HDLs activate ADAM17-dependent shedding
Abstract
The tumor necrosis factor-alpha (TNF) converting enzyme (ADAM17) is a metalloprotease that cleaves several transmembrane proteins, including TNF and its receptors (TNFR1 and TNFR2). We recently showed that the shedding activity of ADAM17 is sequestered in lipid rafts and that cholesterol depletion increased the shedding of ADAM17 substrates. These data suggested that ADAM17 activity could be regulated by cholesterol movements in the cell membrane. We investigated if the membrane cholesterol efflux induced by high-density lipoproteins (HDLs) was able to modify the shedding of ADAM17 substrates. HDLs added to different cell types, increased the ectodomain shedding of TNFR2, TNFR1, and TNF, an effect reduced by inhibitors active on ADAM17. The HDLs-stimulated TNF release occurred also on cell-free isolated plasma membranes. Purified apoA1 increased the shedding of TNF in an ABCA1-dependent manner, suggesting a role for the cholesterol efflux in this phenomenon. HDLs reduced the cholesterol and proteins (including ADAM17) content of lipid rafts and triggered the ADAM17-dependent cleavage of TNF in the non-raft region of the membrane. In conclusion, these data demonstrate that HDLs alter the lipid raft structure, which in turn activates the ADAM17-dependent processing of transmembrane substrates. J. Cell. Physiol. 214: 687–693, 2008. © 2007 Wiley-Liss, Inc.
The plasma concentration of high-density lipoproteins (HDLs) cholesterol is an inverse predictor of future atherosclerotic cardiovascular disease (Gordon et al., 1989; Assmann et al., 1996). The best-known antiatherogenic function of HDLs is to promote the efflux of cholesterol from cells that is necessary for the reverse-cholesterol transport (Fielding and Fielding, 1995), the process by which cholesterol is mobilized from peripheral tissue to the liver for excretion. Two main mechanisms are involved in the HDLs-promoted cholesterol efflux. The first one involves passive diffusion of cholesterol from the plasma membrane to the HDLs particles (Yancey et al., 2003). The second mechanism involves membrane proteins like the ATP binding cassette transporter (ABC) A1 (Wang and Tall, 2003), which accelerates cholesterol export to lipid-poor apoA1 to form nascent HDLs, whereas ABCG1 (Wang et al., 2004) and scavenger receptor BI (Ji et al., 1997) accelerate cholesterol transfer to HDLs. In addition to their role in reverse-cholesterol transport, HDLs also have antioxidant (Navab et al., 2000; Ou et al., 2003; Nicholls et al., 2005), anti-inflammatory (Navab et al., 2005), and antithrombotic (Rosenson and Lowe, 1998) properties that play an important role in their antiatherogenic effects (Barter and Rye, 2006; Choi et al., 2006; Cutri et al., 2006).
It is thought that cholesterol is non-randomly distributed within the membrane, participating in the generation of lipid domains rich in cholesterol and sphingolipids, called lipid rafts (Brown, 1998; Simons and Vaz, 2004). One of the most important properties of lipid rafts is that they can include or exclude proteins to variable extents (Simons and Toomre, 2000; Pike, 2004). This dynamic process that regulates proteins interactions and influences their functions (Golub et al., 2004) is, amongst other things, important for the generation of platforms concentrating signaling molecules.
Our previous results involve the ectodomain shedding of tumor necrosis factor-alpha (TNF) in the formation of early atherosclerosic lesions (Canault et al., 2004). Recently, we showed that the activity of the TNF converting enzyme (TACE or ADAM17), responsible for the shedding of TNF and its receptors (TNFR1 and TNFR2), was sequestered in lipid rafts and that a drastic cholesterol depletion of the cell membrane induced by methyl-β-cyclodextrin increased the release of ADAM17 substrates (Tellier et al., 2006). In the present work, we tested the physiological relevance of these previous results by analyzing the effect of the cholesterol efflux triggered by HDLs on the release of ADAM17 substrates. Our data demonstrate that HDLs alter the lipid raft structure, which in turn activates the ADAM17-dependent processing of transmembrane substrates.
Materials and Methods
Materials
Metalloproteinase inhibitor RU 36156 active on ADAM17 (Gallea-Robache et al., 1997) was donated by Dr. S. Roman-Roman (Aventis Pharma, Romainville, France). Cholesterol-loaded cyclodextrin, cAMP (8-(4-Chlorophenylthio)adenosine 3′,5′-cyclic monophosphate) and purified human apoA1 were from Sigma (L'Isle D'Abeau Chesne, France). ADAM17 polyclonal antibody, TIMP3 and Fluorogenic Peptide Substrate III were from R & D Systems (Lille, France). Actin and Flotillin-1 polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
HDL isolation
Human HDLs (density range 1.063–1.21 g/ml) were isolated by ultracentrifugation from human pooled sera under the previously used conditions (Havel et al., 1955) and dialyzed against PBS containing 100 µM EDTA. LPS content was checked using the Limulus test (Nelson Labs, Salt Lake City, UT).
Cell culture and transfection
The monkey kidney fibroblast cell line COS-7, the rat cardiomyoblast cell line H9C2, were cultured as suggested by ATCC (http://www.lgcpromochem-atcc.com). Endothelial cells from human umbilical vein (HUVEC) were isolated and cultured as previously described (Peiretti et al., 1997) and used at the third passage. Transient transfections were performed with Polyfect reagent (Qiagen, Courtaboeuf, France) essentially as described by the manufacturer. The stable ectopic expression of TNF in the human endothelial cell line ECV-304 (ECVTNF) and culture conditions were described previously (Peiretti et al., 2005). Before experiments, cells were washed with PBS and incubated for 1 h in serum-free medium and experiments were performed in this medium.
Isolation of lipid rafts
Lipid rafts were isolated by sucrose density gradient centrifugation of cells lysed with the non-ionic detergent Brij 98 as previously described (Tellier et al., 2006). After centrifugation, 0.5 ml fractions were collected from the top of the gradient. Aliquots of selected fractions were analyzed by immunoblot and ELISA.
Isolation of plasma membrane
Plasma membranes of ECVTNF cells were prepared as described (Lin et al., 1987). Briefly, cells were collected in a hypotonic buffer (50 mM mannitol, 5 mM Hepes pH 7.4) and homogenized using a Polytron (Kinematica AG); 1 M calcium chloride solution was added to the homogenate to a final concentration of 10 mM and the mixture stirred for 15 min. The calcium-induced microsome aggregates were sedimented at 3,000g for 15 min. Plasma membranes contained in the supernatant were pelleted at 48,000g for 30 min.
Expression vectors
Expression vectors for human TNF, TNFR1, TNFR2 were described previously (Peiretti et al., 2003, 2005).
Fluorogenic assays of metalloprotease activity
Isolated membranes were added to the activity buffer [25 mM Tris/HCl pH 8.0, 2.5 µM ZnCl2] containing 10 µM of pro-TNF mimetic Fluorogenic Peptide Substrate III that was designed as an ADAM17 substrate (Black et al., 2003). Its fluorescence-related enzymatic cleavage was monitored at 320 nm excitation and 405 nm emission wavelength using a microplate fluorescence reader (Chameleon™, Hidex, Turku, Finland). Blank (buffer, membranes and substrate, separately) was subtracted from sample measurements for calculations.
Flow-cytometry analysis
Cells were scraped off the culture dish and surface expression of TNFR2 was determined by flow cytometry by using FITC-conjugated monoclonal antibody (clone 22235 from R & D Systems). Labeled cells were analyzed on a XL-cytofluorograph (Coulter Electronics Inc.).
Cholesterol assay
For cholesterol extraction, a mixture of isopropanol/chloroform (3/2 v/v) was added to the samples. After evaporation, the residue was treated by ethylacetate and the cholesterol was analyzed by gas chromatography-mass spectrometry as previously described (Beaumier-Gallon et al., 1998).
Protein assays
Total amounts (free and bound forms) of soluble human TNFR1, TNFR2, ICAM-1, and soluble and transmembrane TNF were assayed according to the specifications of their respective enzyme-linked immunosorbent assays (ELISA) kits (R & D Systems). ApoA1 concentration was determined by immunonephelometry.
Statistical analyses
Isolations of lipid rafts by sucrose density gradient centrifugation were repeated four times and a representative experiment is shown. All other experiments were performed in triplicate and repeated at least three times. Data are expressed as the mean ± SD. Treatments were compared with their respective controls and statistical calculation of the mean differences was performed with the two-tailed t-test. A value of P < 0.05 was considered statistically significant.
Results
HDLs induce the shedding of ADAM17 substrates
Treatment with HDLs significantly reduced the percentage of COS-7 cells expressing TNFR2 at their surface (from 24.05 ± 0.77 % to 17.54 ± 0.37%; P = 0.0087) (Fig. 1) and concomitantly increased the amount of TNFR2 released in the culture media (Table 1), suggesting that HDLs stimulate the cleavage of transmembrane TNFR2 from the cell surface. HDLs also increased the release of TNF and weakly that of TNFR1 (two other ADAM17 substrates) ectopically expressed in COS-7 and H9C2 cells (Table 1) but the cellular amount of these proteins was not significantly altered (data not shown). Moreover, the release of endogenous ICAM-1 from ECV-304 cells (ICAM-1 is not an ADAM17 substrate in these cells (Peiretti et al., 2005)) was not modified by HDL treatment (control 425.1 ± 21.2 pg/ml; HDLs 450.3 ± 17.5 pg/ml). These results suggest that the HDLs-stimulated ectodomain shedding of transmembrane proteins is at least restricted to ADAM17 substrates and is not a feature of a sole cell type. To verify that the effect of HDLs was not limited to ectopically expressed ADAM17 substrates, we analyzed their effect on the release of endogenous TNFR2 from HUVEC. This substrate was chosen because HUVEC do not synthesize detectable amount of TNF under basal conditions and the release of TNFR1 was only weakly increased by HDLs, as shown with overexpression models above. The release of endogenous TNFR2 from cell surface was significantly increased by HDLs treatment (from 73.5 ± 14.3 pg/ml to 101.6 ± 15.6 pg/ml; P < 0.0001), suggesting that the shedding of endogenous ADAM17 substrates is altered by HDLs.
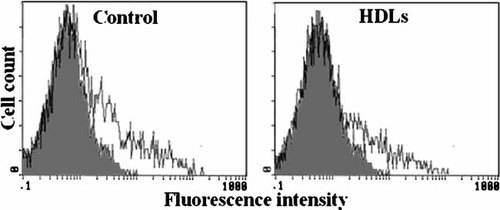
HDLs decrease the cell surface expression of TNFR2. Forty eight hours after transfection, COS-7 cells were incubated for 3 h with HDLs (50 µg/ml) and cell surface expression of TNFR2 was analyzed by flow cytometry as described in “Materials and Methods” section. Shaded and unshaded areas depict untransfected and TNFR2- transfected cells, respectively.
| TNFR2 (pg/ml) | TNFR1 (pg/ml) | TNF (pg/ml) | ||||
|---|---|---|---|---|---|---|
| Control | HDL | Control | HDL | Control | HDL | |
| COS-7 cells | 334.6 ± 38.8 | 552.9 ± 56.6* | 30.0 ± 1.7 | 38.3 ± 3.2* | 15080 ± 4314 | 55247 ± 1327* |
| H9C2 cells | 77.46 ± 11.9 | 226 ± 78.9* | 10.2 ± 0.1 | 12.1 ± 0.2* | 2868 ± 398.7 | 9497 ± 766.1* |
- COS-7 and H9C2 cells were transiently transfected with TNFR2, TNFR1 and TNF. Cells were left untreated or incubated for 3 h with HDL (50 µg/ml) then TNFR2, TNFR1, and TNF contained in the culture media were measured by ELISA. Values represent means ± SD.
- * P < 0.0007, at least, versus control.
Inhibition of HDLs-stimulated ADAM17 substrate shedding
The ECVTNF cell model (human endothelial cell line ECV-304 expressing TNF) was previously generated to specifically study ADAM17-dependent ectodomain-shedding (Peiretti et al., 2005). We showed, with this model that the conclusions drawn about the regulation of TNF shedding were also valid for TNFR1 and TNFR2. We took advantage of this model to evaluate the involvement of ADAM17 in the HDLs-stimulated shedding process. As expected, HDLs increased the release of TNF from ECVTNF cell (Fig. 2A). The factor of increase was maximal 1 h after HDL addition and lasted at least for 24 h. This effect was related to the dose of HDLs added and started to reach a plateau at 100 µg (of apoA1)/ml (a concentration of HDLs in the range of those usually used for in vitro studies (Suc et al., 1997; Robbesyn et al., 2003)). The synthetic inhibitor of metalloproteinases RU 36156 that is active on ADAM17 (Gallea-Robache et al., 1997), reduced the basal and HDLs-stimulated release of TNF by a factor of 12 and 24, respectively. TIMP3, the endogenous inhibitor of ADAM17 (Amour et al., 1998), reduced the basal and HDLs-stimulated release of TNF by a factor of 2 (Fig. 2B). Such a difference between the efficiency of both types of inhibitor was previously reported (Peiretti et al., 2005). These results suggest that ADAM17 is responsible for the HDLs-stimulated shedding of TNF.
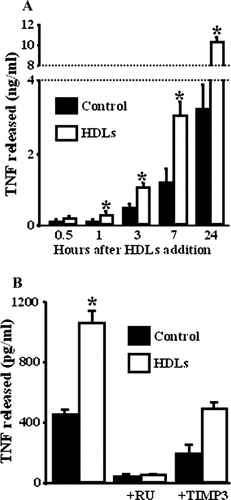
HDLs activate ADAM17-dependent shedding. Time-dependent TNF accumulation in the media of ECVTNF cells treated by HDLs (50 µg/ml) (A). TNF accumulation in the media of ECVTNF cells incubated for 3 h with HDLs in the presence of RU 36156 (RU) (10 µM) or TIMP3 (300 nM) (B). Values represent means ± SD. *P < 0.0001 versus control. The effects of the inhibitors are significant (P < 0.0001).
Role of membranes in the effect of HDLs
We hypothesized that the HDLs-stimulated shedding of ADAM17 substrates was the consequence of alterations of physicochemical properties of the plasma membranes directly induced by HDLs. To test this hypothesis, we isolated ECVTNF cell plasma membranes, incubated them in vitro with HDLs and measured the TNF released. HDLs increased the release of TNF from isolated plasma membranes (Fig. 3A), an effect inhibited by RU 36156 (data not shown), and decreased the amount of TNF contained in plasma membrane (from 516.5 ± 81.3 pg/ml to 282.2 ± 34.2 pg/ml; P = 0.005). These results argue against the involvement of intracellular processes such as an increased synthesis of either TNF (which was unlikely because its expression was driven by the CMV promoter) or ADAM17 (as shown in Fig. 5A) or a stimulated transport of TNF from its storage pool toward the region of cleavage. Furthermore, HDL treatment of isolated membranes did not modify their ADAM17 activity measured in vitro by the cleavage of a TNF mimetic fluorescent peptide (Fig. 3B), which is not in favor of a direct alteration of ADAM17 intrinsic activity by HDLs.
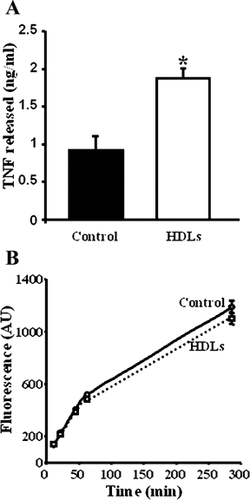
HDLs activate the shedding of TNF from isolated plasma membranes. A: Plasma membranes isolated from ECVTNF cells were incubated for 6 h with HDLs (50 µg/ml). The suspension was then centrifuged 30 min at 48,000g and the supernatant was assayed for TNF. Values represent means ± SD. *P = 0.0042 versus control. B: TNF fluorogenic assay of the metalloproteinase activity contained in isolated membranes treated or not with HDLs was performed as described in the “Materials and Methods” section.
Role of cholesterol in the effect of HDLs
HDL treatment reduced by 30% the amount of total cholesterol of ECVTNF cells (from 89.6 ± 13 ng/µg of protein to 61.8 ± 5.3 ng/µg of protein; P = 0.02). The HDLs-stimulated shedding of TNF was neither observed with cholesterol-loaded ECVTNF cells (Fig. 4) nor with ECVTNF cells treated with cholesterol-loaded HDLs (data not shown), suggesting a role of cholesterol in the increased shedding of ADAM17 substrates induced by HDLs. To further investigate the role of cholesterol efflux in the HDLs-stimulated TNF shedding, we incubated cells in presence of the cholesterol acceptor apoA1. ApoA1 significantly increased the release of TNF (Fig. 4). Overnight incubation of cells with 300 µM cAMP, which stimulates the synthesis of ABCA1 (Oram et al., 2000), was more efficient in stimulating the apoA1-induced TNF shedding than the basal shedding (factors of stimulation: 2.97 ± 0.7 and 1.69 ± 0.4, respectively, P = 0.02), suggesting that the effect of apoA1 involves ABCA1 and likely an efflux of cholesterol. Together, these results suggest that the HDLs-induced cholesterol efflux is responsible for the increased shedding of ADAM17 substrates.
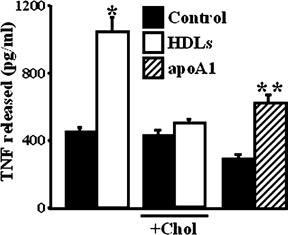
Cholesterol efflux accounts for HDL effect. TNF accumulation in the media of ECVTNF cells incubated for 3 h with HDLs (50 µg/ml) or with apoA1 (20 µg/ml). + Chol indicates that cells were preincubated overnight with 0.02% of cholesterol-loaded cyclodextrin. Values represent means ± SD. *P < 0.0001 versus control; **P = 0.0012 versus control without apoA1.
Effect of HDLs on lipid rafts
Treatment of ECVTNF with HDLs neither modifies the total amount of cellular proteins (not shown) nor those of the mature and immature form of ADAM17 and of actin taken as a control for protein loading (Fig. 5A). However, HDLs reduced the amount of cholesterol (from 6.7 ± 0.4 ng to 5.7 ± 0.3 ng; P = 0.02) and of proteins in lipid rafts without apparent change in the protein content of the non-raft region (Fig. 5B). A comparable effect, although less pronounced, on the protein content of lipid rafts was observed with apoA1 (data not shown). The ability of HDLs to modify lipid rafts was also shown by the reduction in the amount of the specific lipid raft marker: flotillin-1 (from 100 ± 1.3 to 69 ± 2.1 arbitrary units; P < 0.0001) (Fig. 5C). The amount of mature form of ADAM17 contained in lipid rafts was also reduced by HDLs (from 100 ± 2.7 to 85 ± 1.5 arbitrary units; P = 0.015) (Fig. 5C).
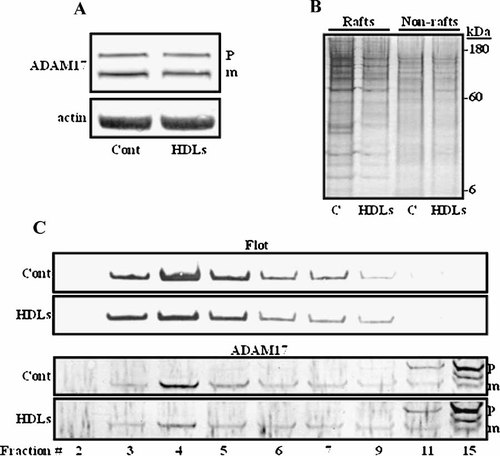
Effect of HDLs on lipid rafts. ECVTNF cells were treated or not with HDLs (as in Figure 2B), lysed and submitted to immunoblot for the detection of ADAM17 and actin (A). Lysates were fractionated by sucrose density gradient centrifugation and fractions containing lipid raft (from 3 to 6) and non-raft (from 11 to 14) domains were pooled, submitted to SDS–PAGE followed by silver staining of the gel (B). Representative immunoblots of flotillin-1 (Flot) and ADAM17 performed on selected fractions of the gradient are shown (C). Signals contained in lipid rafts from 4 immunoblots were quantified using Scion image software and values in arbitrary units are given in the text. Immature and mature forms of ADAM17 are indicated by p and m, respectively.
We investigated the effect of HDLs on the sub-domain repartition of transmembrane TNF. HDL treatment significantly decreased the amount of transmembrane TNF in lipid raft and non-raft regions of the membrane (Fig. 6A,C), an effect abolished by inhibition of metalloproteinases (Fig. 6B,C). This result suggests that the loss of transmembrane TNF is due to its increased shedding by ADAM17 that, upon HDL treatment, occurs in lipid raft and non-rafts region of the membrane. On the contrary and as previously described (Tellier et al., 2006), in control situation (without HDLs), inhibition of metalloproteinases significantly increased the amount of transmembrane TNF in lipid rafts only (Fig. 6C), confirming that constitutive TNF shedding is confined in these structures.
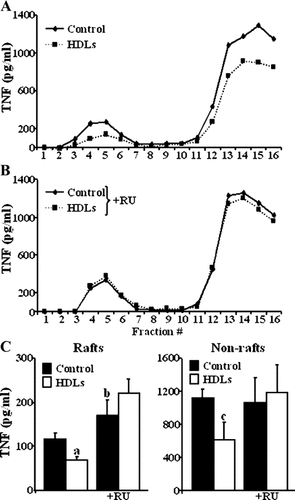
Transmembrane TNF distribution in membrane microdomains. Representative profiles of the transmembrane TNF (measured by ELISA) contained in each fraction of the sucrose density gradient obtained from ECVTNF cells treated or not with HDLs (A) and pretreated with RU 36156 (+RU) (B). TNF measured in the pooled fractions corresponding to lipid raft and non-raft regions from four independent experiments (C). Values represent means ± SD. In lipid rafts: aP = 0.0025 versus control; bP = 0.03 versus control without RU. In the non-raft region: cP = 0.0074 versus control.
HDLs do not bind the shed TNF
We investigated if HDLs could sequester the shed TNF, therefore buffering its activity. ECVTNF cells were stimulated with HDLs, the supernatant was centrifuged and the sedimentation profiles of TNF and HDLs were compared. The TNF released in the presence and absence of HDL followed the same sedimentation profile (Fig. 7), which was comparable to that of recombinant TNF (data not shown). The sedimentation profile of HDLs (followed by apoA1 assay) was dissociated from that of TNF suggesting that TNF was not sequestered by HDLs (Fig. 7).
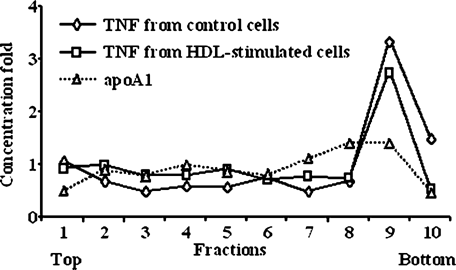
Sedimentation profile of TNF and HDLs. ECVTNF cells were treated or not with HDLs (as in Figure 2B). The supernatant (10 ml) was layered over a 90% sucrose cushion and centrifuged for 3 h at 100,000g. Fractions of the supernatant (1 ml each) were collected from the top and assayed for TNF and apoA1. Without HDLs, apoA1 was not detected. Data are representative of three independent experiments and represent the concentration fold (concentration in a given fraction/concentration of the supernatant before centrifugation) of TNF and apoA1.
Discussion
In this work, we show that the cholesterol efflux induced by HDLs activates the ectodomain shedding of ADAM17 substrates without increasing ADAM17 intrinsic activity. We and others have previously demonstrated that the shedding of ADAM17 substrates normally occurs exclusively in lipid rafts, where the active enzyme is sequestered (Tellier et al., 2006; Thiel and Carpenter, 2006). However, as the major portion of ADAM17 substrates is localized outside lipid rafts one may wonder how a drastic cholesterol depletion activates the shedding process. We propose that lipid raft disruption enhances the enzyme/substrate encounter probability, which results in an increased shedding (Tellier et al., 2006). On the basis of these previous results, we suggested that the HDLs-induced cholesterol efflux from cells could alter the cleavage of ADAM17 substrates. We validated this hypothesis by showing that ADAM17-dependent shedding of TNF, TNFR2 and, less strongly that of TNFR1 is increased by HDLs. The HDLs-dependent stimulation of ADAM17 substrates shedding is, at least, related to alterations of physicochemical properties of the plasma membranes. HDLs reduce the total amount of cellular cholesterol and also that of lipid rafts, leading to a change in their composition. Indeed, after HDLs treatment of the cells, less proteins, including the specific lipid raft marker flotillin-1 and the mature form of ADAM17, were detected in lipid rafts. Our results are in line with those showing that HDLs alter the structure, size and composition of lipid rafts in reconstituted lipid vesicles (Puff et al., 2005) and platelets (Barlage et al., 2006). ApoA1 increased the shedding of TNF in an ABCA1-dependent manner, reinforcing the role of cholesterol efflux in the stimulation of ADAM17 substrate shedding. The HDLs- or apoA1-triggered cholesterol efflux directly from lipid rafts was described to be marginal (Mendez et al., 2001; Gaus et al., 2004) minimizing possible significant effect on lipid raft structure. However, as recently proposed (Sanchez et al., 2007), it is conceivable that the cholesterol content of lipid rafts would be modified in response to an efflux of cholesterol from non-raft regions. Indeed, in order to re-establish the equilibrium between the phases, cholesterol molecules would have to move from the lipid rafts to the non-lipid raft phase.
Moreover, we show that the HDLs-stimulated release of TNF is accompanied by an ADAM17-dependent decreased amount of transmembrane TNF in lipid raft and non-raft regions of the membrane. This result suggests that the HDLs-stimulated shedding of TNF occurs abnormally outside of lipid rafts. The observed reduction of transmembrane TNF in lipid rafts could then result from less transmembrane (uncleaved) TNF coming from the non-raft region. This explanation is strengthened by our data on ADAM17 inhibition showing a restoration of the basal levels of transmembrane TNF in the raft and non-raft regions of the membrane. We can thus propose that the cholesterol efflux triggered by HDLs is sufficient to disorganize lipid rafts which allows ADAM17 to cleave TNF outside lipid rafts. This proposition can be extended to TNFR2 and TNFR1 since we previously showed that the regulation of their shedding is comparable to that of TNF (Peiretti et al., 2005; Tellier et al., 2006). However, the decreased amount of mature form of ADAM17 in lipid rafts was not compensated by an increased amount in the non-raft region of the membrane. This is probably due to the low amount of proteins contained in lipid raft compared with non-raft regions. This difficulty to detect a transfer from lipid raft to non-raft regions is also true for the lipid raft marker flotillin-1 and more generally for the total protein content.
The cellular models of ectopic expression of ADAM17 substrates, used in this study, were designed to highlight the ability of HDLs to stimulate the shedding process. However, to have a more global idea of the effect of HDLs on the physiological regulation of ADAM17 substrates shedding, one should consider the regulation of ADAM17 substrates as a whole. For instance, it was described that HDLs protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac TNF synthesis and content (Calabresi et al., 2003). However, it was observed that this phenomenon was associated with a higher recovery of soluble TNF in the coronary effluent, an effect that might be attributed to the HDLs-stimulated shedding of TNF as documented here. Therefore, since HDLs reduce TNF synthesis, it is unlikely that they could sustain for a long time an increase in TNF release by stimulating its shedding. HDLs can serve as carriers for a wide range of proteins as it was shown by a proteomic approach (Rezaee et al., 2006). To reconcile the anti inflammatory effect of HDLs with their ability to stimulate TNF shedding, one could hypothesize that HDLs stimulate the shedding and sequester the shed products, buffering their activity. However, our results are not in favor to this hypothesis. Indeed, in presence or absence of HDLs, TNF follows the same profile of sedimentation which is dissociated from that of HDLs. For ADAM17 substrates whose synthesis is not reduced by HDLs, a physiological modulation of their shedding by HDLs that would influence their surface expression or the amount of their soluble forms is conceivable. Moreover, it is possible that platelets, which do not synthesize cholesterol and for which membrane cholesterol content depends on plasma lipoproteins (Schick and Schick, 1985), could be more susceptible to HDLs-induced ADAM17-dependent shedding than other cell types. It is noteworthy that lipid rafts were shown to be critical membrane domains for platelet activation (Bodin et al., 2003; Gousset et al., 2004).
Because of the beneficial influence of HDLs on the vascular wall, we attend to the emergence of therapeutic strategies aiming at increasing HDLs for the prevention of cardiovascular diseases (Cutri et al., 2006). We have described for the first time that HDLs stimulate ADAM17 substrate shedding and because of the extremely large diversity of ADAM17 substrates (Smalley and Ley, 2005) it is of interest to investigate the impact of higher levels of HDLs on cellular function that could be altered by the shedding of ADAM17 substrates.
Acknowledgements
The authors are indebted to M.F. Vergnes (Laboratoire de Chimie Analytique, Qualitologie, Nutrition, Faculté de Pharmacie Marseille, France) and A. Campocasso (Université de la Méditerranée) for her technical contributions. E.T. is a recipient of Ministère de l'Enseignement Supérieur, de la Recherche et de la Technologie. M.C. is a recipient of Nouvelle Société Française d'Athérosclérose (Paris). M.P. is a recipient of Groupe de Réflexion sur la Recherche Cardiovasculaire (Paris).




