VDAC2 on ART-induced Bak activation, and found that silencing Bim largely inhibited ART-induced Bak activation that was significantly enhanced by silencing Mcl-1 and more potently by silencing VDAC2 (Fig. 4C). These findings demonstrated the important roles of Bim and VDAC2 as well as to a lesser extent Mcl-1 for activating Bak that triggered intrinsic apoptosis pathway to mediate ART-induced apoptosis.
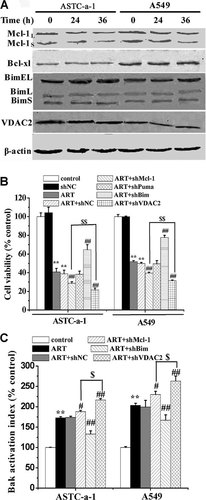
Bim and VDAC2 as well as Mcl-1 play important roles in ART-induced apoptosis. A: ART treatment did not induced significant changes of the expression level of Bcl-xl, Mcl-1, Bim, and VDAC2 determined by Western blotting analysis. Cells were treated with ART for the indicated times. β-actin served as the loading control. B: The effects of silencing Mcl-1, Bim, Puma, and VDAC2, respectively, on the cytotoxicity of ART assessed by CCK-8. Cells were treated with ART for 48 h. ShNC was used as negative control. **P < 0.01, compared with control cells; ##P < 0.01, compared with ART treatment alone. $$P < 0.01, compared with both ART treatment and silencing Mcl-1. C: The effects of silencing Mcl-1, Bim, and VDAC2, respectively, on the ART-induced Bak activation determined by FCM analysis. Cells were treated with ART for 36 h before FCM analysis. ShNC was used as negative control. **P < 0.01, compared with control; #P < 0.05 and ##P < 0.01, compared with ART treatment alone; $P < 0.05, compared with cells with both ART treatment and silencing Mcl-1.
Discussion
Artemisinin and its derivative ART have been reported as potential anticancer agents in many cells (Efferth et al., 2001; Mercer et al., 2007; Sertel et al., 2010). In this report, we dissected the molecular mechanism of ART-induced apoptosis in human lung adenocarcinoma cells. To our best knowledge, this is the first published report demonstrating that ART induces apoptosis dominantly via a Bak- and AIF-mediated caspase-independent intrinsic pathway, in which BH-3 only protein Bim, as a key initiator, participates in activating the Bak that may be released from the binding with VDAC2 and Mcl-1.
Our observations that silencing Bak but not Bax by shRNA significantly prevents ART-induced apoptosis and mitochondrial dysfunction (Fig. 3A,D), and also largely prevents ART-induced AIF release (Fig. 3E) demonstrate that Bak, but not Bax, plays a key role in ART-induced apoptosis. In support of this, we assessed the activation of Bak and Bax, and found that ART treatment induced the activation of Bak but not Bax (Fig. 3F,G). Inactive Bax is a latent monomer in the cytosol, whereas Bak resides in a membrane bound protein complex, which suggests that Bak and Bax are activated by different mechanisms (Gélinas and White, 2005). It has been reported that Bax usually plays a major role and Bak is an auxiliary even a redundant regulator in intrinsic apoptosis pathway upon many stimuli in varies cell lines (Wendt et al., 2005; Lee et al., 2008; Karlberg et al., 2010). However, recent publications reported that there were some apoptotic stimuli, such as SSP, actinomycin D, TRAIL, and dihydroartemisinin, activated preferentially Bak that can lead to the release of Cyt.c in the absence of Bax activation (Neise et al., 2008; Handrick et al., 2010).
Bcl-xl, Mcl-1, and VDAC2 were reported to bind Bak and inhibit its activation (Cheng et al., 2003; Willis et al., 2005). Generally, Bcl-xl inhibits both Bak and Bax to mediate apoptosis (Chipuk and Green, 2008; Edlich et al., 2011). However, the fact that ART treatment neither induces Bax activation (Fig. 3C,F) nor decreases Bcl-xl expression (Fig. 4A) suggests that Bcl-xl is not involved in ART-induced apoptosis. Modest increase of ART-induced apoptosis and Bak activation by silencing Mcl-1 (Fig. 4B,C), in combination with the very modest decrease of Mcl-1 expression after ART treatment (Fig. 4A), suggests that ART treatment modestly neutralizes the binding of Mcl-1 to Bak. Interestingly, VDAC2 showed an important role in ART-induced apoptosis and Bak activation (Fig. 4B,C). Lazarou et al. (2010) suggested that VDAC2 interacted with the hydrophobic tail of Bak to sequester it in an inactive state in the mitochondrial outer membrane. Although ART treatment did not induce significant change of VDAC2 expression level (Fig. 4A), silencing VDAC2 by shRNA increased ART-induced apoptosis and Bak activation more potently compared with silencing Mcl-1(Fig. 4B,C). Therefore, it is reasonable to assume that ART treatment neutralizes the binding of VDAC2 and to a lesser extent Mcl-1 with Bak.
We here found that Bim played an important role in ART-induced Bak activation and apoptosis. It is reported that Bid, Bim and Puma can indirectly activate Bax/Bak by binding to all anti-apoptotic proteins or as activator to directly activate Bax/Bak (Chipuk and Green, 2008). Active caspase-8 cleaves Bid into tBid that translocates to mitochondria to activate Bax/Bak directly or indirectly by inhibiting Bcl-xl (Li et al., 1998). The fact that caspase-8 is not activated (Fig. 2D) suggests that ART does not trigger the extrinsic apoptotic pathway. In combination with the data that Bax and Bcl-xl are not involved in ART-induced apoptosis (Figs. 3A and 4A), it is reasonable to assume that Bid does not participate in ART-induced intrinsic pathway. Interestingly, silencing Bim but not Puma by shRNA significantly prevented ART-induced cytotoxicity (Fig. 4B) and Bak activation (Fig. 4C). Moreover, ART treatment did not induce a significant up-regulation of Bim expression (Fig. 4A). If Bim activates Bak by inhibiting Bcl-xl, Bcl-2, and Mcl-1 in “indirect model,” then it can also activate Bax (Michalak et al., 2009; Sharma et al., 2012). However, ART did not induce Bax activation (Fig. 3C,F), and silencing Bax did not show any inhibitory effect on the ART-induced apoptosis (Fig. 3A). Therefore, we infer that normal level of Bim (endogeneous Bim) activates the Bak released from the binding with VDAC2 and Mcl-1 to mediate ART-induced apoptosis.
Our observations suggest that caspase-9 and caspase-3, the downstream apoptotic caspases of Smac and Cyt.c, do not play important roles in ART-induced apoptosis. Both Smac and Cyt.c released from mitochondria have been reported to activate caspase-9 and -3 to mediated caspase-dependent apoptosis pathway (Autret and Martin, 2009; Rajalingam et al., 2007). ART treatment did not induce Cyt.c release (Fig. 2A and Supplementary Fig. S2). Although we observed the Smac release (Fig. 2A), ART-treated cells showed no caspase-9 activation in ASTC-a-1 cell and a slight caspase-9 activation that was completely prevented by silencing AIF in A549 cells (Fig. 2D). Moreover, inhibition of caspase-9 did not prevent the cytotoxicity of ART (Fig. 2E). We thereby infer that caspase-9 is not involved in ART-induced apoptosis. Interestingly, we found that although ART treatment induced a significant caspase-3 activation which was significantly but partially prevented by silencing AIF (Fig. 2D), inhibition of caspase-3 showed a very faint prevention on the ART-induced cell death (Fig. 2E), suggesting that caspase-3 did not play a potent role in ART-induced apoptosis, in which, however, the molecular mechanism underlying ART-induced a large caspase-3 activation is unclear.
The fact that AIF releases from mitochondria and silencing AIF significantly inhibits apoptosis (Fig. 2A–C) suggests that AIF plays an important role in ART-induced apoptosis. In recent years, AIF is reported to induce caspase-independent apoptosis under various conditions (Gallego et al., 2004; Seiler et al., 2008; Choudhury et al., 2011). Moreover, silencing AIF by shAIF could inhibit the activation of caspase-8, -9 (Fig. 2D). In combination with the data that AIF release can be significantly prevented by silencing Bak but not Bax (Fig. 3E), we are convinced that AIF acts as a central downstream factor in Bak-mediated intrinsic apoptosis pathway to regulate ART-induced apoptosis. It is reported that Ca2+ is involved in AIF cleavage and release from mitochondria (Lorenzo and Susin, 2007; Norberg et al., 2010). However, whether Ca2+ is involved in the ART-induced AIF release and subsequent apoptosis is unclear.
In summary, ART induces apoptosis dominantly via a Bak-dependent AIF intrinsic apoptosis pathway in both ASTC-a-1 and A549 cell lines. On the basis of these findings, we hypothesize that ART treatment neutralizes the interaction of Bak with VDAC2 and to a lesser extent Mcl-1, and Bim directly activates Bak released from VDAC2 and Mcl-1 to trigger the intrinsic pathway. However, the molecular mechanism by which ART neutralizes the interaction between Bak and VDAC2 as well as Mcl-1 is unknown, which will be the task of future investigations to reveal the upstream novel molecular targets triggered by ART and develop its therapeutic potential.
Acknowledgements
We thank Prof. G. J. Gores for providing us with the GFP-Cyt.c plasmid, Prof. R. J. Youle for providing us with the GFP-Bax plasmid and Prof. Y. Gotoh for providing us with the DsRed-Mit plasmid.