Multiple Kv1.5 targeting to membrane surface microdomains†
Ramón Martínez-Mármol and Núria Villalonga contributed equally to this work.
Abstract
Surface expression of voltage-dependent K+ channels (Kv) has a pivotal role in leukocyte physiology. Although little is known about the physiological role of lipid rafts, these microdomains concentrate signaling molecules and their ion channel substrates. Kv1.3 associates with Kv1.5 to form functional channels in macrophages. Different isoform stoichiometries lead to distinct heteromeric channels which may be further modulated by targeting the complex to different membrane surface microdomains. Kv1.3 targets to lipid rafts, whereas Kv1.5 localization is under debate. With this in mind, we wanted to study whether heterotetrameric Kv1.5-containing channels target to lipid rafts. While in transfected HEK-293 cells, homo- and heterotetrameric channels targeted to rafts, Kv1.5 did not target to rafts in macrophages. Therefore, Kv1.3/Kv1.5 hybrid channels are mostly concentrated in non-raft microdomains. However, LPS-induced activation, which increases the Kv1.3/Kv1.5 ratio and caveolin, targeted Kv1.5 back to lipid rafts. Moreover, Kv1.5 did not localize to low-buoyancy fractions in L6E9 skeletal myoblasts, which also coexpress both channels, heart membranes or cardiomyocyes. Coexpression of a Cav3DGV-mutant confined Kv1.5 to Cav3DGV-vesicles of HEK cells. Contrarily, coexpression of Kvβ2.1 impaired the Kv1.5 targeting to raft microdomains in HEK cells. Our results indicate that Kv1.5 partnership interactions are underlying mechanisms governing channel targeting to lipid rafts. J. Cell. Physiol. 217: 667–673, 2008. © 2008 Wiley-Liss, Inc.
Ion channels have differential surface distributions (Misonou and Trimmer, 2004). Lipid rafts are specialized membrane microdomains rich in sphingolipids and cholesterol. Many signal transduction enzymes known to modulate ion channels are concentrated in these domains (Simons and Toomre, 2000; Martens et al., 2004). Caveolae are the best-characterized rafts. These invaginated membrane structures contain caveolin, which interacts directly with several intracellular proteins (Simons and Toomre, 2000). The localization of ion channels in caveolar-lipid rafts triggers rapid regulation of membrane excitability as the caveolar neck opens, thus increasing the number of surface channels (Martens et al., 2004).
Voltage-gated potassium channels (Kv) set the membrane potential, and their subcellular localization is necessary for proper electrical signaling (Misonou and Trimmer, 2004). Several ion channels, and Kv in particular, target to lipid rafts (Martens et al., 2004). Kv2.1 was the first channel identified in rafts (Martens et al., 2000). Next, Kv1.5 was found to be associated with caveolae (Martens et al., 2001). Since then, the list of channels associated with caveolar or non-caveolar lipid rafts has been constantly growing. However, some recent reports on Kv1.5 and Kv4.2 are contradictory, and suggest a complex scenario (Wong and Schlichter, 2004; Eldstrom et al., 2006).
Kv1.3 plays a pivotal role in leukocyte responses (Cahalan et al., 2001). Kv1.3 is involved in the maintenance of the resting membrane potential and plays a critical role during activation and proliferation of leukocytes (Cahalan et al., 2001; Vicente et al., 2003). Upon activation, Kv1.3 concentrates in the immunological synapse together with lipid rafts (Panyi et al., 2004). Cholesterol regulates Kv1.3 gating, and the transformation of these rafts into large ceramide-enriched membrane platforms inhibits channel activity (Bock et al., 2003; Hajdu et al., 2003). On the other hand, Kv1.5, highly expressed in human atria, is involved in the proliferation of astrocytes and myoblasts (MacFarlane and Sontheimer, 2000; Villalonga et al., 2008) and plays a role during activation in mononuclear phagocytes (Vicente et al., 2003, 2005, 2006; Mullen et al., 2006; Pannasch et al., 2006). Kv1.3/Kv1.5 heteromeric association generates a plethora of distinct channels, and the Kv1.3/Kv1.5 ratio plays a pivotal role in macrophage responses (Vicente et al., 2006; Villalonga et al., 2007). Kv1.3 is associated with lipid rafts. However, the association of Kv1.5 with caveolae is controversial. Therefore, our aim was to study the targeting of Kv1.5 and heteromeric Kv1.5-containig channels to rafts in several cell systems, including HEK-293 cells, macrophages, L6E9 myocytes, isolated cardiomyocytes and membrane preparations from rat heart. In this article, we suggest that partnership interaction mechanisms involving heteromeric associations, regulatory subunits or caveolin could alter Kv1.5 targeting to lipid rafts.
Materials and Methods
Cell culture and cardiomyocyte isolation
Raw 264.7 macrophages were cultured in RPMI containing 5% fetal bovine serum (FBS), supplemented with antibiotics 10 U/ml penicillin and streptomycin, and 2 mM L-glutamine (Vicente et al., 2006; Villalonga et al., 2007). Bone marrow-derived macrophages (BMDM) were cultured in DMEM containing 20% FBS and 30% L-cell conditioned media as a source of macrophage colony stimulating factor (Vicente et al., 2003). Macrophages were obtained as a homogeneous population of adherent cells after 7 days in culture. Raw 264.7 and BMDM were cultured in the absence or the presence of 100 ng/ml lipopolysaccharide (LPS) for 24 h. HEK-293 cells were cultured in DMEM containing 10% FBS and supplemented as above (Vicente et al., 2006). L6E9 myoblasts (Dr. B. Nadal-Ginard, Mount Sinai School of Medicine, NY) were grown in DMEM, supplemented as above plus 25 mM Hepes (pH7.4) (Martinez-Marmol et al., 2007).
Hearts from Wistar rats (Harlan Interfauna Iberica) were excised and washed in ice-cold 0.9% NaCl. Calcium-tolerant myocytes were isolated by collagenase digestion. Hearts cut in small pieces in Krebs Buffer (in mM: NaCl 119, KCl 5.4, CaCl2 1.5, KH2PO4 0.6, MgSO4 1.2, NaHCO3 25 and glucose 11.7) were washed 3 times in KB 0Ca (Ca2+ free) for 5 min. Cardiac tissue was further incubated in KB 0Ca, supplemented with 200 U/ml collagenase type IA and 0.03 mg/ml protease XIV (Sigma-Aldrich, Steinheim, Germany). The mixture was oxygenated with 95% O2/5% CO2 for 30 min, maintained at 37°C and gently shaken. Dispersed cells were filtered with a nylon mesh and centrifuged. Cells were washed once in KB 0Ca. Animal handling was approved by the ethics committee of the University of Barcelona, in accordance with EU regulations.
DNA constructs, cell transfection, and immunocytochemistry
Rat pRcCMV-Kv1.3 cDNA was kindly donated by T.C. Holmes (New York University, NY). Kv1.3-YFP and Kv1.5-CFP were generated as described (Vicente et al., 2006). Kvβ2.1 was characterized in Tamkun's laboratory (Uebele et al., 1996). Cav3DGV mutant was from A. Pol (Universitat de Barcelona).
HEK-293 cells were transiently transfected using Lipofectamine 2000 (Invitrogen, Paisley, UK) to nearly 80% confluency, as described (Vicente et al., 2006). Twenty-four hours after transfection, cells were washed with PBS (phosphate-buffered saline) and used for raft isolation or immunocytochemistry. L6E9 myocytes and HEK-293 cells were grown on polylysine-coated glass cover slips. L6E9 cells, fixed with cold methanol, were incubated with blocking solution (10% goat serum/5% non-fat dry milk/PBS) for 1h. Myocytes were reacted with anti-Kv1.5 (Alomone, Jerusalem, Israel), anti-Nav1.5 (Alomone) and anti-pan-caveolin (BD Transduction, San Jose, CA) in 10% goat serum/0.75% Triton X-100/PBS for 90 min. Cells were further incubated for 60 min with CY2 and CY3 secondary antibodies (Amersham Biosciences, Piscataway, NJ). Preparations were fixed and mounted with Aqua Poly/Mount (Polysciences, Warrington, PA).
Crude membrane preparations, raft isolation, and Western blot
Culture cells and cardiomyocytes were washed in cold PBS and lysed with ice-cold lysis solution (1% Triton X-100, 10% glycerol, 50 mmol/L HEPES pH 7.5, 150 mmol/L NaCl) supplemented with 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin and 1 mM phenylmethylsulfonyl fluoride as protease inhibitors. To obtain enriched membrane preparations, homogenates were centrifuged at 3,000g for 10 min and the supernatant was further centrifuged at 150,000g for 90 min. The pellet was resuspended in 30 mM HEPES (pH 7.4).
Fresh hearts were frozen in liquid nitrogen and powdered by a pestle and mortar. The powder was homogenized in 4 mM HEPES (pH 7.0) and 320 mM sucrose supplemented with protease inhibitors in a glass homogenizer. Nuclei and debris were pelleted at 2,000g for 10 min. The supernatant was centrifuged at 100,000g for 1 h. The pellet was resuspended in the same buffer (0.5 ml/g heart).
Culture cells, isolated cardiomyocytes, and crude membrane preparations were used for raft isolation as described (Martens et al., 2000). Samples were homogenized in MES (2-Morpholino ethanesulfonic acid)-buffered saline (24 mM MES, pH 6.5, and 0.15 mM NaCl) plus 1% Triton X-100 and centrifuged at 3,000g for 5 min at 4°C. Then sucrose was added to achieve a final concentration of 40%. A 5–30% linear sucrose gradient was layered on top and further centrifuged (39,000 rpm) for 20–22 h at 4°C in a Beckman SW41 rotor. Gradient fractions (1ml) were collected from the top and analyzed by Western blot. In some experiments, fractions were concentrated using Microcon centrifugal filter devices (Amicon, Bedford, MA).
Sucrose fractions (50 µl) were boiled in Laemmli SDS loading buffer and separated on 10% SDS–PAGE. They were transferred to nitrocellulose membranes (Immobilon-P, Millipore, Bedford, MA) and blocked in 5% dry milk-supplemented 0.1% Tween 20 PBS before immunoreaction. Filters were immunoblotted with antibodies against Kv1.3 (1/200, Alomone), Kv1.5 (1/500, Alomone) and Nav1.5 (1/200, Alomone). Anti-pan-caveolin antibody, which recognizes caveolin 1, 2 and 3, and anti-flotillin-1 were used (1/1,000) as markers of lipid raft fractions (BD Transduction). Since all kinds of lipid rafts are excluded from clathrin-positive regions (Nichols, 2003), filters were also probed with anti-clathrin antibody (Chemicon, Bedford, MA) to characterize non-floating fractions (1/1,000).
Results
Kv1.3, Kv1.5 and Kv1.3/Kv1.5 heteromers target to lipid rafts in HEK-293 cells
Kv1.3 concentrates in raft microdomains in T-cells, and Kv1.5 targets to caveolar-rafts in fibroblasts (Martens et al., 2001; Bock et al., 2003; Folco et al., 2004; Panyi et al., 2004). Since leukocytes and fibroblasts express Kvβ subunits, which are involved in channel surface expression, we expressed Kv1.3 and Kv1.5 in HEK-293 cells (Uebele et al., 1996; Vicente et al., 2005). Kv1.3 and Kv1.5 colocalized with caveolin in HEK-293 cells (Fig. 1A,C). Like fibroblasts and T-cells, channels targeted to low-buoyancy density fractions. However, their presence in non-rafts is noticeable (Fig. 1B,D). Coexpression of Kv1.3 and Kv1.5, which form heteromers, gave similar results (Fig. 1E,F). Similar results (not shown) were obtained by a detergent-free lipid raft isolation protocol (Crossthwaite et al., 2005).
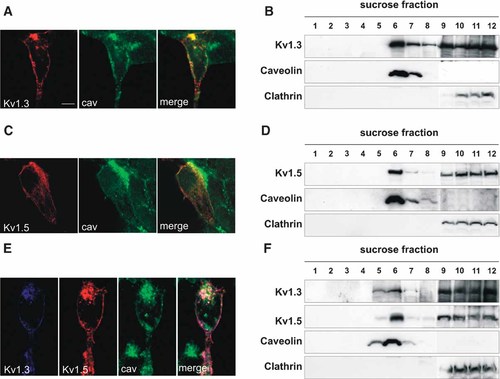
Kv1.3 and Kv1.5 target to lipid rafts in HEK-293 cells. Cells were transfected with either Kv1.3 (A,B) or Kv1.5 (C,D) or doubly-transfected with both channels (E,F). Kv1.3 (A), Kv1.5 (C) and Kv1.3/Kv1.5 (E) channels colocalized with caveolin. Merge colors in E: pink, merge of Kv1.3 and Kv1.5; white, merge of Kv1.3, Kv1.5 and caveolin. (B,D,F) Lipid raft extractions. Kv1.3 (B), Kv1.5 (D) and Kv1.3/Kv1.5 (F) hybrids targeted to low-buoyancy density sucrose fractions. Bars represent 10 µm.
Kv1.3, but not Kv1.5, localized to lipid rafts in macrophages and skeletal muscle myocytes
Heterotetrameric Kv1.3/Kv1.5 channels contribute to the major Kv channel in macrophages (Vicente et al., 2006). Kv1.3 targets to lipid raft in leukocytes, but the Kv1.5 caveolar-localization is questioned in the heart (Bock et al., 2003; Panyi et al., 2004; Eldstrom et al., 2006; Vicente et al., 2008). Figure 2A demonstrates that Raw 264.7 and bone marrow-derived macrophages (BMDM) shared the expression of Kv1.3 and Kv1.5. Unlike BMDM, Raw 264.7 macrophages did not express caveolin and rafts were identified with flotillin (Fig. 2A). Although Kv1.3 colocalized with flotillin in Raw cells (Fig. 2B,C) and caveolin in BMDM (Fig. 2D,E), channels mostly concentrated in non-raft aliquots. Surprisingly, Kv1.5 was restricted to clathrin fractions (Fig. 2B,D). In addition, Kv1.3/Kv1.5 heteromeric association redistributed Kv1.3 to non-floating domains (Fig. 2B–E). However, rafts extractions from LPS-activated macrophages, which increases the Kv1.3/Kv1.5 ratio in the heteromeric channel and induces caveolin expression in BMDM (Vicente et al., 2006), showed that Kv1.5 appeared in low-buoyant density fractions (Fig. 2C,E).
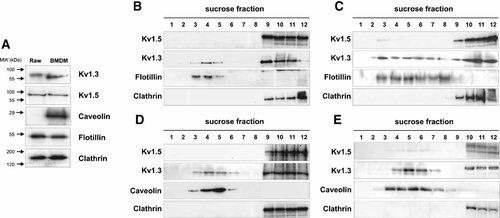
Kv1.3 and Kv1.5 channels differentially target to lipid rafts in macrophages. A: Kv1.3, Kv1.5, clathrin, flotillin and caveolin expression in Raw 264.7 and BMDM macrophages. Sucrose density gradient fractions of Raw 264.7 macrophages (B,C) and BMDM (D,E) cultured in the absence (B,D) or the presence of LPS (C,E). Kv1.3 localized with flotillin (B,C) and caveolin (D,E), whereas Kv1.5 concentrated in non-raft fractions marked with clathrin (B–E). C,F: Kv1.5 appeared in low-buoyant density fractions isolated from LPS-induced macrophages.
Like macrophages, L6E9 myocytes coexpress Kv1.3 and Kv1.5 (Villalonga et al., 2008). Furthermore, myocytes express caveolins and Kvβ subunits (Grande et al., 2003; Martinez-Marmol et al., 2007). Both channels concentrated in non-floating fractions (Fig. 3A). This surprising result is in agreement with a limited Kv1.3 expression in L6E9 cells (Villalonga et al., 2008). Therefore, we concentrated these fractions (Fig. 3B). Concentrated high-sucrose fractions were difficult to electrophorese. Nevertheless, Kv1.3 appeared in fractions 3–5, whereas Kv1.5 did not colocalize with caveolin. This result was in contrast to that observed in HEK cells, but clearly in agreement with macrophage results. Therefore, we analyzed Kv1.5 and caveolin colocalization in HEK and L6E9 cells (Fig. 4). The results confirmed the raft-isolation studies. Unlike HEK cells, Kv1.5 did not colocalize with caveolin in myoblasts (Fig. 4A). In contrast, Nav1.5 colocalized with caveolin in L6E9 cells (Fig. 4B) indicating that, similarly to Kv1.3, this may be a regular location for this channel (Martens et al., 2004; Martinez-Marmol et al., 2007).
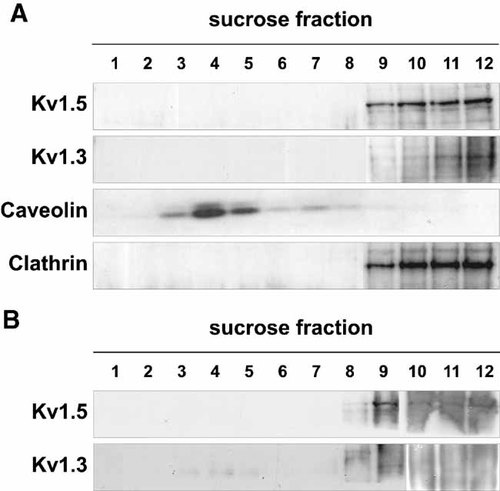
Kv1.3 and Kv1.5 channels differentially target to lipid rafts in L6E9 myocytes. Sucrose density gradient fractions of L6E9 cells. A: Fifty microliters of each fraction were analyzed. B: Five hundred microliters of each sucrose fraction were concentrated to 25 µl with Amicom devices. While Kv1.3 appeared in fractions 3 to 5, Kv1.5 only concentrated in the non-raft fractions. Longer film exposures impaired the visualization.
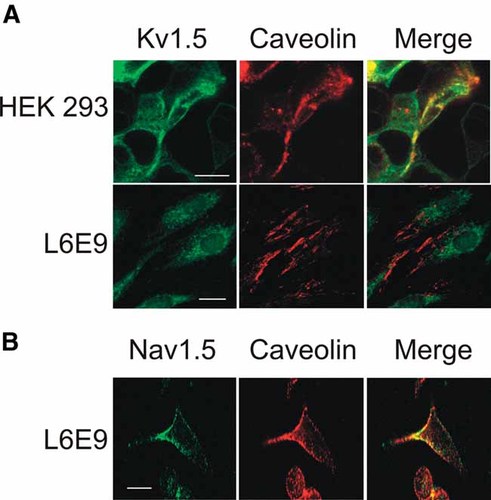
Kv1.5 colocalizes with caveolin in HEK but not in L6E9 cells. A: HEK cells transfected with Kv1.5 and L6E9 cells were immunoreacted with anti-Kv1.5 and anti-pan-caveolin. B: Nav1.5 colocalizes with caveolin in L6E9 cells. Green, channels; red, caveolin; yellow, colocalization. Bars represent 10 µm.
Kv1.5 does not target to lipid rafts in heart and cardiomyocytes
Kv1.5 targets to lipid rafts (Martens et al., 2001; Folco et al., 2004; Cogolludo et al., 2006; Abi-Char et al., 2007; McEwen et al., 2008). However, Fedida and coworkers failed to detect Kv1.5 in rafts from cardiac samples (Eldstrom et al., 2006). Our results confirmed these discrepancies. We investigated whether this channel targeted to low-buoyancy fractions in isolated cardiomyocytes and crude membranes from rat heart (Fig. 5). Unlike Nav1.5, Kv1.5 did not colocalize with the lipid raft in isolated cardiomyocytes (Fig. 5A) and heart membranes (Fig. 5B).
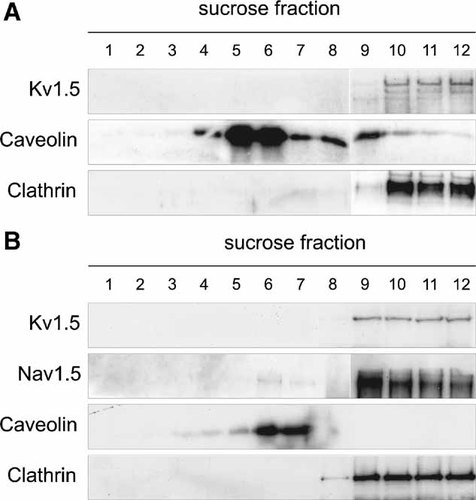
Kv1.5 does not localize with lipid rafts in isolated cardiomyocytes and rat heart membranes. A: Cardiomyocytes and (B) membranes from rat heart were used for the lipid raft isolation. While Kv1.5 does not target to low-buoyancy fractions, Nav1.5 is found in both raft and non-raft fractions in rat heart membranes.
Cav3DGV confines Kv1.5, but not Kv1.3, to cholesterol-rich vesicles
While Kv1.5 localized in low-buoyancy fractions when expressed in HEK cells, Kv1.5 neither targeted to lipid rafts nor colocalized with caveolin in tissues and native cells. However, an increase of Kv1.3 in heteromeric channels and an augmentation of caveolin targeted Kv1.5 back to lipid rafts (see Fig. 2). Therefore, we coexpressed Kv1.3 and Kv1.5 with a Caveolin-3DGV mutant. This mutant generates Cav3-endoplasmic reticulum-related and cholesterol-rich vesicles (Pol et al., 2001). Unlike Kv1.3 (Fig. 6A–C), Kv1.5 concentrated in Cav3DGV-vesicles (Fig. 6D–F). The association of Kv1.3 and Kv1.5 generated an intermediate pattern (Fig. 6G–J), confirming that, as in biophysics and pharmacology, heteromerization modifies targeting and localization (Vicente et al., 2006, 2008). Cav3DGV-vesicles are enriched in cholesterol (Pol et al., 2001). Therefore, channels were localized in low-buoyancy density fractions (Fig. 6K–M).
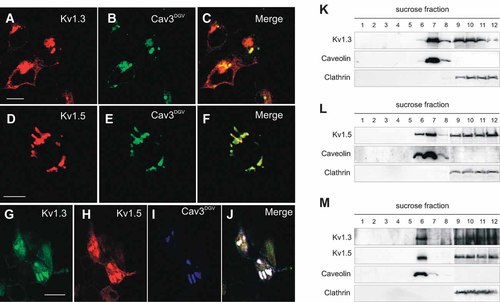
Expression of Cav3DGV confines Kv1.5, but not Kv1.3, in Cav3DGV-vesicles. HEK cells were cotransfected with Cav3DGV and Kv1.3, Kv1.5 or both. A–C: Cav3DGV did not modify Kv1.3 localization. D–F: Coexpression of Kv1.5 and Cav3DGV. Kv1.5 concentrated in Cav3DGV-vesicles. Colors: red, channels; green, Cav3DGV; yellow, colocalization of channel and Cav3DGV. G–J: Coexpression of Cav3DGV with Kv1.3 and Kv1.5 generated an intermediate pattern. G: Kv1.3; (H) Kv1.5; (I) Cav3DGV; (J) merge. Yellow, colocalization of channels; white, triple colocalization of channels and Cav3DGV. Bars represent 10 µm. K–M: Kv1.3 and Kv1.5 target to low-buoyancy density fractions in Cav3DGV-transfected HEK-293 cells. HEK cells were cotransfected with Cav3DGV together with Kv1.3 (K), Kv1.5 (L) or doubly-transfected with both (M).
Kvβ2.1 redistributes Kv1.5 to non-floating domains
Contrary to what observed in HEK cells, Kv1.5 channels mostly localized out of lipid rafts in native cells and tissues. While macrophages, myoblasts and heart share the expression of cytoplasmic Kvβ subunits (Grande et al., 2003; Vicente et al., 2005), HEK-293 cells do not (Uebele et al., 1996). Therefore, we coexpressed Kv1.5 and Kvβ2.1 in HEK cells. Kv1.5 and Kvβ2.1 colocalized (Fig. 7A) and the presence of Kvβ2.1 impaired the Kv1.5 targeting to raft fractions (Fig. 7B,C). In addition, some Kvβ2.1 localized in low-buoyancy density fractions.
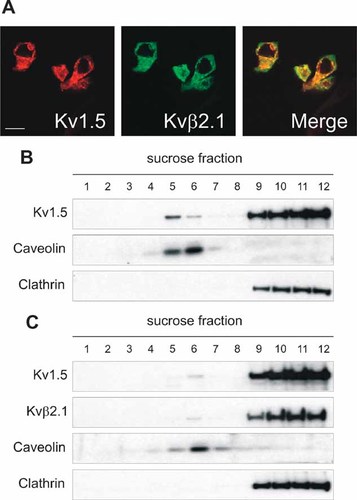
Regulatory Kvβ2.1 subunit impairs Kv1.5 targeting to raft microdomains. HEK cells were transfected with either Kv1.5-YFP or doubly-transfected with Kv1.5-YFP and Kvβ2.1-CFP (ratio1:1). A: Kv1.5 and Kvβ2.1 colocalized. Red, Kv1.5; green, Kvβ2.1; yellow, merge. B,C: Lipid raft extractions. Kv1.5 (B), Kv1.5/ Kvβ2.1 (C). The presence of Kvβ2.1 redistributes Kv1.5 to non-floating fractions. Bar represents 10 µm.
Discussion
Kv1.5 targets to lipid raft in HEK-293 cells but not in native tissues and cells
In this work, we show that Kv1.5 targets to multiple membrane microdomains. Kv1.5 locates in low-buoyant density fractions in HEK 293 cells, but not in native cells (macrophages and myocytes) and tissues (heart). However, while the regulatory Kvβ2.1 subunit redistributes Kv1.5 to non-floating domains in HEK cells, selective induction of interacting proteins, such as Kv1.3 and caveolins, targeted Kv1.5 back to lipid rafts in macrophages. This is in accordance with what we found by overexpressing Cav3DGV, suggesting that partnership interactions regulate Kv1.5 trafficking to cholesterol-rich membrane microdomains.
Kv1.3 and Kv1.5 locate in lipid rafts and caveolae in fibroblasts and leukocytes (Martens et al., 2001; Bock et al., 2003; Folco et al., 2004; Panyi et al., 2004; Cogolludo et al., 2006). Caveolae are specialized lipid rafts, which contain caveolins (Simons and Toomre, 2000). Besides caveolins, fibroblasts also express Kvβ2.1 subunits, which are involved in surface channel expression (Uebele et al., 1996). Not only Kv1.3 but also Kv1.5 associate to Kvβ subunits (Uebele et al., 1996; Vicente et al., 2005). Therefore, we expressed Kv1.3 and Kv1.5 in HEK-293 cells, which lack Kvβ (Uebele et al., 1996). In addition, HEK-293 cells express caveolin 2, but the presence of caveolin 1 is scarce (Scherer et al., 1997). Therefore, the number of caveolae is low (not shown). Like in fibroblasts and T-cells, channels targeted to low-buoyancy density fractions in HEK cells. However, their presence in non-rafts was abundant and was even higher in the presence of Kvβ2.1. Fedida and coworkers claim that Kv1.5 does not target to rafts in HEK cells (Eldstrom et al., 2006). There is no clear explanation for this discrepancy. However, it could be a consequence of caveolin-deficient expression in the HEK-293 cell clone (Eldstrom et al., 2006). Indeed, Martens and coworkers recently described that caveolins govern Kv1.5 targeting to lipid rafts (McEwen et al., 2008).
Heterotetrameric Kv1.3/Kv1.5 channels contribute to the major Kv channel in macrophages (Vicente et al., 2006). Subunit association modulates channel targeting, leading to proper electrical signaling (Misonou and Trimmer, 2004; Vicente et al., 2008). Since Kv1.3, but not Kv1.5, was found in low-buoyant density fractions, our results confirm that Kv1.3 exists as a homomeric channel in macrophages (Vicente et al., 2006; Villalonga et al., 2007). In addition, Kv1.3/Kv1.5 heteromers redistributed Kv1.3 to non-floating domains (Fig. 2A,B). However, on activation, selective induction of Kv1.3 and caveolin targets heteromeric channels back to rafts. Similarly, in myocytes, where Kv1.5 is much more abundant (Villalonga et al., 2008), channels mostly concentrated in non-raft microdomains.
Does Kv1.5 target to lipid raft microdomains?
Kv1.5 co-immunoprecipitates with caveolin-1 in pulmonary artery smooth muscle cells (Cogolludo et al., 2006). In addition, caveolin-3 and SAP97 form a scaffolding protein complex in CHO cells (Folco et al., 2004). However, Eldstrom et al. (2006) described that Kv1.5 does not target to rafts (Eldstrom et al., 2006). Our data in no way contradict previous observations. While Kv1.5 localized in low-buoyancy fractions when expressed in HEK cells, Kv1.5 neither targeted to lipid rafts nor colocalized with caveolin in tissues and native cells. Similar discrepancies have been described with Kv4.2, which suggests the involvement of underlying factors (Martens et al., 2000; Wong and Schlichter, 2004). A possible mechanism links channels and nearby interacting elements.
Protein–protein interactions are implicated in the confinement of channels in lipid rafts. Postsynaptic, disc large, zonula occludens (PDZ) domain-containing proteins play a role in targeting Kv channels to caveolar-rafts (Tiffany et al., 2000). PSD-95 and SAP97 interact with Kv1.5 and recruit channels to a complex which includes caveolin-3 (Eldstrom et al., 2002; Folco et al., 2004). Caveolins present binding domains (CBD) and signaling proteins and channels with CBD localized in caveolae (Couet et al., 1997). Kv1.5 and Kv1.3 have CBD near the T1 domain (FXXXXWXXF). It is not known to what extent these elements are involved in caveolin-channel interactions. However, similar to what was found by coexpressing Cav3DGV, Kv1.5 follows caveolin after microtubule disruption (Martens et al., 2001) and exogenous caveolin expression (McEwen et al., 2008). Further partnership mechanisms are Kvβ subunits, which stabilize channels on the cell surface (Martens et al., 1999). Kvβ bind to channels at the T1 domain near CBDs (Gulbis et al., 2000). Therefore, both proteins may compete.
Why does Kv1.5 target differently in heterologous expression and native systems? Our results suggest that the presence of Kvβ subunits may impair the Kv1.5 localization in caveolar-lipid rafts. Unlike native systems, HEK-293 cells lack Kvβ subunits (Uebele et al., 1996). Therefore, the Kv1.5-CBD may be accessible. Fedida and coworkers found that Kv1.5 did not target to rafts in their Kv1.5-HEK cell (Eldstrom et al., 2006). However, as the authors point out, their HEK-293 cell line was defective in caveolin. We demonstrated that overexpression of Cav3DGV concentrated Kv1.5. In fact, specific induction of Kv1.3 in Kv1.3/Kv1.5 hybrid channels and caveolin targets back Kv1.5 to lipid rafts. On the contrary, differential Kvβ expression may interfere with Kv1.5 targeting to rafts microdomains.
The findings of the present study are of interest, since K+ channels in leukocytes are pharmacological targets in autoimmune diseases. While Kv1.3 efficiently targets to the lipid raft, the presence of Kv1.5 in these microdomains depends on several factors, including PDZ domain-containing proteins, caveolins, and Kvβ subunits. In macrophages, Kv1.3 associates with Kv1.5, and the Kv1.3/Kv1.5 ratio may change, leading to biophysically and pharmacologically distinct channels (Vicente et al., 2006; Villalonga et al., 2007). Furthermore, different subunit stoichiometry may modulate the channel targeting to microdomains enriched in signaling molecules. Kv1.3 redistributes in the presence of Kv1.5 (Vicente et al., 2008). Under different physiological stimuli, changes in the oligomeric composition of functional Kv could have crucial effects on intracellular signals, determining the specific macrophage response.
Acknowledgements
NV and RMM hold fellowships from the Ministerio de Educación y Ciencia (Spain). LS is supported by “Fundación La Caixa”. We thank the editorial assistance of American Journal Experts.




