The role of the EGFR signaling in tumor microenvironment†
A. De Luca was supported by a fellowship from Associazione Italiana per la Ricerca sul Cancro (AIRC).
Abstract
The epidermal growth factor receptor (EGFR) family comprehends four different tyrosine kinases (EGFR, ErbB-2, ErbB-3, and ErbB-4) that are activated following binding to epidermal growth factor (EGF)-like growth factors. It has been long established that the EGFR system is involved in tumorigenesis. These proteins are frequently expressed in human carcinomas and support proliferation and survival of cancer cells. However, activation of the EGFR in non-malignant cell populations of the neoplastic microenvironment might also play an important role in cancer progression. EGFR signaling regulates in tumor cells the synthesis and secretion of several different angiogenic growth factors, including vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), and basic fibroblast growth factor (bFGF). Overexpression of ErbB-2 also leads to increased expression of angiogenic growth factors, whereas treatment with anti-EGFR or anti-ErbB-2 agents produces a significant reduction of the synthesis of these proteins by cancer cells. EGFR expression and function in tumor-associated endothelial cells has also been described. Therefore, EGFR signaling might regulate angiogenesis both directly and indirectly. In addition, activation of EGFR is involved in the pathogenesis of bone metastases. Within the bone marrow microenvironment, cancer cells stimulate the synthesis of osteoclastogenic factors by residing stromal cells, a phenomenon that leads to bone destruction. It has been shown that EGFR signaling regulates the ability of bone marrow stromal cells to produce osteoclastogenic factors and to sustain osteoclast activation. Taken together, these findings suggest that the EGFR system is an important mediator, within the tumor microenvironment, of autocrine and paracrine circuits that result in enhanced tumor growth. J. Cell. Physiol. 214: 559–567, 2008. © 2007 Wiley-Liss, Inc.
It has been long established that the epidermal growth factor receptor (EGFR) and its ligands, belonging to the epidermal growth factor (EGF)-like family of growth factors, play an important role in tumorigenesis. These proteins are frequently expressed in the majority of human carcinomas (Salomon et al., 1995; Normanno et al., 2003). More importantly, the EGFR and its cognate growth factors support autocrine and paracrine circuits that regulate several mechanisms underlying tumor pathogenesis and progression, including proliferation, survival, tissue invasion, and ability to form metastases (Salomon et al., 1995; Normanno et al., 2003, 2005a).
Tumor progression is a complex phenomenon that involves the interaction of tumor cells with surrounding normal tissues. The EGFR is expressed in almost all types of non-transformed cells, with the only exception of mature cells of the lymphohemopoietic system (Salomon et al., 1995; Normanno et al., 2006). Therefore, activation of the EGFR signaling in non-malignant cell populations of the tumor microenvironment might play an important role in cancer progression. In particular, evidence suggests that the EGFR system is involved in two important phenomena that promote tumor progression such as angiogenesis and bone destruction that is necessary for the development of metastatic bone disease.
Angiogenesis, the formation of new blood vessels from the existing vasculature, is essential for the growth of the primary tumor and for the formation of metastasis (Folkman, 1971). A number of different growth factors and cytokines can affect the proliferation and migration of endothelial cells and have been implicated in tumor-associated angiogenesis (Jain, 2003; Ferrara and Kerbel, 2005). Actually, angiogenesis is mediated by positive and negative regulators whose balance determines the rate of vessel formation (Ferrara and Kerbel, 2005). Vascular endothelial growth factor (VEGF) is the main inducer of tumor-associated neo-angiogenesis, although additional growth factors such as interleukin-8 (IL-8) and basic fibroblast growth factor (bFGF) might in turn play a role in this phenomenon (Ferrara and Kerbel, 2005). As we will discuss, the EGF receptor/ligand system regulates tumor-associated angiogenesis by different mechanisms, which include both direct and indirect effects on endothelial cells.
Tumor-induced stimulation of osteoclast-mediated bone resorption is the main mechanism responsible for bone destruction in cancer patients (Roodman, 2001). In this respect, bone marrow stromal cells (BMSCs) represent critical mediators for the pathogenesis of bone metastases. In fact, these cells are able to synthesize different growth factors that can induce differentiation and activation of osteoclast (Boyle et al., 2003). Interestingly, recent findings suggest that BMSCs express functional EGFR, whose signaling regulates the ability of these cells to sustain osteoclast activation (Normanno et al., 2005b).
In this short review, we describe the role of the EGFR and its ligands in the cross-talk between tumor cells and the above-mentioned non-malignant cell types of the tumor microenvironment. In particular, we will illustrate how these interactions might affect tumor growth and progression and how these mechanisms may offer important potential targets for novel therapeutic approaches.
The ErbB Receptor/Ligand Network
The ErbB family of tyrosine kinase receptors includes four members, namely EGFR, ErbB-2, ErbB-3, and ErbB-4 (Table 1; Normanno et al., 2005a, 2006). These proteins have an extracellular ligand-binding domain, a single hydrophobic transmembrane domain and a cytoplasmic tyrosine kinase-containing domain (Olayioye et al., 2000). The intracellular tyrosine kinase domain of ErbB receptors is highly conserved. However, the kinase domain of ErbB-3 contains substitutions of critical amino acids and therefore lacks kinase activity (Guy et al., 1994). The extracellular domains of the ErbB receptors are less conserved, suggesting that they are able to bind different ligands (Olayioye et al., 2000; Yarden, 2001; Yarden and Sliwkowski, 2001). In fact, the receptors of the ErbB family are activated following binding to peptide growth factors of the EGF-family (Table 1). EGF-like growth factors can be produced by the same cells that express ErbB receptors (autocrine secretion) or by surrounding cells (paracrine secretion), including stromal cells (Olayioye et al., 2000; Yarden and Sliwkowski, 2001). The EGF-like peptides are characterized by the presence of an EGF-like domain composed of three disulfide-bonded intramolecular groups, which confers binding specificity, and additional structural motif such as immunoglobulin-like domains, heparin-binding sites and glycosylation sites. Proteins of this family can be divided into three groups (Table 1; Yarden and Sliwkowski, 2001; Normanno et al., 2003). The first group includes EGF, transforming growth factor α (TGF-α), amphiregulin (AR), and the recently identified ligand epigen that binds specifically the EGFR (Strachan et al., 2001). The second group includes betacellulin (BTC), heparin-binding growth factor (HB-EGF), and epiregulin (EPR), which are capable of binding both EGFR and ErbB-4. The third group is composed of the neuregulins (NRGs) and tomoregulin, and can be divided in two subgroups based upon their capacity to bind ErbB-3 and ErbB-4 (NRG-1 and NRG-2) or only ErbB-4 (NRG-3, NRG-4, and tomoregulin) (Carraway et al., 1997; Chang et al., 1997; Zhang et al., 1997; Harari et al., 1999). None of the EGF family of peptides binds ErbB-2.
| ErbB receptor | Ligand |
|---|---|
| EGFR | Epidermal growth factor (EGF) |
| Transforming growth factor α (TGF-α) | |
| Amphiregulin (AR) | |
| Epigen | |
| EGFR and ErbB-4 | Betacellulin (BTC) |
| Heparin-binding growth factor (HB-EGF) | |
| Epiregulin (EPR) | |
| ErbB-2 | None |
| ErbB-3 and ErbB-4 | Neuregulin 1 (NRG-1) |
| Neuregulin 2 (NRG-2) | |
| ErbB-4 | Neuregulin 3 (NRG-3) |
| Neuregulin 4 (NRG-4) | |
| Tomoregulin |
The ErbB receptors form either homo- or hetero-dimers following ligand binding. Formation of dimers is essential for receptor activation and subsequent transmission of intracellular signals (Olayioye et al., 2000). In fact, following dimerization, auto- and trans-phosphorylation in tyrosine residues of the ErbB receptors occur. The tyrosine-phosphorylated receptors become able to interact with adaptor proteins that couple the receptors to intracellular signaling pathways (Olayioye et al., 2000). The ErbB receptors are able to activate different intracellular signaling cascades, including the phosphatidylinositol 3-kinase (PI3K)/Akt, the ras/raf/MEK/mitogen-activated protein kinase (MAPK), and the signal transducer and activator of transcription (STAT) pathways. Activation of these signaling proteins regulates cellular functions involved in cancer pathogenesis and progression (Normanno et al., 2006).
The Role of EGFR in Angiogenesis
It has long been demonstrated that EGFR ligands such EGF and TGFα are potent pro-angiogenic factors (Schreiber et al., 1986). However, the effects of these peptides on tumor-associated angiogenesis depend on two distinct phenomena: an indirect effect, due to the ability of the EGFR system to regulate the production of angiogenic factors in tumor cells, and a direct effect on endothelial cells.
Indirect effects
A number of in vitro studies have demonstrated that EGFR signaling regulates the production of pro-angiogenic factors in tumor cells of different histological origin (Table 2). For example, stimulation of glioma cells with EGF resulted in a significant increase of the secretion of bioactive VEGF (Goldman et al., 1993). Conditioned media prepared from EGF-stimulated glioma cells was able to activate human umbilical vein endothelial cells (HUVECs), and this effect was blocked by an anti-VEGF antibody (Goldman et al., 1993). In this respect, Maity et al. (2000) demonstrated that EGFR activation regulates VEGF mRNA expression in glioblastoma cells through a Ras/PI3K pathway. Mutations of PTEN, a negative regulator of PI3K/AKT signaling that is frequently inactivated in glioblastoma cells, were found to cooperate with EGFR activation to increase VEGF mRNA expression (Pore et al., 2003). In this regard, the ability of the EGFR tyrosine kinase inhibitor gefitinib to inhibit VEGF production in hepatocellular carcinoma cells was significantly reduced in cells with low levels of PTEN and constitutive activation of AKT (Ueda et al., 2006). Interestingly, transcriptional regulation of VEGF promoter by EGFR in glioma cells appeared to be distinct from signals induced by hypoxia (Maity et al., 2000). However, blockade of EGFR with the tyrosine kinase inhibitors gefitinib or erlotinib in squamous cell carcinoma led to reduced VEGF mRNA expression by decreasing Sp1 binding to the VEGF promoter and by downregulating HIF-1α expression (Pore et al., 2006). These latter findings suggest that different mechanisms are involved in the ability of anti-EGFR agents to decrease VEGF expression in tumor cells.
| Upregulated | Downregulated |
|---|---|
| Vascular endothelial growth factor (VEGF) | Thrombospondin-1 (TSP-1) |
| Interleukin-8 (IL-8) | |
| Basic fibroblast growth factor (bFGF) | |
| Angiopoietin-1 (Ang-1) | |
| Angiopoietin-2 (Ang-2) | |
| Plasminogen-activator–inhibitor-1 (PAI-1) |
Treatment with EGF induced in EGFR-positive gastric cancer cell lines the expression of mRNA for both VEGF and neuropilin-I (NRP-I), which acts as co-receptor for VEGF-165 and increases its affinity for VEGFR-2 in endothelial cells (Akagi et al., 2003). EGF induction of NRP-I was prevented by blockade of both MAPK and AKT signaling in these cells. Similar findings were obtained in pancreatic cancer cell lines, and expression of NRP-I was demonstrated in human pancreatic adenocarcinoma specimens (Parikh et al., 2003). In contrast, EGF-dependent regulation of NRP-1 expression in colon carcinoma cells was mediated by MAPK signaling but not the PI3K pathway (Parikh et al., 2004). Interestingly, overexpression of NRP-1 in colon and prostate carcinoma cells increased their motility in response to VEGF (Miao et al., 2000; Parikh et al., 2004). NRP-1 transduced tumor cells also showed increased tumor growth and angiogenesis in nude mice (Miao et al., 2000; Parikh et al., 2004). However, the mechanism through which NRP-1 promotes tumor cell growth and angiogenesis has not been clarified yet.
Treatment of nude mice bearing pancreatic carcinoma cells implants into the pancreas with the EGFR tyrosine kinase inhibitor PKI 166 produced a significant reduction in tumor cell production of both VEGF and IL-8 and in microvascular density (Bruns et al., 2000; Solorzano et al., 2001). The ability of anti-EGFR agents to reduce the secretion of both VEGF and IL-8 was confirmed by using in vitro assays in which pancreatic cancer cells were exposed to PKI 166 (Bruns et al., 2000).
EGF significantly induced production of both VEGF and IL-8 in A431 vulvar squamous cancer cells and in KB3-1 epidermoid cancer cells (Hirata et al., 2002). In agreement with these findings, gefitinib inhibited both constitutive and EGF-induced production of VEGF and IL-8 in the above-mentioned cancer cell lines (Hirata et al., 2002). Similar results were obtained with the anti-EGFR C225 antibody. In fact, treatment of A431 squamous carcinoma cells with C225 resulted in a significant reduction in their ability to produce VEGF protein and mRNA (Petit et al., 1997). Analogously, treatment of head and neck squamous carcinoma cells (HNSCC) with EGF induced secretion of both VEGF and IL-8, and this phenomenon was prevented by blockade of EGFR with C225 or with the EGFR tyrosine kinase inhibitor PD15035 (Bancroft et al., 2002). Interestingly, blockade of MEK signaling with the specific inhibitor U0126 produced a significant reduction of both VEGF and IL-8 expression, while the PI3K inhibitor LY294002 blocked expression of IL-8 but not VEGF in HNSCC cells (Bancroft et al., 2002).
In human transitional cell carcinoma (TCC) of the bladder, treatment with exogenous EGF produced a significant increase in the secretion of VEGF, IL-8, and bFGF (Perrotte et al., 1999). Analogously, in vitro treatment of TCC with C225 antibody resulted in decreased secretion of VEGF and IL-8, and in a significant reduction of cell-associated bFGF. The anti-EGFR antibody also induced a twofold to 10-fold reduction in the levels of mRNA expression for the three angiogenic factors. In agreement with these findings, a significant reduction of protein and mRNA expression of VEGF, IL-8, and bFGF as well as a reduction in tumor microvessel density was observed, following treatment with C225, in TCC cells orthotopically implanted in nude mice (Perrotte et al., 1999; Inoue et al., 2000).
Regulation of angiogenic factors by EGFR in prostate cancer cells has also been demonstrated. In particular, EGF significantly increased the secretion of VEGF in prostate cancer cells, while treatment with gefitinib inhibited both basal and EGF-induced production of VEGF (Sini et al., 2005). Accordingly, in a different study treatment with gefitinib was found to reduce the synthesis of both VEGF and bFGF in prostate cancer cells cultured in vitro or implanted in the flank of nude mice (Bianco et al., 2004). However, no significant effects of the EGFR tyrosine kinase PKI 166 on the expression of VEGF, bFGF, and IL-8 in prostate cancer cells injected in the tibia of nude mice were observed (Kim et al., 2003b). Furthermore, treatment of prostate cancer cells growing orthotopically in nude mice with the anti-EGFR antibody C225 produced a significant reduction in the levels of expression of IL-8 mRNA, whereas EGFR blockade had no effect on bFGF and VEGF transcripts (Karashima et al., 2002). Since different cell lines and different experimental approaches were used in these experiments, we might conclude that the effects of EGFR blockade on the expression of angiogenic factors in prostate cancer cells in vivo depend on different not fully defined factors.
In human GEO colon cancer cells, in vitro treatment with the anti-EGFR antibody C225 or with gefitinib produced a significant reduction in the secretion of TGF-α, bFGF, and VEGF. In agreement with these findings, immunohistochemical analysis of GEO tumor xenografts in nude mice showed a significant decrease of TGF-α, bFGF, and VEGF protein expression and of microvessel count following treatment with both anti-EGFR agents (Ciardiello et al., 2000, 2001). Ciardiello et al. (2001) also analyzed the effects of gefitinib on VEGF and bFGF expression in a panel of cancer cell lines of different histological origin, including colon, breast, ovary, and gastric tumor cells. A dose-dependent inhibition of VEGF and bFGF secretion in the conditioned medium of all the cancer cell lines examined was observed following treatment with gefitinib (Ciardiello et al., 2001).
Finally, blockade of EGFR in human renal cancer cells with PKI 166 decreased the secretion of both VEGF and bFGF (Kedar et al., 2002). Treatment with PKI 166 of renal cancer cells implanted orthotopically in the kidney of nude mice was also associated with a significant downregulation of VEGF, IL-8, and bFGF and a reduction of microvessel density (Kedar et al., 2002). However, PKI 166 produced a significant reduction in the levels of VEGF but not bFGF in a renal cell line established from a human renal cancer bone metastasis and implanted into the tibia of nude mice (Weber et al., 2003).
Taken together, the above-summarized findings demonstrate that the EGFR system regulates in cancer cells the expression of different pro-angiogenic growth factors through transcriptional and/or post-transcriptional mechanisms.
Interestingly, it has also been demonstrated that ErbB receptors other than EGFR might play a role in tumor-associated angiogenesis. Transformation of NIH3T3 fibroblasts with a rat oncogenic mutant of ErbB-2 resulted in a significant induction of VEGF production, and this phenomenon was enhanced by hypoxia (Petit et al., 1997). In addition, treatment of breast cancer cells that overexpress ErbB-2 with a neutralizing anti-ErbB-2 antibody produced a dose-dependent reduction of VEGF protein expression (Petit et al., 1997). This early report has been confirmed by several studies. Overexpression of ErbB-2 in breast cancer cells led to induction of the basal level of VEGF (Yen et al., 2000). In addition, heregulin β1 (NRG-1), the ligand for ErbB-3 and ErbB-4, induced VEGF secretion in a panel of breast and lung cancer cell lines with constitutive ErbB-2 overexpression or engineered to stably overexpress ErbB-2, but not in normal human mammary and bronchial cells (Yen et al., 2000). Similar findings were reported by Xiong et al. (2001), who found that heregulin β1 upregulates VEGF mRNA expression in SK-Br-3 and MCF-7 breast cancer cell lines through p38 MAPK. ErbB-2-mediated transcriptional upregulation of VEGF was found to involve a HIF-1α independent responsive region located between nucleotides −88 to −66 of VEGF promoter (Yen et al., 2002). However, a functional correlation between ErbB-2 signaling, HIF-1α and VEGF expression has also been demonstrated. In fact, it has been described that ErbB-2 signaling increases the levels of HIF-1α protein and VEGF mRNA expression through a PI3K/AKT dependent pathway (Laughner et al., 2001). In addition, ErbB-2-induced expression of HIF-1α and VEGF has been shown to require Stat3 signaling in breast cancer cells (Xu et al., 2005). Finally, expression of a constitutively active form of ErbB-2 in MDA-MB-435 breast cancer cells produced a significant, AKT/mTOR/p70S6K-dependent, increase in the levels of VEGF protein synthesis without any significant effect on VEGF mRNA levels (Klos et al., 2006). These findings suggest that different molecular mechanisms might be involved in the ability of ErbB-2 to induce VEGF expression in cancer cells.
Tumor cells frequently co-express different ErbB receptors that can form either homodimers or heterodimes with different signaling abilities. In this regard, comparison of all ErbB receptors, expressed singly or as paired receptor combinations in NIH3T3 cells, showed that the EGFR/ErbB-2 and ErbB-2/ErbB-3 heterodimers are the most potent inducers of VEGF mRNA expression (Yen et al., 2002). In vivo angiogenesis was also evaluated by immunohistochemical analysis of tumor xenografts of NIH3T3 cells expressing different ErbB dimers in nude mice. These experiments confirmed that EGFR/ErbB-2 and ErbB-2/ErbB-3 heterodimers induce a more significant increase of VEGF protein expression and vessel density as compared with other ErbB dimers (Yen et al., 2002).
Evidence suggests that ErbB-2 modulates the expression of different growth factors involved in tumor angiogenesis. For example, overexpression of ErbB-2 in MCF-7 and T47D breast cancer cells produced a significant increase of VEGF and IL-8 expression, whereas decreased the expression of the anti-angiogenic factor thrombospondin-1 (TSP-1) (Wen et al., 2006). In agreement with these findings, inhibition of ErbB-2 with the anti-ErbB-2 antibody trastuzumab or with a retrovirus-mediated small interfering RNA (siRNA) against ErbB-2, resulted in a reduction of VEGF and IL-8 mRNA and protein expression, and in increase of TSP-1 mRNA expression in BT474 breast cancer cells that constitutively overexpress ErbB-2 (Wen et al., 2006). Trastuzumab-induced upregulation of TSP-1 was associated with activation of p38 MAPK, but not of the PI3K/AKT pathway in BT474 cells in vitro and in vivo (Wen et al., 2006). In addition, a correlation was found in human breast cancer cell lines between high levels of ErbB-2 expression and the levels of angiopoietin-2 (Ang-2) (Niu and Carter, 2007), an angiogenic factor that was recently demonstrated to play a positive role in tumor angiogenesis (Oliner et al., 2004). These results were confirmed by immunoistochemical analysis of primary breast cancer tissues (Niu and Carter, 2007). More importantly, blockade of ErbB-2 with trastuzumab or with an ErbB-2 siRNA produced a reduction of Ang-2 expression at both the mRNA and protein levels in ErbB-2 positive breast cancer cells (Niu and Carter, 2007). Expression of Ang-2 was found to be regulated by both PI3K/AKT and MAPK signaling (Niu and Carter, 2007).
The ability of trastuzumab to affect the expression of different angiogenesis-related genes in tumor cells has also been demonstrated by gene array analysis of MDA-MB-361 breast cancer cells (Izumi et al., 2002). Treatment with trastuzumab produced downregulation of VEGF, TGF-α, angiopoietin-1 (Ang-1), and plasminogen-activator–inhibitor-1 (PAI-1), and upregulation of TSP-1. However, differences were found between in vitro and in vivo treatment. In particular, no significant reduction of VEGF mRNA expression was revealed in tumor xenografts, suggesting that host derived VEGF might be upregulated in breast cancer xenografts following trastuzumab treatment (Izumi et al., 2002).
Finally, the EGFR system also regulates the expression of VEGF in smooth muscle cells (SMCs) that are implicated in vessel maturation. In particular, EGF stimulated the release of VEGF by SMCs, and this phenomenon resulted in an increase of endothelial cell sprouting in a co-culture assay (Sini et al., 2005). Treatment with gefitinib of the EGF-stimulated co-cultures produced a significant reduction of VEGF secretion, suggesting that gefitinib exerts an indirect effect on sprouting through the blockade of EGFR activity in SMCs (Sini et al., 2005).
Direct effects on endothelial cells
Although evidence suggests a direct effect of ErbB ligands on endothelial cells, the expression and function of ErbB receptors in this cell type is still debated. The models of endothelial cells that have been employed to study the role of ligands and receptors of the EGF family are the already cited HUVEC and human microvascular endothelial cells (MVEC).
Several reports demonstrated expression of EGFR in MVECs. For example, Baker et al. (2002) showed that human MVECs isolated from the dermis express a functional EGFR protein that can be activated upon stimulation with either EGF or TGF-α. Moreover, Hirata et al. (2002) demonstrated that human MVECs derived from omentum express EGFR and are able to migrate in response to EGF. Treatment with gefitinib inhibited the EGF-induced migration of these cells (Hirata et al., 2002). In particular, EGFR phosphorylation, MAPK, and AKT signaling and formation of tube-like structures were significantly inhibited in MVECs by gefitinib (Hirata et al., 2002). EGF was also shown to activate MAPK and AKT in MVECs derived from neonatal dermis (Hirata et al., 2004). In contrast with these findings, Amin et al. (2006) did not find EGFR expression in commercially available MVEC nor in endothelial cells derived from normal mouse and human tissues. However, normal endothelial cells were found to express ErbB-2, ErbB-3, and ErbB-4, and their growth was inhibited by treatment with NRG. In contrast, tumor-derived endothelial cells expressed EGFR, ErbB-2, and ErbB-4, and their growth was not inhibited by treatment with NRG (Amin et al., 2006).
Several studies failed to demonstrate expression of EGFR in HUVEC (Hirata et al., 2002; Amin et al., 2006). In agreement with these findings, EGF was not able to stimulate migration of HUVECs, when cultured alone (Hirata et al., 2002). However, when HUVECs were co-cultured in presence of EGFR-overexpressing cancer cells, EGF enhanced their migration (Hirata et al., 2002). Treatment with gefitinib inhibited the migration of these cells indirectly, through blockade of EGF-induced VEGF production in cancer cells (Hirata et al., 2002). Moreover, Kim et al., 2003a showed that HUVECs express ErbB-2, ErbB-3, and ErbB-4, but not EGFR. Stimulation of these cells with BTC induced activation of MAPK and AKT through activation of ErbB-4 homodimer, ErbB-2/ErbB-3, or ErbB-2/ErbB-4 heterodimers (Kim et al., 2003a). In contrast, Sini et al. (2005) detected expression of EGFR and ErbB-2, but not of ErbB-3 and ErbB-4, in HUVECs and MVECs. Stimulation of HUVECs with EGF, HB-EGF, and BTC led to an increase of EGFR phosphorylation and ERK 1/2 activation (Sini et al., 2005). In addition, Wu et al. (2007) found EGFR and VEGFR2 protein expression and activation in HUVECs. Treatment with ZD6474, a small-molecule inhibitor of both EGFR and VEGFR2 kinases, blocked proliferation, migration and invasion of these cells (Wu et al., 2007). Finally, the expression of EGFR has been shown in tumor-associated endothelial cells in different in vivo experimental models: bone metastases from renal cell carcinoma, androgen-independent prostate cancer and follicular thyroid carcinoma; an orthotopic model of pancreatic carcinoma; tumor xenografts of prostate and lung cancer cells (Weber et al., 2003; Kim et al., 2003b; Sini et al., 2005; Yokoi et al., 2005; Younes et al., 2005).
In conclusion, heterogeneous results on the expression of ErbB receptors and on the activity of EGF-like growth factors in endothelial cells have been reported. Such heterogeneity might be related to the different tissue of origin of the endothelial cells, to differences between individuals as well as to cell culture variability. However, activation of EGFR signaling in endothelial cells could occur in specific physiological situation, being also conditioned by the surrounding microenvironment. As above-mentioned, analysis of tumor-derived endothelial cells showed that they express EGFR, are stimulated by EGFR-ligands and are not inhibited by NRG, whereas their normal counterparts do not express the EGFR and their growth is inhibited by NRG (Amin et al., 2006). Therefore, a switch of sensitivity to EGFR-ligands in endothelial cells might promote angiogenesis. Interestingly, Baker et al. (2002) demonstrated that only tumor-associated endothelial cells obtained from EGF/TGF-α positive bladder, pancreatic, and renal carcinomas express EGFR and its activated form. Tumor-associated endothelial cells obtained from a EGF/TGF-α negative renal cancer cell line implanted in nude mice were found to lack expression of EGFR (Baker et al., 2002). Therefore, tumor cells might induce expression and activation of EGFR-dependent pathways in endothelial cells, and this phenomenon might be involved in tumor-associated angiogenesis. Finally, EGF stimulation might turn of a greater relevance in tumors with low levels of VEGF production. In fact, EGF was found to promote the proliferation of endothelial cells in presence of low levels of VEGF, whereas it has little activity on these cells in presence of high levels of VEGF (Sini et al., 2005).
The Role of EGFR in the Pathogenesis of Bone Metastases
The pathogenesis and progression of bone metastases represent complex phenomena that involve the interaction of tumor cells with different cell types of the bone microenvironment (Roodman, 2001). Although it has been shown that tumor cells can directly resorb bone, evidence suggests that the main mechanism responsible for bone destruction in cancer patients is tumor-induced stimulation of osteoclast-mediated bone resorption (Roodman, 2001). Osteoclasts are the specialized progeny of hemopoietic precursors committed to the monocyte/macrophage lineage that, upon appropriate stimuli, fuse by giving rise to mature bone resorbing cells (Boyle et al., 2003). Development and functional activation of mature osteoclasts are regulated through the interplay of several cytokines and growth factors. However, two factors are both necessary and sufficient for osteoclast formation and activation: macrophage colony stimulating factor (M-CSF), which induces proliferation and differentiation of pre-osteoclast cells, and receptor activator of NF-kB ligand (RANKL) that is involved in fusion and activation of these cells (Boyle et al., 2003). Activation of osteoclasts is also regulated by osteoprotegerin (OPG), a soluble decoy receptor for RANKL that functions by sequestering the secreted form of RANKL and, therefore, by preventing the binding to its cognate receptor (Boyle et al., 2003).
Cancer cells are able to synthesize many growth factors and cytokines that can lead to the activation of osteoclasts (Roodman, 2001). In detail, parathyroid hormone related protein (PTHrP) is believed to represent the main mediator of breast cancer-induced bone resorption (Burtis et al., 1990). PTHrP has been purified from breast cancer cells, and it is expressed in up to 60% of human primary breast carcinomas (Burtis et al., 1990; Southby et al., 1990). However, most osteotropic factors do not directly stimulate osteoclast, but rather act indirectly by binding to accessory cells of the bone marrow microenvironment (Roodman, 2001). This heterogeneous cell compartment comprehends specialized endothelial cells, as well as mesenchymal stem cells (MSC), which maintain a level of self renewal and give rise to different specialized connective tissue cells such as “reticular cells,” osteoblasts, chondrocytes, adipocytes, and SMCs (Clark and Keating, 1995; Deans and Moseley, 2000). MSC and their progeny are collectively referred to as the BMSC compartment that is known to support hemopoiesis.
MSC, marrow stromal cells, and osteoblasts support osteoclast differentiation within the bone (Takahashi et al., 1988; Udagawa et al., 1989; Mbalaviele et al., 1999). In this regard, tumor cells have been shown to regulate the synthesis of osteoclastogenic factors in BMSC. In fact, PTHrP induces in BMSC the expression of RANKL (Horwood et al., 1998). Interestingly, no expression of RANKL was demonstrated in breast cancer cell lines (Thomas et al., 1999). In agreement with these data, these cells did not act as surrogate osteoblasts to support osteoclast formation in co-culture experiments (Thomas et al., 1999). However, when breast cancer cells overexpressing PTHrP were added to co-cultures of murine osteoblasts and hematopoietic cells, osteoclast formation took place without the addition of any osteotropic agents (Thomas et al., 1999). RANKL has also been shown to increase in BMSC the production of IL-6 that is involved in activation of osteoclasts; IL-6 in turn can upregulate RANKL and downregulate OPG in osteoblasts through PGE2 (Giuliani et al., 2004; Liu et al., 2005). Finally, it has been demonstrated that human MSC positively regulate osteoclastogenesis as undifferentiated progenitor cells (Mbalaviele et al., 1999). Co-culture with CD34+ hematopoietic stem cells induces MSC to secrete cytokines that support osteoclast formation, such as IL-6, IL-11, leukemia inhibitory factor (LIF), and M-CSF (Mbalaviele et al., 1999).
Several findings suggest a potential role of the EGFR pathway in regulating the ability of BMSC to promote osteoclast formation and activation. Addition of recombinant TGFα to long-term human bone marrow cultures, which contain MSC, stromal cells, osteoblasts, and osteoclast-precursors, has been shown to stimulate the formation of bone resorbing osteoclasts (Takahashi et al., 1986; Tanaka et al., 1998). In addition, the ability of TGFα and EGF to stimulate bone resorbing activity in organ culture systems, such as the fetal rat long bone and neonatal mouse calvarial systems, has long been described (Tashjian et al., 1985; Ibbotson et al., 1986). Several studies have also demonstrated expression of EGFR in BMSC and in osteoblast-like cells, as well as biological effects of EGF and/or TGFα in osteoblasts (Ibbotson et al., 1986; Drake et al., 1994; Sodek et al., 1995; Satomura et al., 1998). For example, EGF and TGFα caused a concentration-dependent inhibition of alkaline phosphatase activity in osteoblast-like cells (Ibbotson et al., 1986). EGF has also been found to upregulate the expression of mRNA for osteopontin in osteoblasts; osteopontin is a bone matrix protein that is synthesized by osteoblastic cells and mediates osteoclast adherence to the bone matrix (Sodek et al., 1995). Interestingly, PTH induced an increase in the levels of expression of EGFR in osteoblast-like cells, suggesting that PTH-induced growth of osteoblasts might be mediated by EGFR (Drake et al., 1994). Since expression of EGF has been demonstrated to occur in osteoclasts (Symons, 2003), it is conceivable that paracrine circuits involving EGFR and its ligands are operating between osteoclasts and osteoblasts. More recently, the functional role of EGFR signaling in MSC has been investigated. Krampera et al. (2005) reported that activation of EGFR by HB-EGF increased cell proliferation and prevented adipogenic, osteogenic, and chondrogenic differentiation in human bone marrow-derived MSC. In contrast, a different study described that the differentiation of human MSC into bone forming cells was stimulated by EGF but not PDGF (Kratchmarova et al., 2005). The use of different EGFR-ligands might at least in part justify such contrasting results. Furthermore, slight differences among preparations of primary MSC might also give rise to cells with divergent ability to differentiate in specialized bone marrow populations.
The involvement of the EGFR in the pathogenesis of bone metastases is suggested by an unexpected phenomenon that was observed in clinical trials of gefitinib in breast cancer patients. Albain et al., 2002 enrolled in their study of gefitinib in breast cancer 12 patients with bone metastases and bone pain. Surprisingly, 5 out of 12 patients had a significant relief of bone pain, leading to the complete withdrawal of all scheduled narcotics in several cases. Due to the impressive effects on bone pain palliation, two patients were maintained on gefitinib despite objective progression of the disease. A significant improvement in bone pain was also reported in a patient enrolled in a different trial of gefitinib in metastatic breast cancer (von Minckwitz et al., 2005).
Recent findings from our group have shed light on the role of EGFR signaling in the pathogenesis of bone metastases. In fact, we found that treatment of MSC-like cells with gefitinib resulted in significant reduction of the basal levels of activation of the EGFR and AKT, without affecting MSC proliferation (Normanno et al., 2005b). However, gefitinib treatment produced a significant decrease in the levels of secreted M-CSF and cell-associated RANKL in MSC (Normanno et al., 2005b). Furthermore, the ability of conditioned medium from gefitinib-treated MSC-like cells to sustain the differentiation of pre-osteoclasts was significantly reduced as compared with untreated cells (Normanno et al., 2005b). These results demonstrated for the first time that the EGFR regulates the ability of MSC to induce osteoclast differentiation. Our findings were confirmed by Angelucci et al. (2006) who demonstrated that treatment with gefitinib significantly reduced the ability of conditioned medium from prostate cancer cells to induce expression of RANKL in osteoblasts. In this regard, it is well established that EGFR and several of its ligands are expressed by prostate cancer cell lines and human primary prostatic carcinomas (Leverton and Gullick, 2000). Interestingly, expression of EGF-like peptides in BMSC has been also demonstrated (Mahtouk et al., 2004; Normanno et al., 2005b).
Although the above-mentioned study suggest a direct role of the EGFR in MSC-mediated osteoclast differentiation, different mechanisms are likely to be involved in the effects of anti-EGFR agents on the pathogenesis and progression of bone metastases (Normanno and Gullick, 2006). In fact, anti-EGFR agents might have a direct effect on cancer cells. In this regard, it has been shown in an experimental model of prostate cancer cells implanted in the bone of nude mice that tumor cells growing adjacent to bone tissues express high levels of activated EGFR whereas tumor cells invading the muscle do not (Kim et al., 2003b). This observation might suggest that activation of EGFR is essential for the ability of prostate cancer cells to grow within the bone. In addition, EGFR signaling modulates in tumor cells the expression of molecules that play an important role in the metastatic cascade. For example, the urokinase-type plasminogen-activator (uPA)/uPA receptor (uPAR) system and matrix metalloproteinases (MMPs) are involved in the invasive ability of tumor cells and in the formation of bone metastases (Guise and Mundy, 1998; Nemeth et al., 2002). In this regard, treatment with gefitinib significantly reduced the expression and activation of uPA and MMP-9 in prostate cancer cells, and this phenomenon was associated with a significant decrease in the ability of these cells to form bone metastases (Angelucci et al., 2006). Furthermore, treatment of human squamous cell carcinoma of head and neck (SCCHN) cells with the anti-EGFR blocking antibody C225 (cetuximab) significantly reduced their ability to invade surrounding tissues, including bone, and this inhibition was associated with downregulation of MMP-9 expression (Huang et al., 2002). Neo-angiogenesis is also essential for the formation of bone metastases. Expression of the EGFR in the endothelial cells of experimental models of bone metastases has been described (Kim et al., 2003b; Weber et al., 2003). The ability of anti-EGFR agents to inhibit the formation of bone metastases might be related at least in part to the induction of apoptosis in EGFR-expressing endothelial cells. Interestingly, it has been also demonstrated that osteoclasts express the VEGF receptor-1 (VEGFR-1), and that VEGF can induce osteoclast differentiation and activation (Niida et al., 1999). Therefore, the decrease in VEGF synthesis that is induced by anti-EGFR agents might lead to a reduced activation of osteoclasts. Finally, expression of EGFR mRNA in osteoclasts has been described (Wang et al., 2004). The EGFR tyrosine kinase inhibitor AG1478 reduced the formation of osteoclasts from bone marrow cells. However, expression of functional EGFR protein in osteoclasts has not yet been formally documented.
Conclusions and Perspectives
The above-summarized data demonstrate that the ErbB receptors and their ligands are involved in the cross-talk between cancer cells and different cell types of the tumor microenvironment (Fig. 1). In fact, several different studies have shown that the signaling of ErbB receptors regulates the production of pro-angiogenic factors in tumor cells. In addition, the majority of human carcinomas are able to synthesize and to secrete EGF-like growth factors that can bind to ErbB receptors expressed in accessory cells of the tumor microenvironment (Normanno et al., 2001). This interaction has been shown to regulate important mechanisms of tumor progression, such as proliferation and motility of endothelial cells and production of pro-angiogenic and pro-osteoclastogenic cytokines in tumor and stromal cells. Interestingly, expression of ErbB ligands has also been demonstrated to occur in endothelial cells (Arkonac et al., 1998; Iivanainen et al., 2003, 2007). Endothelial-derived EGF-like growth factors can have multiple functions: (a) regulation of VEGF synthesis in tumor cells; (b) activation of autocrine circuits in endothelial cells; and (c) direct activity on SMCs that are implicated in vessel maturation. In fact, SMCs express EGFR and ErbB-2, and their migration is induced by activation of the EGFR (Iivanainen et al., 2003). Furthermore, the EGFR signaling regulates the synthesis of VEGF in SMCs (Sini et al., 2005). Finally, BMSCs secrete EGF-like peptides and angiogenic growth factors that can contribute to boost the above-mentioned paracrine circuits (Gupta et al., 2001; Mahtouk et al., 2004; Normanno et al., 2005b).
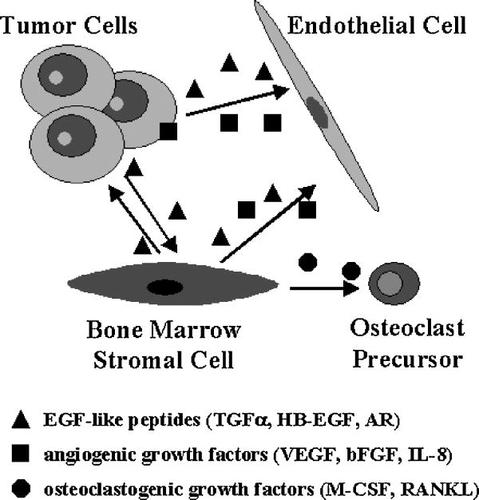
Autocrine and paracrine circuits involving the EGFR signaling in tumor microenvironment. Activation of the EGFR and ErbB-2 regulates in tumor cells the synthesis of angiogenic growth factors. Cancer cells also secrete EGF-like growth factors that can act directly on endothelial cells. In addition, EGFR ligands induce in bone marrow stromal cells the expression of osteoclastogenic factors that promote maturation and activation of osteoclasts, leading to bone destruction and to the formation of bone metastases. Finally, bone marrow stromal cells produce EGF-like peptides and angiogenic growth factors that can act on both endothelial cells and tumor cells.
The receptors and ligands of the EGFR family mediate complex interactions between tumor cells and the neoplastic environment that may ultimately result in enhanced tumor growth and progression. This observation makes of the EGFR an interesting target for therapeutic intervention even in tumors with EGFR-independent growth. Interestingly, clinical activity of anti-EGFR agents in patients carrying EGFR-negative tumors has been already demonstrated (Normanno et al., 2003). In this respect, the finding that EGFR-targeting drugs can affect mechanisms that support cancer progression in the tumor microenvironment might at least in part explain this phenomenon.
Two different hypotheses on the mechanism of action of anti-EGFR agents with respect to inhibition of angiogenesis have been formulated. Several studies have shown that anti-EGFR agents produce a significant reduction in the synthesis of pro-angiogenic cytokines in experimental tumors. It has also been demonstrated that downregulation of angiogenic factors precede the reduction of microvessel density (Perrotte et al., 1999). Therefore, these data suggest that the anti-angiogenic effect of EGFR targeting drugs is mainly indirect. On the other hand, it has been suggested that tumor-associated endothelial cells express the EGFR and that blockade of this receptor can lead to apoptosis of endothelial cells (Baker et al., 2006). It is conceivable that both these mechanisms might be involved in the anti-tumor effect of anti-EGFR agents. Finally, the observation that the EGFR regulates the production of pro-osteoclastogenic cytokines in BMSC suggests that anti-EGFR drugs might be employed to control the progression of bone metastases. However, clinical trials of anti-EGFR agents in cancer patients with metastatic bone disease are definitely required to confirm this hypothesis.
An interesting therapeutic approach in cancer patients is the combined blockade of EGFR and VEGF receptor signaling, which both contribute to tumor progression. In this regard, different tyrosine kinase inhibitors that are able to block the activation of both receptors have been synthesized and are in clinical development. The anti-tumor efficacy of these agents has been demonstrated in different experimental models of human tumors including bone metastases (Ciardiello et al., 2003; Yokoi et al., 2005; Younes et al., 2005; Morelli et al., 2006; Wu et al., 2007). Preliminary results of clinical trials with these drugs suggest a good tolerability and a significant anti-tumor activity (Morabito et al., 2006). However, larger clinical trials are definitely required to confirm the efficacy of these compounds.
In conclusion, the evidence summarized in this article suggests that the EGFR system is an important mediator of autocrine and paracrine circuits within the tumor microenvironment. Since the EGFR is expressed in all the cell types with the exception of mature hemopoietic cells, these findings also highlight the importance of studying the effects of anti-EGFR agents in the different non-cancer cell types of the neoplastic microenvironment that might be involved in tumor growth and progression. In this respect, targeting EGFR-mediated circuits of tumor microenvironment might represent a novel important therapeutic approach.




