The MAP kinase JNK-1 of Caenorhabditis elegans: Location, activation, and influences over temperature-dependent insulin-like signaling, stress responses, and fitness†
Marc Wolf and Frank Nunes contributed equally to this work.
Abstract
The mitogen-activated protein kinase (MAPK) pathways and insulin-like signaling play pivotal roles in cellular stress response. Using an anti-phospho-SAPK/JNK antibody and a daf-16::GFP-based reporter assay, the present study shows in Caenorhabditis elegans that ambient temperature (1–37°C) specifically influences the activation (phosphorylation) of the MAP kinase JNK-1 as well as the nuclear translocation of DAF-16, the main downstream target of insulin-like signaling. Activated JNK-1 was detected only in neuronal cells, and JNK-1 was found to be controlled by the MAPK JKK-1 under heat stress. Comparative analyses on the wildtype and a jnk-1 deletion mutant revealed a promoting influence of JNK-1 on both nuclear DAF-16 translocations and DAF-16 target gene (superoxide dismutase 3, sod-3) expressions within peripheral, non-neuronal tissue. Consequently, the mutant exhibited a reduced thermal tolerance and reproductive fitness at higher temperatures. These results provide evidence of indirect interactions between neuronal MAPK and peripheral insulin-like signaling in response to environmental stimuli (temperature, H2O2). J. Cell. Physiol. 214: 721–729, 2008. © 2007 Wiley-Liss, Inc.
The mitogen-activated protein kinase (MAPK) pathways and the insulin-like receptor pathway (DAF-2 pathway) are important signaling cascades involved in cellular stress responses. MAP kinases are a widely conserved family of serine/threonine protein kinases. They act as central elements of signaling cascades, which are essential for many cellular processes such as cell proliferation and differentiation, cell movement, cell death, and cellular stress response (Cobb, 1999; Davis, 2000; Pearson et al., 2001; Kim et al., 2004). These cascades transmit environmental and cellular signals by a sequence of phosphorylations of different MAP kinases. In the nematode Caenorhabditis elegans, the DAF-2 pathway plays a pivotal role in the formation of dauer larvae, determination of lifespan and regulation of stress response toward various stressors (Barsyte et al., 2001; Lithgow and Walker, 2002; Garsin et al., 2003; Lamitina and Strange, 2005). The main downstream target of DAF-2 signaling is DAF-16, a transcription factor of the forkhead box O (FOXO) protein family (Murphy et al., 2003). An activation of the DAF-2 pathway by stimulatory insulin-like peptides leads to the phosphorylation of DAF-16, which prevents the nuclear translocation of this transcription factor. Low food supply, high population density or abiotic stressors cause a downregulation of insulin-like signaling and a subsequent dephosphorylation of DAF-16, which then translocates from the cytoplasm into the cell nucleus, to either initiate or repress the expression of target genes (Henderson and Johnson, 2001). DNA microarray analyses as well as chromatin immunoprecipitation experiments revealed that DAF-16 particularly participates in the upregulation of genes involved in the cellular stress response including genes encoding for superoxide dismutases (Murphy et al., 2003; Gems and McElwee, 2005; Oh et al., 2006).
Recently, it has been shown in transfected COS-7 culture cells (Oh et al., 2005) that the activated C. elegans MAPK JNK-1 is able to directly phosphorylate DAF-16. In addition, a generally higher level of nuclear DAF-16 translocation was reported for a jnk-1 overexpressing C. elegans strain. JNK-1 is a homolog of human stress-activated protein kinases (SAPK) and by GFP reporter gene assays, jnk-1 expression in the worm's nervous system has been demonstrated (Kawasaki et al., 1999). C. elegans jnk-1 deletion mutants are short-lived and more susceptible to heavy metal stress. Overexpression of jnk-1 increases the resistance to oxidative stress and prolongs the lifespan of the worms (Villanueva et al., 2001; Oh et al., 2005).
In the present study, we focused on the role of ambient temperatures below, at, and above the presumptive optimum range (15–20°C) of C. elegans for nuclear DAF-16 translocations as well as on the hitherto not linked results of a neuronal expression of jnk-1 (Kawasaki et al., 1999) and an enhanced DAF-16 translocation due to jnk-1 overexpression (Oh et al., 2005). These results indicate an indirect impact of neuronal JNK-1 on DAF-16 translocations in non-neuronal tissue. First, we localized activated (phosphorylated) JNK-1 and studied the temperature dependence of JNK-1 activation using an anti-phospho-SAPK/JNK antibody. Then, we studied the role of temperature for the nuclear DAF-16 translocation in peripheral cells (intestine) of the wildtype and a jnk-1 deletion mutant using a daf-16::GFP-based reporter assay, and investigated the role of JNK-1 for the expression of DAF-16 target genes (superoxide dismutase 3, sod-3) in peripheral tissues. Finally, we looked for the consequences of jnk-1 deletion in terms of thermal tolerance and reproductive fitness.
Materials and Methods
Organisms
The nematode C. elegans was kept as described by Brenner (1974) at 20°C on agar plates containing E. coli OP50 as food source. The following strains were used for the experiments: (i) C. elegans N2 (Bristol variety), (ii) VC8 jnk-1 (gk7) IV, (iii) CF1139 daf-16::GFP (mu86) I; muIs61, (iv) KU2 jkk-1 (km2), (v) FK171 mek-1 (ks54), and (vi) GR1307 daf-16 (mgDf50) (cf. http://www.wormbase.org/). All strains were kindly provided by the Caenorhabditis Genetics Center (Minneapolis, MN).
Microscopy
An inverse microscope (Axiovert 100 with 40× PlanNeofluar objective; Zeiss, Germany) equipped with a CCD camera (Kappa CF 8/1 FMC; Kappa, Gleichen, Germany) was used for fluorescence microscopy. Images were captured at identical exposure time by a frame grabber (DT2867; Data Translation, Marlboro, MA). Image processing and enhancement was made using Adobe Photoshop© (Adobe Systems Incorporated, San Jose, CA).
Whole-mount antibody staining of phosphorylated JNK-1
The wildtype and the jnk-1 (gk7) deletion mutant strain were heat stressed on standard NGM plates for 45 min at 37°C to stimulate JNK-1 activation (phosphorylation). The incubated worms were washed off the plates with sterile water and centrifuged. The obtained pellets were transferred to Poly-L-Lysin (Sigma, Deisenhofen, Germany) coated microscope slides, on which the worms were fixed with methanol and acetone (−20°C; 20 min each) followed by rehydration via an ethanol dilution series (90, 60, 30%; 10 min each). The slides were washed afterwards three times with TBS-Tween (20 mM Tris-HCl, 150 mM NaCl, 1% Tween 20, pH 8) and incubated overnight at 4°C with the 1:100 diluted rabbit anti-phospho-SAPK/JNK (Thr183/Tyr185) antibody (mAb#4668; Cell Signaling Technology, Beverly, MA). The antibody is specific for a highly conserved protein motif of human JNK, which is identical in C. elegans JNK-1α/β. Then, the slides were washed three times again with TBS-Tween, and the staining was detected using a 1:100 diluted Texas-red-conjugated anti-rabbit antibody (Jackson ImmunoResearch Europe, Cambridgeshire, UK), which was applied for 4 h at room temperature, before the slides were finally washed with TBS-Tween and sealed for analysis. To additionally test the specificity of the used antibody for phosphorylated JNK-1, Δjkk-1 (KU2) and Δmek-1 (FK171) mutant worms were probed as well.
Western blot analysis of JNK-1 phosphorylation
To check for a temperature-dependent activation of JNK-1, wildtype worms were incubated for 45 min at 1, 15, 20, and 37°C respectively. The worms were washed off the NGM plate with sterile water, centrifuged, and the resulting pellet was quick-frozen in liquid nitrogen. After thawing, PMSF was added to a final concentration of 2 mM, and the extracts were resuspended in 0.5 vol. 3× SDS–PAGE sample buffer (188 mM Tris, pH 6.8, 300 mM SDS, 30% glycerin, and 75 mM DTT). The samples were heat denaturated for 5 min at 95°C and finally sonicated.
For Western analysis, equal amounts of protein were separated by SDS–PAGE (protein marker: PageRuler Prestained Protein Ladder; Fermentas, St. Leon-Roth, Germany) and transferred to nitrocellulose membranes. The membranes were probed overnight with the rabbit anti-phospho-SAPK/JNK (Thr183/Tyr185) antibody (Cell Signaling Technology) for immunodetection of phosphorylated JNK-1. Alkaline phosphatase-conjugated (AP) goat anti-rabbit IgG (Dianova, Hamburg, Germany) was subsequently used for 1 h as secondary antibody. Proteins were detected by NBT/BCIP precipitation. As an antibody for non-phosphorylated JNK-1 was not available, protein-loading control was carried out using a mouse anti-tubulin antibody (Sigma) and a secondary HRP-conjugated anti-mouse antibody followed by ECL detection.
Construction of a jnk-1 deletion mutant strain expressing daf-16::GFP
To generate a daf-16::GFP expressing jnk-1 (gk7) deletion mutant strain, males of the mutant were mated with CF1139 [daf-16::GFP (mu86) I; muIs61] hermaphrodites. Males of the F1 progeny showing the CF1139 roller phenotype were then mated with jnk-1 (gk7) deletion mutant hermaphrodites. Resulting F2 hermaphrodites, which exhibited the roller phenotype, were then separated on NGM plates. The F1 progeny of the separated worms showing also the roller phenotype were tested for homozygote jnk-1 deletion via PCR (94°C, 20 sec; 50°C, 30 sec; 72°C, 30 sec; 30 cycles) using the primers AGCTCTCCAAATATACACCC and GTAGAAGCGTGGAAGAGGA. The obtained homozygote jnk-1 deletion mutant and daf-16::GFP expressing strain was termed MS7. The MS7 strain was checked for the presence of a wildtype daf-16 gene copy via PCR analysis using the following primers: CTCGTTCTCTCCGTATTTCCACA and CCATTAAGTGTCGAGTGAAGGGA. No wildtype daf-16 gene copy was found additionally proving the correct genetic background of the MS7 strain.
Construction of a jnk-1 rescue strain
To generate a jnk-1 rescue strain, genomic DNA of the jnk-1 gene was amplified via PCR (94°C, 20 sec; 59°C, 30 sec; 72°C, 6.5 min; 35 cycles) using the following primers: GTCCATACTTAAAGTTATCTCACCAGTCCAG and GCATTGTAGGCGGTTGAAGTTGCCAAAGTCA. The obtained PCR product was cloned into the vector pJET1 (Fermentas) and injected into Δjnk-1 (VC8) deletion mutant worms (see the paragraph on the sod-3::DsRed fusion construct for microinjection procedures). Expression of the jnk-1 transcript was confirmed via RT-PCR analysis using the primers AGCTCTCCAAATATACACCC and GTAGAAGCGTGGAAGAGGA.
DAF-16 translocation assay
Translocation of DAF-16-GFP from the cytoplasm into the cell nuclei of intestinal cells was studied by analyzing the degree of nuclear GFP fluorescence. Three different states can easily be distinguished (no, weak, or strong nuclear GFP fluorescence), which are related to a cytoplasmic, intermediate, or nuclear location of DAF-16-GFP. To study the temperature-dependent nuclear translocation of DAF-16, at least 20 young adult worms of both, the CF1139 and the MS7 strain, were transferred to fresh NGM plates and incubated for 2 h at 1, 8, 15, 20, 25, and 30°C, respectively. After incubation, 10 worms were randomly picked into 12.5 µl of Levamisole (1 mM in M9 buffer) on a microscope slide to anaesthetize the animals, sealed with a cover slip and inspected under a fluorescence microscope. An image of the posterior intestinal segments (int 8 and int 9) was taken of each worm, and only intestinal cells were analyzed for DAF-16 translocation. The degree of nuclear translocation of DAF-16 was evaluated by counting the number of worms showing an either weak or strong nuclear GFP fluorescence and computing the relative share (%) of these worms in relation to the total number of worms observed. Neuronal or hypodermal cell nuclei were not taken into account as DAF-16 translocation in these tissues may occur irrespectively of the applied temperature stress due to handling and/or microscopy procedures. The analysis of 10 worms took about 5–10 min. Hydrogen peroxide was used to analyze reactive oxygen species (ROS)-induced DAF-16 translocation. Five hundred microliter of a H2O2 solution was applied and allowed to diffuse into the agar of bacteria-free standard NGM plates with the H2O2 concentration of the solution adjusted to obtain a final concentration of 1 mM within the agar. At least 60 young adult CF1139 and MS7 worms were transferred to these plates and incubated at 20°C in the dark. After 60, 90, 120, and 150 min, respectively, 10 worms were picked off the plate and analyzed for DAF-16 translocation. Analysis was conducted as described previously for the temperature-dependent DAF-16 translocation.
Generation of a sod-3::DsRed fusion construct and microinjection of C. elegans
For plasmid construction, a genomic DNA fragment of the sod-3 gene was amplified via PCR (94°C, 20 sec; 50°C, 30 sec; 72°C, 2 min; 32 cycles) using the following primers: TGCAAAACGAGCAGGAAAGTCA and AGTGGTACCATTCCTTCCAAAG. The latter primer contained a wobble to insert a KpnI restriction site. The fragment contained 1,146 bp of the sod-3 gene (524 bp without introns) and 1,078 bp of the 5′ region including the predicted SKN-1 and DAF-16 binding sites. The fragment was cloned after PstI/KpnI digestion into the DsRed containing vector pPromrp2-3.8, which was also cut by PstI and KpnI.
Germline transformation was carried out as described by Mello et al. (1991). sod-3 reporter plasmids were injected at concentrations varying from 5 to 15 ng/µl. As selectable marker for the isolation of transgenic worms under stereo-microscopical observation (green fluorescence at blue excitation), the plasmid pPD118.33, which expresses GFP under the myo-2 promoter in pharyngeal muscles (Mello and Fire, 1995), was co-injected at a concentration of 50 ng/µl. Single worms of the F1 progeny showing GFP expression were transferred to separate NGM plates after 3 days of incubation at 20°C. After an additional incubation for 3 days at 20°C, F2 animals showing pharyngeal GFP expression were selected and maintained to isolate heritable transgenic lines. The obtained sod-3::DsRed expressing wildtype (showing red fluorescence at green excitation under fluorescence-microscopical observation) and the jnk-1 (gk7) deletion mutant strain were termed MS8 and MS9. Three independent lines were analyzed in the course of the experiments.
sod-3::DsRed expression and co-location
Expression of the sod-3::DsRed fusion construct was studied in wildtype (MS8) and jnk-1 deletion mutant (MS9) worms after 2 days of incubation at 10, 15, 20, 25, and 30°C, respectively. The sod-3::DsRed expressing worms were photographed under identical imaging conditions (e.g., intensity of excitation light, exposure time). The expression of sod-3 was quantified and checked for DAF-16 dependency by semi-quantitative RT-PCR analyses (94°C, 20 sec; 50°C, 20 sec; 72°C, 45 sec; 28 cycles) using wildtype, Δjnk-1 (VC8) and Δdaf-16 (GR1307) mutant worms and the following primers: ATGCTGCAATCTACTGCTCG and AAGTAGTAGGCGTGCTCCC. In addition, sod-3 expression was quantified by real-time quantitative RT-PCR analyses. For it, we used the iQ5 Real-Time PCR Detection System (Biorad, Munich, Germany) and the iQ™ SYBR® Green Supermix (Biorad). Optimal reaction conditions were obtained with 10 µl 2× iQ™ SYBR® Green Supermix, 5 µM of each primer, and 2 µl of cDNA (obtained from 1 µg total RNA) in a total volume of 20 µl. Amplifications were performed starting with a 2 min template denaturation step at 95°C followed by 45 cycles of denaturation at 95°C for 10 sec, and a combined primer annealing/extension at the gene-specific primer temperature (60°C) for 30 sec. At the end of each PCR, a melting curve with increasing temperature from 60 to 95°C in steps of 3°C min−1 was generated. The fluorescence increase was automatically measured every 10 sec to detect fluorescence alterations and to confirm the specificity of the amplification. The data were analyzed using the iCycler iQ Software Version 3.1 (Biorad). For the relative quantification, the obtained threshold cycle (CT) values were normalized with three housekeeping genes (consensus primers for actin genes act-1 to act-4) as reference index. Expression level comparisons were performed in Microsoft Exel© by applicating the 2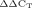 method (Livak and Schmittgen, 2001). The wildtype expression level was set to 100%. The sod-3 expression levels were calculated in relation to the wildtype's expression level. For each condition and strain, we performed three independent experiments. All samples were analyzed twice.
method (Livak and Schmittgen, 2001). The wildtype expression level was set to 100%. The sod-3 expression levels were calculated in relation to the wildtype's expression level. For each condition and strain, we performed three independent experiments. All samples were analyzed twice.
Aqueous Rhodamine123 solution (50 µM) was injected in the pseudocoelomic cavity of 25°C incubated worms to selectively stain mitochondria. Images taken of the red sod-3::DsRed fluorescence and the green Rhodamine123 fluorescence were later merged using Adobe Photoshop© (Adobe Systems Incorporated).
Thermal tolerance test
Thermal tolerance of the wildtype, the jnk-1 (gk7) deletion mutant and the jnk-1 rescue strain was tested at 36°C. For each experiment, 25 L4 larval stages of each strain were transferred to fresh NGM plates and incubated at 36°C. Animals were tapped every hour with a platinum wire pick. Worms showing no reaction were scored as dead.
Reproduction test
At least 30 eggs of the wildtype and the jnk-1 (gk7) deletion mutant strain were transferred onto fresh NGM plates to synchronize the worms. After 2 days of incubation at 20°C, six L4 larvae of each strain were transferred separately onto new NGM plates and incubated at 10, 15, 20, as well as 25°C. Progeny (worms and eggs) was counted after 3 days (20 and 25°C), 4 days (15°C), and 6 days (10°C) to take into account the slow development and egg laying of the latter worms. In addition, progeny of the jnk-1 rescue strain was counted at 25°C.
Statistics
Statistical analyses (two-way ANOVA, t-test) were performed using SIGMAPLOT 8.0 and SIGMASTAT 3.04 (Systat Software, Erkrath, Germany). Significant differences (P < 0.05) were marked with an asterisk or indicated in the figure legends.
Results
In a study based on reporter gene assays (Kawasaki et al., 1999), the MAP kinases JKK-1 and JNK-1 were found only in neurons of C. elegans. To investigate the location of active (phosphorylated) JNK-1 also in wildtype animals, whole-mount antibody staining was carried out. Under heat stress, phosphorylated JNK-1 was detected only in the nerve ring of the animals (Fig. 1A). In a jnk-1 deletion mutant (gk7), which lacks approximately 1 kb of the jnk-1 gene including the antibody's epitope, the nerve ring was not stained (Fig. 1B). Control experiments in the wildtype (Fig. 1C) indicated the specificity of the primary antibody. The specificity of the primary antibody for phosphorylated JNK-1 was further tested by staining mutant worms with a deficiency in the upstream MAPK kinases JKK-1 and MEK-1. The absence of a staining signal in the jkk-1 mutant (Fig. 1D) proves that the utilized primary antibody discriminates between phosphorylated and non-phosphorylated JNK-1, and shows in addition that JNK-1 phosphorylation in response to heat stress depends on JKK-1 activity but not on MEK-1 activity, because a staining of the nerve ring was detected in the mek-1 mutant (Fig. 1E). In conclusion, JNK-1 was found to be localized in the nervous system of C. elegans and to be controlled by JKK-1 under heat stress.
The MAPK JNK-1 is located in the nerve ring of C. elegans and is controlled by the upstream MAPK JKK-1. A: Activated (phosphorylated) JNK-1 (pJNK-1) in the nerve ring (encircled area) of the wildtype (wt) under heat stress revealed by whole-mount stainings using an anti-pJNK antibody. A weak staining of grinder and pharyngeal lumen can be regarded as an artifact as it was also found at these locations in a jnk-1 deletion mutant. B: Whole-mount antibody staining demonstrating the absence of activated JNK-1 in the nerve ring of a jnk-1 deletion mutant (Δjnk-1) under heat stress. C: Control experiments with omitted primary antibody, which resulted in a non-stained nerve ring in the wildtype (wt) under heat stress, proved the specificity of antibody and staining pattern. Whole-mount antibody stainings revealed that JNK-1 is controlled by JKK-1 ((D) no antibody staining of pJNK-1 in the nerve ring of a jkk-1 deletion mutant, Δjkk-1), but not by MEK-1 ((E) antibody staining of pJNK-1 in the nerve ring of a mek-1 deletion mutant, Δmek-1) under heat stress, and additionally proved the specificity of the primary antibody for pJNK-1.
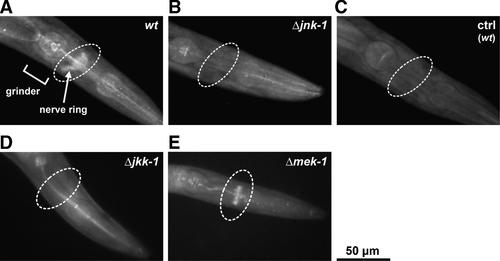
Western blot analyses of protein extracts of C. elegans (Fig. 2A) allowed to differentiate between the two alternative isoforms JNK-1α and JNK-1β of the gene jnk-1 (Villanueva et al., 2001). In a jnk-1 deletion mutant, activated JNK-1 isoforms could not be detected. In the wildtype, the degree of phosphorylation of JNK-1α and JNK-1β increased with ambient temperatures between 1 and 37°C and rose particularly between 15 and 20°C (Fig. 2C). The detected quantities of phosphorylated JNK-1β were generally higher than those of JNK-1α. Accordingly, JNK-1 activation and ambient temperature are more or less linearly related.
Activation of the MAPK JNK-1α and JNK-1β isoforms increases with ambient temperature. A: Western blot analyses of the activated jnk-1 gene products JNK-1α (53 kDa) and JNK-1β (43 kDa) using an anti-pJNK antibody revealed increasing amounts of pJNK-1α/β in the wildtype with rising ambient temperature (1–37°C). A jnk-1 deletion mutant (Δjnk-1) did not show a pJNK-1-specific signal. (M: prestained marker proteins). B: Protein loading was controlled using an anti-tubulin antibody, which confirmed the application of unaltered protein quantities. C: Quantifying the relative band intensities (with the intensity of the JNK-1β band at 37°C set to 100%) showed that the degree of JNK-1α/β activation increased more or less constantly with rising ambient temperature (mean ± SD; n = 3 experiments at each temperature; between the different temperatures, the increase of JNK-1α/β activation was always statistically significant: P ≤ 0.05, two-way Anova).
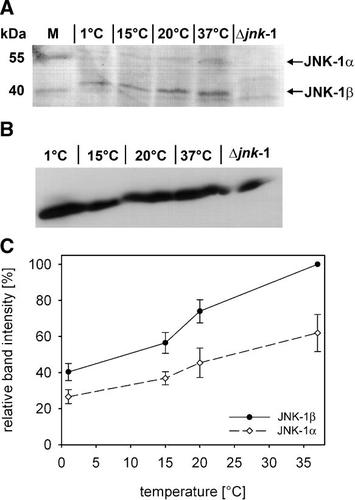
Apart from MAPK signaling, insulin-like (DAF-2) signaling plays a pivotal role for stress signal processing in C. elegans (Lithgow and Walker, 2002; Kim et al., 2004). Using a specific C. elegans strain (CF1139), which carries a daf-16::GFP construct, cellular stress responses in a cell, tissue, or an organ, such as the intestine, can be studied by analyzing the translocation of the transcription factor DAF-16 from the cytosol into the nucleus of a cell (Fig. 3A). In the intestine of the transgenic wildtype (CF1139), the degree of nuclear translocation of DAF-16 was minimal at 15°C and increased at lower (down to 1°C) and even more at higher (up to 30°C) temperatures (Fig. 3B). A transgenic jnk-1 deletion mutant (MS7) was generated by crossing the transgenic strain CF1139 (daf-16::gfp) with the jnk-1 (gk7) deletion mutant strain VC8. The obtained progeny possessed a homozygote non-functional jnk-1 gene and the above-mentioned daf-16 reporter gene construct. In the intestine of the transgenic deletion mutant, the pattern of nuclear translocation of DAF-16 as a function of ambient temperature was similar to the transgenic wildtype; however, the degree of nuclear translocation was generally and statistically significantly lower in the mutant than in the wildtype (Fig. 3B). Under ROS stress, a similar result was found (Fig. 3C): with an increasing period of incubation at 1 mM H2O2, the degree of nuclear translocation of DAF-16 increased in the intestinal cells of transgenic wildtype and mutant worms. However, this translocation was constantly and statistically significantly higher in the wildtype in respect to the mutant during the whole incubation period. These results show that JNK-1 modulates the intestinal stress-induced translocation of DAF-16 from the cytosol into the cell nucleus. Both, cold and warm temperatures (relative to 15°C) induce nuclear translocations of DAF-16.
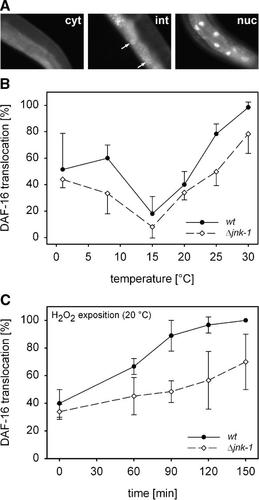
The temperature- and H2O2-induced nuclear translocation of DAF-16 within intestinal cells of C. elegans is lower in a jnk-1 deletion mutant (Δjnk-1) than in the wildtype (wt). A: Depending on the degree of nuclear GFP fluorescence, three different states of translocation of DAF-16::GFP from the cytoplasm into the cell nuclei of intestinal cells can be distinguished: cytoplasmic location (cyt; no nuclear GFP fluorescence), intermediate location (int; weak nuclear GFP fluorescence), and nuclear location (nuc; strong nuclear GFP fluorescence). B: After incubation at different ambient temperatures (1–30°C), the degree of nuclear DAF-16 translocation within intestinal cells was minimal at 15°C and increased toward lower and higher temperatures both in wildtype and mutant worms (mean ± SD, n = 5 experiments at each temperature on N = 10 worms (wt, Δjnk-1) each). In the mutant, however, DAF-16 translocation was significantly reduced in comparison to the wildtype (P ≤ 0.05, two-way ANOVA). C: The degree of nuclear DAF-16 translocation also increased with the incubation period (0–150 min) on NGM plates containing 1 mM H2O2 (mean ± SD, n = 3 experiments at each incubation period on N = 10 worms (wt, Δjnk-1) each). Again, this cellular response was significantly lower in the mutant than in the wildtype (P ≤ 0.05, two-way ANOVA).
One of the DAF-16 target genes is sod-3 (Murphy et al., 2003). To follow-up the processing of thermal stress signals also downstream of the signaling cascade, we generated a sod-3::DsRed reporter gene construct. This construct was injected into wildtype and jnk-1 deletion mutant worms to generate strains (MS8, MS9) that carry the reporter gene. Under standard temperature conditions (20°C), the transgenic wildtype showed a relatively strong constitutive sod-3 expression within the pharyngeal muscles and a weaker one within the rectum (Fig. 4A). The sod-3 expression increased drastically under heat stress, and sod-3::DsRed could now be observed in all muscle and hypodermis cells. SOD-3 was predicted to be located within the mitochondria (Hunter et al., 1997). We confirmed the mitochondrial location of sod-3::DsRed by double-staining experiments. By injecting a solution of the mitochondria-selective and membrane-permeable dye Rhodamine123 (green fluorescence) into the pseudocoelom of worms expressing sod-3::DsRed (red fluorescence), a co-localization of red and green fluorescence resulting in yellow-colored subcellular structures could be proven.
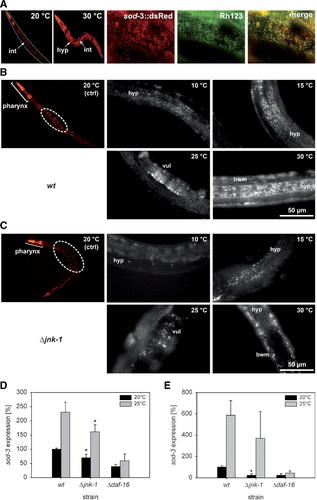
The temperature-induced expression of the superoxide dismutase SOD-3 is lower in a jnk-1 deletion mutant (Δjnk-1) than in the wildtype (wt) of C. elegans. A: Worms carrying a sod-3::DsRed reporter gene construct showed a constitutive pharyngeal and rectal sod-3 expression at 20°C and a strongly enhanced sod-3 expression in all tissues at 30°C. The mitochondrial location of SOD-3 was verified by staining the mitochondria of worms expressing sod-3::DsRed (25°C incubation) with Rhodamine123. The red fluorescence of SOD-3::DsRed (left) and the green fluorescence of Rhodamine123 (center) resulted in yellow-colored co-localized areas (right). B: Transgenic wildtype worms carrying a sod-3::DsRed construct mainly showed pharyngeal sod-3 expression at 20°C (control animals: ctrl; encircled areas indicate photographed regions). At 15°C, the expression of sod-3 increased in the hypodermis (hyp). At 10°C, sod-3 expression generally decreased again. At warmer temperatures (25, 30°C), sod-3 expression increased strongly in the hypodermis and appeared in the vulva (vul; 25°C) as well as in all body wall muscles (bwm; 30°C). C: The expression level of sod-3::DsRed was generally lower in transgenic jnk-1 deletion mutants; however, the expression pattern was similar to the wildtype. The expression of sod-3 was also quantified by (D) semi-quantitative RT-PCR analyses as well as by (E) real-time quantitative RT-PCR analyses at 20 and 25°C in wildtype as well as in Δjnk-1 and Δdaf-16 (GR1307) mutant worms. These analyses confirmed the reduced expression of sod-3 in jnk-1 deletion mutants and showed a strong DAF-16 dependency of sod-3 expression (asterisks indicate significant differences between wildtype and jnk-1 deletion mutant worms: P ≤ 0.05, t-test). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The expression of sod-3 (sod-3::DsRed) was studied in transgenic wildtype and jnk-1 deletion mutant worms as a function of ambient temperatures between 10 and 30°C. Wildtype worms (Fig. 4B) exhibited a low sod-3 expression level under standard temperature conditions (20°C) with the pharynx being the main expression site. At 15°C, there was a distinct increase of sod-3 expression, which was mainly found in the anterior half of the animals. At 10°C, the sod-3 expression level decreased again with the remaining expression located also in the anterior part of the worm. At warm-to-high temperatures (25, 30°C), the expression of sod-3 increased drastically, and sod-3::DsRed became detectable in the vulva (25°C) and finally in all body wall muscle cells as well as hypodermis cells throughout the entire worm (30°C). The transgenic jnk-1 deletion mutant (Fig. 4C) showed at each tested temperature a sod-3 expression pattern similar to the transgenic wildtype. The sod-3 expression level, however, was constantly lower in the mutant than in the wildtype, which was also confirmed by semi-quantitative RT-PCR analyses (Fig. 4D) as well as by real-time quantitative RT-PCR analyses (Fig. 4E). The DAF-16 dependency of sod-3 expression was reflected in the reduced quantities of sod-3 mRNA as well as in the almost absent increase of sod-3 expression between 20 and 25°C in the daf-16 mutant.
Consequences of the jnk-1 deletion mutation on the animals' thermal tolerance were studied by carrying out lethality tests. The capability to survive high temperatures (36°C) for several hours differed between the wildtype and the mutant. At each incubation period lasting longer than 2 h, the relative share of living worms at 36°C was lower for the mutant than for the wildtype (Fig. 5). The resulting, statistically different lethal times at 50% mortality (Lt50) were 5.7 ± 0.2 h for the wildtype and 5.0 ± 0.3 h for the mutant (P ≤ 0.01, t-test). A wildtype-like level of heat tolerance was regained after the injection of a jnk-1 rescue plasmid into the jnk-1 deletion mutant.
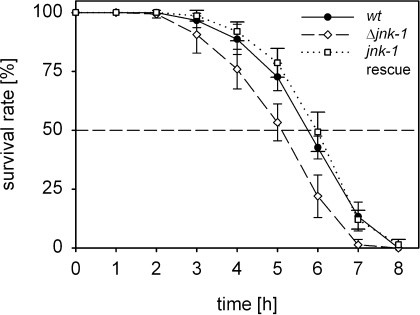
Heat tolerance is lower in a jnk-1 deletion mutant (Δjnk-1) than in the wildtype (wt) of C. elegans. The survival rate (mean ± SD) of wildtype, jnk-1 deletion mutant, and jnk-1 rescue animals decreased with the period of incubation at 36°C (n = 6 experiments each in wildtype and Δjnk-1 mutant, n = 3 experiments in jnk-1 rescue animals; N = 25 worms each). However, the jnk-1 deletion mutant proved to be more heat susceptible than the wildtype (P ≤ 0.05, two-way ANOVA). Heat tolerance was fully regained in jnk-1 rescue animals carrying a jnk-1 rescue plasmid (P ≤ 0.05, two-way ANOVA).
Fitness consequences of the jnk-1 deletion mutation were studied by measuring reproduction rates at different ambient temperatures. The number of offspring increased between 10 and 20°C, but decreased again at 25°C (Fig. 6B). Above 28°C, the worms became sterile and reproduction was not observed (data not shown). At 10 and 15°C, no significant differences in the number of offspring and the developmental stage were found between the wildtype and the mutant. At 20 and 25°C, however, the mutant showed a statistically significantly lower reproduction rate than the wildtype. The reduction in the number of progeny in the jnk-1 mutant in the warmth could be reversed by the injection of a jnk-1 rescue plasmid (Fig. 6C). In addition to the difference in reproduction rate, the development of mutant worms was retarded in comparison to the wildtype by one or two larval stages (Fig. 6A). After 3 days at 25°C, the wildtype progeny had reached maturity, whereas the offspring of the mutant was mainly at the L3 or L4 larval stage.
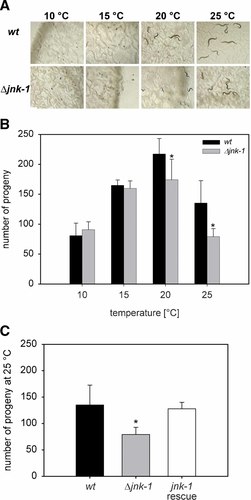
The reproductive fitness at moderate-to-warm ambient temperatures is lower in a jnk-1 deletion mutant (Δjnk-1) than in the wildtype (wt) of C. elegans. A: F1 progeny (worms and eggs) of single L4 larvae of wildtype and jnk-1 deletion mutant incubated at 10°C (6 days), 15°C (4 days), 20 and 25°C (3 days). After these periods, we found only eggs at 10°C, worms and eggs in the wildtype, and mainly eggs in the mutant at 15°C, and a delay by one or two larval stages in the mutant at 20 and 25°C. B: At 20 and 25°C, the number of progeny (mean ± SD) of the jnk-1 deletion mutant was reduced in comparison to the wildtype (n = 6 experiments at each temperature on N = 6 L4 larvae (wt, Δjnk-1) each; asterisks indicate significant differences between wildtype and mutant: P ≤ 0.05, t-test). C: In jnk-1 rescue animals carrying a jnk-1 rescue plasmid, the reproduction rate in the warmth increased to wildtype levels (n = 6 experiments on N = 6 L4 larvae (wt, Δjnk-1, jnk-1 rescue) each). In comparison to wildtype, the number of progeny was lower in the jnk-1 deletion mutant (asterisk) and similar in the jnk-1 rescue animal (P ≤ 0.05, t-test). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
The MAPK pathways and the insulin-like pathway are independent signaling cascades important to signal transduction and control of cellular responses to environmental stimuli (stressors) as well as the determination of development and lifespan (Wolkow et al., 2000; Lithgow and Walker, 2002; Kim et al., 2004; Inoue et al., 2005). In the present study, we investigated the role of ambient temperature in activation and interactions of both types of signaling cascades in the nematode C. elegans.
In a first step, we focused on the activation (phosphorylation) of the C. elegans MAPK JNK-1. In wildtype worms, whole-mount antibody staining using an anti-phospho-SAPK/JNK antibody revealed activated JNK-1 in the worm's nerve ring around the pharynx under heat stress (Fig. 1A), which confirmed a previous report on the neuronal expression of jnk-1::GFP in C. elegans (Kawasaki et al., 1999). The nerve ring showing phospho-JNK-1 contains cell bodies of sensory neurons including the AFD neuron pair, which is involved in thermotaxis and other temperature-dependent behavior (Mori and Ohshima, 1995; Tsalik and Hobert, 2003). Experiments in jnk-1, jkk-1, and mek-1 deletion mutants demonstrated a JKK-1-dependent JNK-1 activation upon heat stress apart from proving the specificity of the staining pattern based on the primary antibody (Fig. 1B–E). Western blot analyses showed an increasing activation of both JNK-1 isoforms (JNK-1α and JNK-1β) (Villanueva et al., 2001) with rising temperature over a wide thermal range (Fig. 2). The almost linear relationship between phosphorylated JNK-1 and temperature, including the preferred temperature range (15–20°C) of C. elegans in a thermal gradient (Yamada and Ohshima, 2003), may indicate that JNK-1 is involved in thermal perception in the nervous system beside its already known function in the regulation of synaptic vesicle transport (Byrd et al., 2001) and in the co-ordination of locomotory behavior (Kawasaki et al., 1999).
In a next step, the temperature-dependent nuclear translocation of the FOXO transcription factor DAF-16, which is the main downstream target of insulin-like signaling (Ogg et al., 1997), was analyzed in intestinal cells. The intestine was chosen for these experiments, because in this tissue the DAF-16 translocation proved to be much less susceptible to side effects of experimental handling than in other tissues (see Materials and Methods). In wildtype worms, a major accumulation of DAF-16 within the intestinal cell nuclei was found at both low (<10°C) and high (≥20°C) temperatures, whereas a minimal translocation was measured at 15°C (Fig. 3B). Since a nuclear location of DAF-16 is an indicator of stress (Henderson and Johnson, 2001), the temperature of 15°C most likely represents the natural optimum for the soil-dwelling nematode C. elegans, which also correlates with the above-mentioned preference temperature (Yamada and Ohshima, 2003). In a jnk-1 deletion mutant, the overall pattern of temperature-dependent intestinal DAF-16 translocation was similar, however, the degree of nuclear translocation was statistically significantly lower (Fig. 3B). This result is indirectly supported by a previous study (Oh et al., 2005), in which a jnk-1 overexpressing C. elegans strain exhibited a generally higher level of nuclear DAF-16 accumulation after heat stress (35°C, 30 min) than the wildtype. Deletion of the JNK-1-activating MAP kinase-kinase JKK-1 (Kawasaki et al., 1999) in the jnk-1 overexpressing strain resulted in a wildtype-like DAF-16 translocation after heat stress, which suggests that under this condition, the phosphorylation state of JNK-1 influences the nuclear translocation of DAF-16. As the target genes of DAF-16 include catalase and superoxide dismutases (McElwee et al., 2003; Murphy et al., 2003), we exposed worms directly to ROS by applying hydrogen peroxide. With increasing H2O2 incubation period, the nuclear DAF-16 translocation rose in the wildtype at a higher rate than in the jnk-1 deletion mutant (Fig. 3C). Accordingly, the promoting influence of JNK-1 on DAF-16 translocation is not only limited to the processing of thermal information. As other abiotic environmental factors, such as UV radiation, which also contribute to ROS production, are known not to cause a translocation of DAF-16 (Henderson and Johnson, 2001), specificity in the ROS-mediated DAF-16 translocation is implied.
Because activated JNK-1 (Fig. 1), which is controlled by JKK-1 under heat stress, and jkk-1/jnk-1::GFP expression (Kawasaki et al., 1999) were found only in neurons of C. elegans, it is possible that neuronal JNK-1 modulates peripheral insulin-like (DAF-2) signaling via the utilization of a yet unidentified signal substance within the pseudocoelomic cavity for the signal transfer from neuronal to peripheral cells (intestine) which are not directly connected to the nervous system. Consequently, the thermal activation of neuronal JNK-1 (Fig. 2) would promote nuclear DAF-16 translocations within the intestinal cells of the wildtype, whereas in the jnk-1 deletion mutant the stimulating influence of activated neuronal JNK-1 would be absent (Fig. 3). However, the basic patterns of intestinal DAF-16 translocation under stress, which are shown by the jnk-1 deletion mutant (Fig. 3), are independent of activated JNK-1. Possible candidates for such a signal substance could be a neuronally expressed and secreted inhibitory insulin-like peptide (ILP) such as INS-1, which is known to antagonize DAF-2 signaling (Pierce et al., 2001) or, alternatively, a biogenic amine such as octopamine or tyramine representing invertebrate counterparts to vertebrate adrenergic transmitters (Roeder, 2005). Octopamine, for example, has been reported to possess a thermo-protective function in insects (Armstrong and Meldrum Robertson, 2006). An involvement of ILPs in this context has been demonstrated in Drosophila melanogaster, where the neuron-specific activation of the Drosophila JNK-1 homolog caused a reduced neuronal release of stimulatory insulin-like peptides (Wang et al., 2005) and an increase of the animals' stress tolerance (Wang et al., 2003). It has been concluded that a decrease in insulin-like signaling in the peripheral body cells and thus an activation of stress-related genes is initiated by this mechanism. Further evidence for a neuronal modulation of DAF-2 signaling comes from laser ablation experiments, which revealed that ASI gustatory neurons regulate lifespan in a DAF-16-dependent manner (Alcedo and Kenyon, 2004). Mutants for the neuron-specific protein OSM-3 are long-lived and show an increased constitutive DAF-16-GFP translocation rate (Apfeld and Kenyon, 1999; Lin et al., 2001). Furthermore, ttx-1 mutants are abnormally cryophilic and lack finger-like cilia on AFD neurons, which are responsible for thermotaxis (Perkins et al., 1986). ttx-1 mutants also exhibit a longer lifespan than the wildtype (Apfeld and Kenyon, 1999). In spite of much indications of a neuronal control of peripheral stress signaling via extracellular signal substances, however, more direct evidence is yet needed to completely exclude non-neuronal JNK-1 expression and direct interactions between JNK-1 and DAF-16 within the intestinal cells of C. elegans.
To follow-up subsequent events in the periphery, the expression of a DAF-16 target gene (sod-3) (Murphy et al., 2003) was studied in vivo and via RT-PCR analyses (Fig. 4). SOD-3, which is located within the mitochondria, plays an important role in detoxifying ROS. Detoxification of ROS is an essential component of cellular reactions toward environmental changes due to the negative influence of ROS on lifespan or fitness (Vanfleteren, 1993; Melov et al., 2000; Ishii et al., 2001). The generated sod-3::DsRed reporter gene construct also included the predicted DAF-16 and SKN-1 (another stress-related transcription factor) binding sites to ensure native gene expression control. Similar to the nuclear DAF-16 translocations within intestinal cells, the expression of sod-3::DsRed increased strongly both in the higher (25 and 30°C) and lower (15°C) temperature range within hypodermic and muscle cells of wildtype and mutant worms. In contrast to the wildtype (Fig. 4B), however, the jnk-1 deletion mutant showed a generally lower level of sod-3::DsRed expression (Fig. 4C), which is in correspondence with its lower level of DAF-16 translocation (Fig. 3). The difference in temperature of minimal DAF-16 translocation (15°C) and minimal sod-3 expression (20°C) may be related to the investigation of different tissues (intestine vs. hypodermis/body wall muscles).
Due to its important role in ROS detoxification, the increase of SOD-3 expression at lower and higher temperatures should reflect an increased ROS generation within the mitochondria at these conditions. At high temperatures, ROS is generated by a reduction in phosphorylation and mitochondrial coupling (lower ADP/O ratio) due to an increase in non-phosphorylating state 4 oxygen consumption, which in turn is the result of an increasing leakiness of the mitochondrial membrane (Abele et al., 2002; Abele and Puntarulo, 2004). At low temperatures, the decrease in metabolic rate (Van Voorhies and Ward, 1999) and consequently ATP consumption may cause a lack of the mitochondrial “substrate” ADP. This shortage may cause an increasingly reduced state of the complex III ubiquinone pool as the electron transport chain further generates a high proton potential. As a consequence, the lifetime of semiquinole (CoQH•) is prolonged, thereby increasing the chance of CoQH• autoxidation, which finally results in the release of ROS (Abele et al., 2002; Abele and Puntarulo, 2004).
Fitness consequences of jnk-1 deletion were determined by studying thermal tolerance and reproduction rate. In comparison to the wildtype, the mutant showed an increased susceptibility to high temperatures (Fig. 5) as well as a retarded development and reduced reproduction rate at 20 and 25°C (Fig. 6). The loss of JNK-1-mediated DAF 16 translocation with the consequence of a reduced sod-3 expression resulted in an overall decreased fitness. Rescue experiments proved the JNK-1 dependency of the increase in overall fitness. At low temperatures (10 and 15°C), no differences in reproduction rate were found between mutant and wildtype. The reduced sod-3 expression in the mutant could obviously be compensated by other mechanisms. The difference in the fitness of the mutant at low and high temperatures indicates that C. elegans is primarily adapted to low temperatures, which seems reasonable for a soil-dwelling organism of temperate clime.
The present study has provided evidence of indirect interactions between JNK-1 and DAF-16. Activation of the MAPK JNK-1, which was found only in the neuronal system, modulates the nuclear translocation of DAF-16 within peripheral cells (intestine) possibly via a hormonal messenger within the pseudocoelomic cavity. More precisely, the results of the present study suggest that thermal information (particularly higher temperatures) percepted by the neuronal system promote acclimatory responses in the periphery including the activation of anti-oxidative defense mechanisms with the consequence of an increase in overall fitness.
Acknowledgements
We thank Prof. Michael Hippler, Institut für Biochemie und Biotechnologie der Pflanzen WWU Münster, for critically reading the article as well as the Caenorhabditis Genetics Center for providing C. elegans strains. We thank Dr. Stefan Weinl for supporting the real-time quantitative RT-PCR analyses.




