Abstract
Human neutrophil peptides (HNP) kill microorganisms but also modulate immune responses through upregulation of the chemokine IL-8 by activation of the nucleotide P2Y6 receptor. However, the intracellular signaling mechanisms remain yet to be determined. Human lung epithelial cells (A549) and monocytes (U937) were stimulated with HNP in the absence and presence of the specific kinase inhibitors for Src, extracellular signal-regulated kinase-1 and -2 (ERK1/2), p38 mitogen-activated protein kinase (MAPK), c-Jun-N-terminal kinases (JNK), and Akt. HNP induced a rapid phosphorylation of the kinases in both cell types associated with a dose-dependent, selective production of IL-8 among 10 cytokines assayed. The HNP-induced IL-8 production was blocked by the Src tyrosine kinase inhibitor PP2, MEK1/2 inhibitor U0126, and the phosphatidylinositol 3 kinase (PI3K) inhibitor LY294002, but not by the JNK inhibitor SP600125 in both cell types. Treatment with the p38 inhibitor SB203580 attenuated the HNP-induced IL-8 production only in monocytes. Blockade of Src kinase blunted HNP-induced phosphorylation of the ERK1/2 and Akt but not p38 in monocytes. In contrast, Src inhibition had no effect on phosphorylation of the other kinases in the lung epithelial cells. We conclude that the activation of ERK1/2 and PI3K/Akt pathways is required for HNP-induced IL-8 release which occurs in a Src-independent manner in lung epithelial cells, while is Src-dependent in monocytes. J. Cell. Physiol. 214: 820–827, 2008. © 2007 Wiley-Liss, Inc.
Human neutrophil peptides (HNP) are known for their antimicrobial properties (Ganz, 2003). HNP level in plasma ranges from undetectable to 0.3 µg/ml in healthy volunteers but increases to 170 µg/ml in patients with bacterial infections (Panyutich et al., 1993; Ihi et al., 1997). These clinical studies suggested that HNP may play an important role contributing to immune response in inflammatory lung diseases. Increasing evidence indeed suggests that HNP also exert immune modulating function (Chen et al., 2006). We were particularly interested in the fact that HNP selectively induce the CXC chemokine IL-8 in human lung epithelial cells and other cell types (Okrent et al., 1990; Van Wetering et al., 1997; Sakamoto et al., 2005; Khine et al., 2006; Vaschetto et al., 2007), since it is a major mediator to modulate inflammatory responses. We have demonstrated that intratracheal instillation of HNP induced production of chemokines resulting in neutrophil infiltration and lung injury in mice (Zhang et al., 2001).
We have previously shown that both purified and synthetic HNP induce IL-8 production through the G-protein coupled nucleotide purinoceptor P2Y6 signaling in human lung epithelial cells (Khine et al., 2006). However, the downstream signaling pathways were not addressed (Khine et al., 2006). It is important to understand the intracellular mechanisms by which HNP modulate inflammation in order to develop novel strategies for therapeutic interventions in inflammatory lung diseases.
The Src family kinases are activated following engagement of several different classes of cell surface receptors, including immuno-receptors, cytokine receptors, and G-protein coupled receptors (GPCR) (Thomas and Brugge, 1997). Interestingly, a functional interaction between the nucleotide P2Y2 receptor and Src tyrosine kinases is facilitated by the Src homology 3 (SH3) binding motif in the C-terminus of the P2Y2 receptor (Liu et al., 2004; Weisman et al., 2005). Src protein tyrosine kinase mediates downstream signal transduction in many different ways. In response to extracellular stimuli, activation of Src kinases could further activate other downstream signals, such as phosphatidylinositol 3 kinase (PI3K)/Akt pathway, MAP kinases, and NF-κB pathway, depending upon the stimuli, cell types, and other confounding factors (Okutani et al., 2006). For example, Src activation triggered by reactive oxygen species can induce IκBα degradation resulting in NF-κB nuclear translocation (Fan et al., 2003). In response to a variety of stimulation Src kinases mediate mitogen-activated protein kinase (MAPK) activation (Kitagawa et al., 2002). Currently, the best described MAPK members are the extracellular signal-regulated kinase-1 and -2 (ERK1/2), the p38 MAPKs, and the c-Jun-N-terminal kinases (JNKs). MAPKs can also be activated in response to extracellular stimuli independent of Src modulation (Puddicombe and Davies, 2000).
Since the human P2Y6 protein sequence lacks the SH3 binding motif (PXXP) domain (NCBI, 2007) thus whether HNP-induced IL-8 production involves activation of Src kinases is yet to be examined. In the present study, we sought to investigate whether lung structural and immune cells share common pathways to release IL-8 by using human lung epithelial cells (A549) and monocytes (U937). We demonstrated that Src kinases play an important role in the HNP-induced IL-8 release that is independent of ERK1/2 and PI3K/Akt activation in lung epithelial cells while is dependent of these kinases in monocytes.
Materials and Methods
Reagents
All primary antibodies used were from Cell Signaling Technology (Danvers, MA), and secondary antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA). Enhanced chemiluminesence (ECL) kit was from Amersham Pharmacia (Piscataway, NJ). The kinase inhibitors PP2, U0126, SB203580, SP600125, and LY294002 were purchased from Calbiochem (La Jolla, CA). The process of purification of HNP has been described previously (Khine et al., 2006; Vaschetto et al., 2007).
Cell culture and treatment
Human lung epithelial type II like cells (A549 cell, ATCC, Manassas, VA) were cultured and maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS). Human U937 monocytes (ATCC) were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated FBS (Gibco), penicillin (100 U/ml), streptomycin (100 U/ml), 0.5% L-glutamine (Sigma, St. Louis, MO), and 1 mM sodium pyruvate (Sigma) and maintained at 37°C with 5% CO2 in a humidified incubator. All experiments were conducted within four passages of the cells used.
To measure cytokines, confluent A549 cells cultured in 24-well plates (Corning Costar, Cambridge, MA) or U937 cells in suspension (1 × 106 cells/ml) were incubated overnight with serum-free medium followed by stimulation with HNP at indicated concentrations. For blocking assays, the cells were treated with the various concentrations of kinase inhibitors 30 min prior to stimulation with HNP. The cell supernatants were collected, centrifuged and aliquots were stored at −80°C until further analysis for cytokines.
For Western blot analysis, A549 cells in six-well plates (Corning Costar) after reaching confluence or U937 cells in suspension (1 × 106 cells/ml) were serum-starved overnight. Quiescent cells were then subjected to HNP stimulation as detailed in the figure legends. For blocking experiments, cells were pre-treated with inhibitors 30 min prior to HNP stimulation. The A549 cells were then lysed in Nonidet P-40 (NP-40) lysis buffer (1% NP-40, 137 mM NaCl, 20 mM Tris, 2 mM EDTA (pH 8.0), 10% glycerol, 1 mM sodium orthovanadate (Na3VO4), 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 µg/ml leupeptin, and 10 µg/ml aprotinin) and incubated on ice for 10 min followed by centrifugation at 4°C. The total protein concentrations of cell lysates were determined by Bio-Rad DC protein assay kits (Bio-Rad, Hercules, CA) using bovine serum albumin (BSA) as standard.
Western blot analysis
Equal amounts of protein were subjected to 10% SDS–PAGE gel for Western blotting. The supernatants of cell lysates were further prepared in Laemmli sample buffer (100 mM DTT, 10% glycerol, 3% SDS, and 0.025% bromophenol blue). The U937 cells were lysed in triton lysis buffer (1% Triton X-100, 10 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 1 mM PMSF, 1 mM Na3VO4, 10 µg/ml leupeptin, 10 µg/ml aprotinin). Cell lysates were further mixed with gel loading buffer (5% β-mercaptoethanol, 10% glycerol, 2% SDS, and 0.025% bromophenol blue). The samples were separated in SDS–PAGE, and transferred into a nitrocellulose transfer membrane (Bio-Rad) in 20% methanol buffer containing 1 mM Na3VO4. The membrane was blocked in 5% (w/v) BSA in 1 × TBST buffer (25 mM Tris, pH 7.4, 150 mM NaCl, 0.4 % Tween-20, 0.05 % NP-40, 1 mM Na3VO4) at 4°C overnight followed by incubation with a primary phospho-specific antibody in 5% (w/v) BSA overnight at 4°C. The membrane was washed three times at 15 min intervals with TBST wash buffer (TBST buffer without Na3VO4) and then incubated with an HRP-conjugated second antibody for 1 h at room temperature. The membrane was extensively washed again, developed with an ECL kit (Amersham Phamacia), and reprobed with an antibody against the total protein of the same kinase. The band density was evaluated by a Kodak image station 2000 MM (Mandel, Guelph, Ont., Canada). The phosphorylation/total (p/t) ratio was measured and expressed as fold increase from control conditions.
Transfection of antisense oligonucleotides
Transfection of U937 cells with the sense or antisense oligonucleotides (2.5 µM final concentration) corresponding to the translation initiation sites of human P2Y6 were performed by using Lipofectamine reagent (Gibco). The sequences included P2Y6 sense, 5′-GCCATGGAATGGGACAATG-3′; and P2Y6 antisense, 5′-CATTGTCCCATTCCATGGC-3′. The transfection medium was replaced with complete DEME 4 h after transfection and the cells were incubated overnight at 37°C. The cells were washed with phosphate-buffered saline (PBS), and serum free medium prior to stimulation with HNP.
Cytokine assay
Cell supernatants were simultaneously analyzed for ten cytokines including TNFα, IFNγ, GM-CSF, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, and IL-12 on a multiplex station using the Biorad Multiplex kit (Biorad, Hercules, CA). A human IL-8/NAP1 ELISA kit (Biosource International, Camarillo, CA) was used subsequently to quantify IL-8 in the culture medium.
Cytotoxicity measurement
To evaluate the cell viability in the conditions where HNP or/and the kinase inhibitors were present, LDH activity was measured at 490 nm using a cytotoxicity detection kit (Roche Applied Science, Penzberg, Germany). Both cell culture supernatants and cell lysates were used and a < 5% of cytotoxicity was ensured in all experimental conditions reported.
Statistical analysis
The statistical analysis was performed using GraphPad Prism. One-way analysis of variance with post hoc analysis by Student–Neuman–Keuls test was used, and P < 0.05 is considered significant. Results are expressed as the mean ± SEM.
Results
HNP selectively induce IL-8 release in a dose-dependent manner
Figure 1A,B show that stimulation with HNP at10 µg/ml selectively induced the release of IL-8 out of 10 cytokines assayed in both the epithelial cells at 8 h and the monocytes at 4 h, respectively. These results confirm and extend the previous observation where we demonstrated that HNP selectively induced IL-8 release in A549 cells (Khine et al., 2006). The lung epithelial cells appear to be more resistant to stimulation with HNP than the monocytes, because higher doses and longer stimulation are required for IL-8 induction (Fig. 1C,D). In subsequent experiments, we thus used IL-8 as a biological readout in response to HNP stimulation. Similar to lung epithelial cells (Khine et al., 2006), we observed that P2Y6 signaling was involved in the HNP-induced IL-8 production in the monocytes that was blocked by using specific antisense (Fig. 1E).
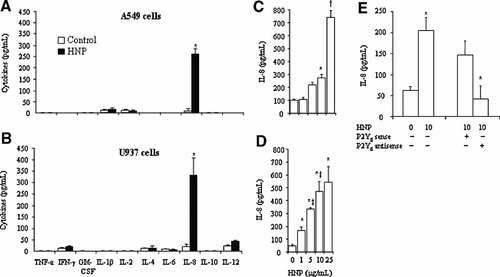
HNP induce IL-8 release. Cells were incubated overnight with serum-free medium, and exposed to either 0.01% acetic acid as control or HNP (10 µg/ml) for 8 h in A549 cells (A) or for 4 h in U937 cells (B). Cell supernatants were then collected for multiple cytokine assays. A549 cells or U937 cells were incubated in the presence of HNP at indicated concentrations for 8 h (C) and 4 h (D), respectively. In separate experiments, U937 cells were transfected with P2Y6 sense and antisense, respectively, prior to HNP stimulation for 8 h (E). IL-8 levels were measured in cell supernatants by an ELISA specific for human IL-8. N = 6 from three experiments. *P < 0.05 vrsus 0 control; †P < 0.05 versus other groups at identical conditions; and ‡P < 0.05 versus the immediate previous group, respectively.
Src tyrosine kinase is required for HNP-induced IL-8 production
The Src phosphorylation at tyrosine 416 (Y416) was used as a marker to determine its activation (Xu et al., 2007), in response to HNP stimulation in the two cell types. Figure 2A,D illustrates that stimulation with HNP for up to 30 min resulted in a dose-dependent Src phosphorylation. Treatment with the PP2, a specific inhibitor of Src family kinases, markedly attenuated Src phosphorylation (Fig. 2B,D) that was associated with a reduction of the HNP-induced IL-8 production (Fig. 2C,E). These data suggest that Src tyrosine kinases play an important role in mediating HNP-induced IL-8 production in lung epithelial cells and monocytes.
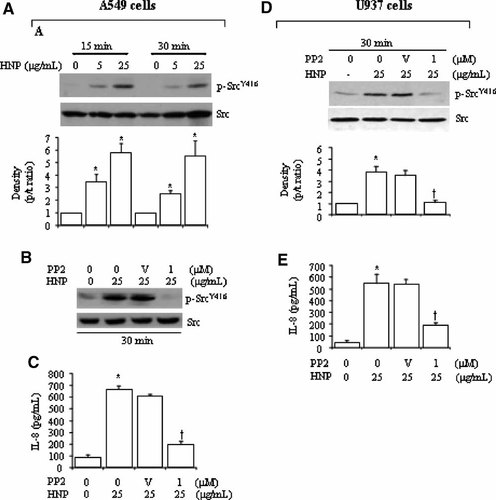
Src tyrosine kinase is required for HNP-induced IL-8 production. A549 cells and U937 cells were incubated with either control solution (0.01% acetic acid, 0 µg/ml) or HNP at indicated concentrations and time points in the presence and absence of PP2, given 30 min prior to HNP stimulation. The cell lysates were analyzed by Western blot to determine phosphorylated and total levels of Src. A: A representative blot showing HNP-induced Src activation and the mean band density analysis from three experiments in A549 cells. The use of the Src inhibitor PP2 decreased HNP-induced Src activation (B) and attenuated IL-8 production (C). D: A representative blot showing HNP-induced Src activation and the mean band density analysis from three experiments in U937 cells. The use of PP2 blunted the HNP-induced Src activation and attenuated IL-8 production (E). V: 0.01% DMSO vehicle control of PP2; *P < 0.05 versus 0 control at identical conditions, respectively; †P < 0.05 versus vehicle control (V) in the presence of HNP at identical conditions, respectively.
PI3K/Akt signaling in HNP-induced IL-8 production and its relation to Src protein kinases
PI3K/Akt is a downstream pathway for P2Y2 signaling in monocytes (Santiago-Perez et al., 2001) and for other P2 purinoreceptor signaling in human glioma cells (Jacques-Silva et al., 2004). We examined the PI3K/Akt pathway in the HNP/P2Y6-induced IL-8 production. Figure 3A,D show a phosphorylation of Akt in response to HNP stimulation in the two cell types. Treatment with LY294002, a potent and specific inhibitor of PI3K that is considered as upstream kinase of the Akt pathway, resulted in a dose-dependent attenuation of the HNP-induced IL-8 production (Fig. 3B–E). Interestingly, inhibition of Src by PP2 did not significantly alter the HNP-induced phosphorylation of Akt in A549 cells (Fig. 4A), but abrogated the Akt phosphorylation in U937 cells (Fig. 4B). This suggests that the Src activation is not required for HNP-induced PI3K/Akt activation in the epithelial cells but is required in the monocytes.
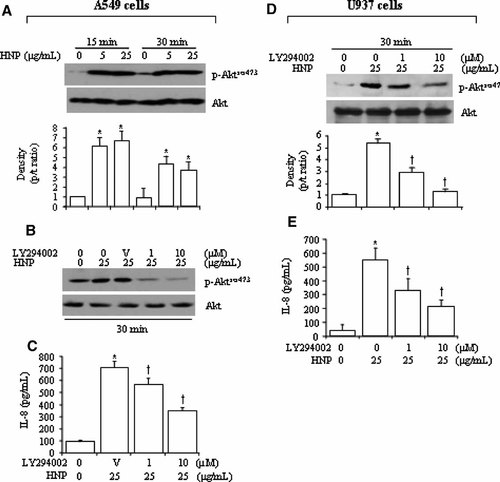
PI3K/Akt pathway in HNP-induced IL-8 production. A549 and U937 cells were incubated with either control solution (0.01% acetic acid, 0 µg/ml) or HNP at indicated concentrations for 15–30 min in the presence and absence of LY294002 given 30 min prior to HNP. The cell lysates were analyzed by Western blot. A: Representative blots of HNP-induced Akt activation, and density analysis. B: Blockade of HNP-induced Akt phosphorylation by LY294002. C: Inhibition of PI3K by LY294002 attenuated the HNP-induced IL-8 production. D: LY294002 decreased the HNP-induced Akt phosphorylation. E: LY294002 attenuated the HNP-induced IL-8 production. N = 6 from three independent experiments. *P < 0.05 versus 0 control at identical conditions; and †P < 0.05 versus control with no LY294002 in the presence of HNP at identical conditions, respectively.
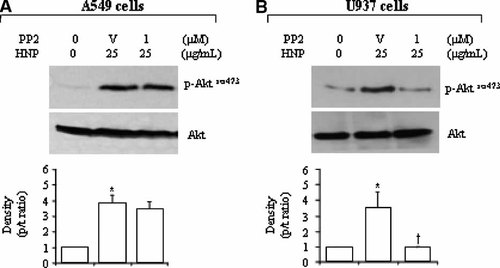
Effect of Src kinase blockade on HNP-induced Akt activation. A549 and U937 cells were treated with PP2 for 30 min and stimulated with HNP for additional 30 min. The cell lysates were analyzed to detect phosphorylated and total levels of Akt. Representative blots from three independent experiments, along with densitometric analysis. V: DMSO vehicle control of PP2; *P < 0.05 versus 0 control (0.01% acetic acid, 0 µg/ml HNP) at identical conditions; and †P < 0.05 versus vehicle control in the presence of HNP at identical conditions, respectively.
MAPK signaling in HNP-induced IL-8 production and their relationships to Src protein kinases
The MAPK family members such as ERK1/2 and p38 have been shown to modulate downstream signaling in response to P2Y2 activation in human monocytes (Marcet et al., 2007; Santiago-Perez et al., 2001). We thus investigated the role of these signaling mechanisms in HNP-mediated IL-8 production.
Figure 5A–C and E–G show that HNP stimulation activated the ERK1/2, p38, and JNK in the lung epithelial cells and monocytes. The HNP-mediated MAPK activation was blocked by the specific inhibitors in a dose-dependent manner in the two cell types (Fig. 5D–G). However, in A549 cells the HNP-induced IL-8 production was blunted only by the MEK inhibitor U0126. In U937 cells, not only the MEK inhibitor U0126 but also the p38 inhibitor SB203580 blocked the HNP-induced IL-8 production. The JNK inhibition by using SP600125 had no effect on the HNP-induced IL-8 release in the two cell types (Fig. 6A,B).
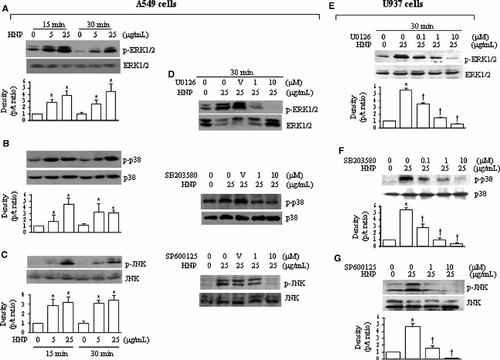
HNP induces MAPK activation. A549 and U937 cells incubated with either control solution (0.01% acetic acid, 0 µg/ml) or HNP at indicated concentrations for 15 and 30 min in the presence and absence of either U0126 (MEK1/2 inhibitor), SB203580 (p38 inhibitor), or SP600125 (JNK inhibitor) given 30 min prior to HNP. A–C: Representative Western blots of the HNP-induced MAPKs activation in A549 cells. D: The specific MAPK inhibitors decreased the HNP-mediated MAPK activation. E–G: Representative Western blots of the HNP-induced MAPKs activation in U937 cells, and the specific MAPK inhibitors decreased the HNP-mediated MAPK activation. V: DMSO vehicle control; *P < 0.05 versus 0 control at identical conditions; and †P < 0.05 versus control in the presence of HNP at identical conditions, respectively.
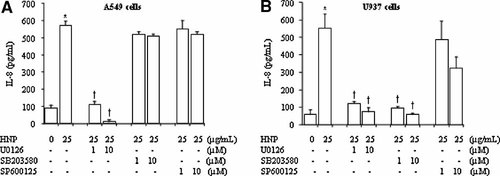
Involvement of MAPKs in the HNP-mediated IL-8 production. A549 and U937 cells were treated with the specific inhibitors of MAPKs 30 min prior to HNP stimulation for 6 h in A549 cells (A) and 4 h in U937 cells (B). IL-8 levels were measured in cell supernatants by ELISA. N = 8 from four independent experiments. V: DMSO vehicle control; *P < 0.05 versus 0 control (0.01% acetic acid) at identical conditions; and †P < 0.05 versus control in the presence of HNP at identical conditions, respectively.
We went further to examine the relationship between Src kinase and the MAPKs in response to HNP. The Src blockade by using PP2 did not influence the HNP-induced activation of ERK1/2 in the lung epithelial cells (Fig. 7A), but decreased phosphorylation of ERK1/2 without affecting p38 in the monocytes (Fig. 7B,C). Since p38 signal was not involved in the HNP-induced IL-8 production in the lung epithelial cells, the effect of Src inhibition on p38 was not further studied. These findings suggest that Src is not required for activation of ERK1/2 in the lung epithelial cells, but the activation of ERK1/2 and p38 is dependent on Src in the monocytes.
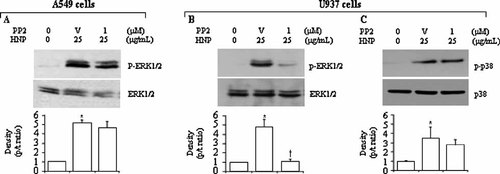
Effect of Src kinase on HNP-induced MAPK activation. A549 and U937 cells were treated with PP2 30 min prior to HNP stimulation for additional 30 min. The cell lysates were analyzed to detect phosphorylated and total levels of ERK1/2 and p38. Representative Western blots along with densitometric analysis are illustrated. N = 8 from four independent experiments. V: DMSO vehicle control of PP2; *P < 0.05 versus 0 control (0.01% acetic acid) at identical conditions; and †P < 0.05 versus vehicle control in the presence of HNP at identical conditions, respectively.
Discussion
The main findings of the present study are that: (1) both Src-dependent and -independent mechanisms are involved in HNP-induced IL-8 release; (2) the activation of PI3K/Akt and ERK1/2 are required for HNP-induced IL-8 production in the lung epithelial cells while PI3K/Akt, ERK1/2, and p38 are involved in the monocytes; and (3) the activation of PI3K/Akt and ERK1/2 is independent of Src in the lung epithelial cells and is partially dependent on Src in the monocytes in response to HNP stimulation.
We have previously reported that HNP selectively induce IL-8 production out of 10 common cytokines and chemokines assayed in the epithelial cells (Khine et al., 2006). The present study expands the previous finding by showing that HNP induce only IL-8 production in the monocytes. Also noteworthy is that HNP were able to upregulate the expression of TNF-α and IL-1β in monocytes activated with the Gram-positive spherical bacteria Staphylococcus aureus or phorbol myristate acetate (PMA), while HNP alone was unable to induce TNF-α and IL-1β expression in resting monocytes (Chaly et al., 2000). We also demonstrate that the lung epithelial cells are relatively more resistance in response to HNP stimulation compared to the monocytes with respect to IL-8 production. A longer time of stimulation (8 h vs. 4 h) and a higher dose (25 µg/ml vs. 1 µg/ml) of HNP are required for a significant IL-8 release in the lung epithelial cells than in the monocytes. The different responses between the two cell types are yet to be determined. The doses of HNP used are similar to the HNP concentrations detected in patients with acute respiratory distress syndrome (Ashitani et al., 2004), pneumonia (Ashitani et al., 2007), and sepsis (Panyutich et al., 1993), and did not induce cytotoxicity in the two cell types assessed by a LDH assay (data not shown).
We have previously demonstrated that HNP induce IL-8 production via P2Y6 signaling (Khine et al., 2006). The present study was to examine the downstream signaling mechanisms. Since Src tyrosine kinases is known to be a downstream effector of P2Y2 signaling (Santiago-Perez et al., 2001; Katz et al., 2006; Shankar et al., 2006), thus we thought the HNP induced-IL-8 expression is independent of Src activation because of a lack of SH3 binding motif to Src kinase in the P2Y6 (NCBI, 2007). Interestingly, we demonstrate that stimulation with HNP resulted in Src kinase phosphorylation that is required for IL-8 production in both cell types studied but the specific phenotype(s) of the Src family kinases is yet to be identified. We have previously demonstrated that stimulation with HNP of lung tissues resulted in an enhanced production of H2O2 (Porro et al., 2001) that has been reported to potentate activation of Src kinases (Fan et al., 2003; Okutani et al., 2006). Although the Src family members in these two cell types are yet to be identified in mediating the HNP signaling, blocking Src kinases by PP2 blunted the HNP-induced IL-8 release suggesting an important role of this pathway in mediating HNP-induced inflammatory responses.
We also demonstrate that HNP induce rapid activation of multiple kinases including ERK1/2, p38, JNK, and PI3K/Akt in the two cell types. However, only ERK1/2 and PI3K/Akt are functionally involved in the HNP-induced IL-8 release in the lung epithelial cells. Interestingly, a recent study showed that the active peptide LL-37 of the human cathelicidin cationic antimicrobial protein-18 expressed in neutrophils activates ERK1/2 to induce IL-8 production in lung epithelial cells (Tjabringa et al., 2003). Another study reported that the HNP-mediated proliferation of lung epithelial cells is dependent on ERK1/2 signaling (Aarbiou et al., 2002). Taken together, the present and other studies suggest that HNP can activate ERK1/2 in the lung epithelial cells.
When the above experiments were repeated in the monocytes, we found that not only ERK1/2 and PI3K/Akt but also p38 are required for the HNP-induced IL-8 production. Interestingly, LL-37 released from neutrophils also induce IL-8 production through activation of ERK1/2 and p38 in human monocytes (Bowdish et al., 2004). These data suggest that the antimicrobial peptides stored in the neutrophils may share similar intracellular signaling pathway to induce IL-8 production in the target cells.
We went to further to examine the protein–protein crosstalk between Src protein tyrosine kinases and the MAPK cascades in the HNP-induced IL-8 release by blocking Src kinase with the PP2. It appeared that neither ERK1/2 nor Akt phosphorylation was affected by PP2 in the lung epithelial cells, suggesting that the Src kinases modulate the HNP-induced IL-8 production independent of MEK/ERK1/2 and PI3K/Akt pathways. As mentioned above, we speculate that an alterative signaling pathway by which Src kinase activation induce IL-8 resulted from the generation reactive oxygen species following HNP stimulation in lung tissue (Porro et al., 2001). There is evidence that Src can be activated by reactive oxygen species leading to NF-κB activation (Fan et al., 2003; Okutani et al., 2006). On the contrary, blockade of Src in the monocytes attenuated the HNP-induced ERK1/2 and Akt phosphorylation. This suggests that Src kinases are upstream of ERK1/2 and PI3K/Akt in mediating HNP-induced IL-8 production in monocytes (Fig. 8). It is noteworthy that a recent study reported that Src is directly associated with Akt through the interaction between its SH3 domain and the PXXP motif in the C-terminal regulatory region of Akt (Jiang and Qiu, 2003).
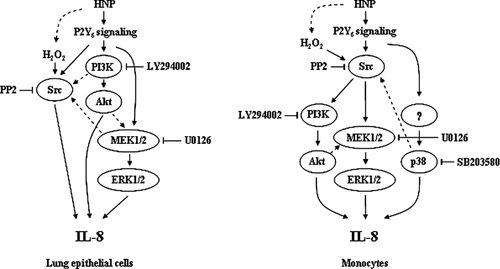
Differential signaling mechanisms of HNP-induced IL-8 production in human lung epithelial cells and monocytes. MEK1/2/ERK1/2 and PI3K/Akt activation is required for the HNP-induced IL-8 release that is independent of Src kinases in lung epithelial cells, while is dependent of these kinases in monocytes. The p38 signal is involved in the HNP-induced IL-8 production only in monocytes, which is independent of Src kinases. Dashed lines suggest other possible interrelated signal pathways.
In conclusion the activation of ERK1/2 and PI3K/Akt pathways is required for HNP-induced IL-8 release which occurs in a Src-independent manner in lung epithelial cells, while is Src-dependent in monocytes. These findings provide further insights into the molecular mechanisms regulating HNP-induced IL-8 release and may aid in designing novel therapeutic strategies to reverse the unwanted HNP-induced inflammatory responses.
Acknowledgements
This work is supported by Canadian Institutes of Health Research (CIHR) operating grants to ML, AS, and HZ and Ontario Thoracic Society (OTS) grant-in-aid to HZ.




