Cyclin D1/cdk4 can interact with E2F4/DP1 and disrupts its DNA-binding capacity
Abstract
The E2F family of transcription factors regulate the expression of many growth-related genes in a cell cycle-dependent manner. These transcription factors can activate or, in conjunction with an Rb-related protein, repress transcription. E2F transcriptional activity is regulated at several different levels that are each linked to cell cycle progression. In many cell types, E2F4 and E2F5 are the predominant E2F species during G0 and early G1 and function primarily as repressors of E2F-regulated genes. In this study, co-immunoprecipitation techniques were used to demonstrate that cyclins D1, D2, and D3 are capable of interacting with E2F4, E2F5, and DP1. Overexpression of cyclin D1/cdk4 reduced E2F4-mediated transcription in a simple reporter gene assay and electrophoretic mobility shift analyses using nuclear extracts from transfected cells indicated that cyclin D1/cdk4 disrupts the DNA-binding ability of E2F4. Cell cycle analysis following stimulation of serum-starved 3T3 cells indicated that E2F4 undergoes changes in its phosphorylation pattern coincident with the synthesis of cyclin D1. Examination of a series of E2F4 deletion mutants indicated that a cyclin D1-binding site located close to the carboxyl terminus of E2F4 was critical for the disruption of DNA binding by cyclin D1/cdk4. These data support a model in which E2F4 DNA binding is abolished during mid-G1 at the same time when E2F interactions with pRb-related proteins are disrupted by cyclin D1/cdk4. J. Cell. Physiol. 214: 568–581, 2008. © 2007 Wiley-Liss, Inc.
E2F transcriptional activity was originally characterized as a cellular factor capable of binding to and activating the E2 promoter of adenovirus. Initial research revealed that E2F is an important cellular factor controlling the expression of many genes involved in cell cycle progression (for a review see Attwooll et al., 2004; Dimova and Dyson, 2005). More recently, the activities attributed to E2F have become more diverse and include regulating genes involved in differentiation and apoptosis. E2F transcriptional activity is mediated by heterodimers comprised of components from two related protein families known as E2F and DP. There are eight known genes encoding at least nine E2F proteins that share amino acid sequence similarity and are capable of binding directly to the E2F DNA consensus site. The three DP family members, DP1, DP2/3, and DP4, encode proteins that are related to the E2F family with homology in the DNA-binding and dimerization domains of E2F. The DP proteins lack the ability to bind autonomously to DNA and function as the heterodimeric partners for the E2F family. DP1 and DP2/3 increase the DNA-binding efficiency and transactivation potential of the E2F proteins, whereas, DP4 appears to interfere with E2F-mediated transcription.
Transient transfection assays with simple E2F-reporter plasmids have demonstrated that heterodimers of E2F's 1–5 with DP1 can function as activators of transcription when overexpressed (Helin et al., 1992; Kaelin et al., 1992; Lees et al., 1993; Beijersbergen et al., 1994; Ginsberg et al., 1994; Buck et al., 1995; Hijmans et al., 1995). E2F/DP heterodimers also can participate in repression of transcription through interactions with an Rb-related protein (pRb, p107, or p130). E2F-mediated recruitment of an Rb-related protein to the DNA facilitates the formation of a transcriptional repression complex at the E2F site (reviewed in Cobrinik, 2005).
Evidence from a number of studies has suggested that E2F proteins can be classified according to function as either “activators” (E2F1, E2F2, and E2F3a) or “repressors” (E2F3b, E2F4, E2F5, E2F6, E2F7, and E2F8). E2F1, E2F2, and E2F3a can bind to pRb (Helin et al., 1992; Ivey-Hoyle et al., 1993; Lees et al., 1993), are predominantly nuclear (Magae et al., 1996; Lindeman et al., 1997; Muller et al., 1997; Verona et al., 1997) and contain a cyclin A-binding motif (Krek et al., 1994; Xu et al., 1994). The E2F1, E2F2, and E2F3a genes are transcribed in a cell cycle-dependent manner with levels low in G0 and early G1 and then rising late in G1 (Hsiao et al., 1994; Johnson et al., 1994; Sears et al., 1997; Leone et al., 1998). An additional level of E2F1 regulation occurs through a direct interaction with cyclin A/cdk2 (Dynlacht et al., 1994; Krek et al., 1994; Xu et al., 1994; Kitagawa et al., 1995). After the onset of S phase, cyclin A/cdk2 phosphorylates both E2F1 and DP1 resulting in a loss of DNA-binding ability. The cyclin A/cdk2 interaction site on E2F1 is conserved in E2F2 and E2F3 but not in E2F4 and E2F5 suggesting that E2F2 and E2F3 may also be regulated in a similar manner. These observations are consistent with a role for E2F1, E2F2, and E2F3 in late G1 and at least part of S phase, a time when many E2F-regulated genes are actively transcribed. Using chromatin immunoprecipitation assays, promoter occupation primarily by E2F1 and E2F3 has been correlated with histone acetylation and active transcription for several E2F-regulated genes (Takahashi et al., 2000; Wells et al., 2000). Indeed, the loss of all three activating E2Fs results in acute cell cycle arrest indicating that activator E2F activity is essential for cell cycle progression (Wu et al., 2001).
In contrast to the “activator” E2Fs, E2F3b, E2F4, and E2F5 are transcribed throughout the cell cycle (Ginsberg et al., 1994; Beijersbergen et al., 1995; Buck et al., 1995; Hijmans et al., 1995; He et al., 2000; Leone et al., 2000). E2F4 and E2F5 differ from E2F1–3 in that they are localized primarily in the cytoplasm (Magae et al., 1996; Lindeman et al., 1997; Muller et al., 1997; Verona et al., 1997). Regulation of E2F4 and E2F5 nuclear localization appears to be complex and it may involve direct nuclear import, Crm1-dependent export, and facilitated nuclear import through interactions with proteins such as pRB, p107, and p130 (Magae et al., 1996; Gaubatz et al., 2001; Apostolova et al., 2002). Through the work of many studies, a model has emerged in which E2F4 and E2F5 function primarily as transcriptional repressors during G0 and early G1 in conjunction with pRB and p130 (Attwooll et al., 2004; Dimova and Dyson, 2005).
E2F6, E2F7, and E2F8 appears to form a distinct class of E2F repressors as they lack both the transactivation domain and the binding site for the RB family (Morkel et al., 1997; Cartwright et al., 1998; Gaubatz et al., 1998; Trimarchi et al., 1998; Di Stefano et al., 2003; de Bruin et al., 2003; Logan et al., 2004, 2005; Maiti et al., 2005). E2F6 dimerizes with DP proteins and interacts with several chromatin-remodeling proteins that are homologs of the polycomb family (Trimarchi et al., 2001; Ogawa et al., 2002). E2F7 and E2F8 function as repressors in a manner that is independent of DP and RB family proteins (Cartwright et al., 1998; Di Stefano et al., 2003; de Bruin et al., 2003; Logan et al., 2004, 2005; Maiti et al., 2005).
The association of E2F proteins with the pRb family is governed by cyclins and cyclin-dependent kinases (cdks) (reviewed in Cobrinik, 2005). During G0 and early G1, both pRb and p130 are present in the cell and capable of binding to E2F proteins. Cyclins D1, D2, and D3 complex with cdk4 or cdk6 to initiate phosphorylation of pRb beginning in mid G1 (Matsushime et al., 1992; Dowdy et al., 1993; Ewen et al., 1993; Kato et al., 1993). Disruption of the pRb interaction with E2F requires additional phosphorylation events by cyclin E/cdk2 (Lundberg and Weinberg, 1998; Harbour et al., 1999). The phosphorylation of p130 and p107 is not as well studied as pRb; however, several studies suggest that D cyclin/cdk4 activity is sufficient to disrupt E2F interactions (Beijersbergen et al., 1995; Johnson, 1995; Mayol et al., 1995; Dong et al., 1998; Hansen et al., 2001; Farkas et al., 2002; Leng et al., 2002).
In G0 and early G1, when most E2F-regulated genes are repressed, E2F4/p130 complexes are bound to E2F regulatory sites in promoters of genes that are transcriptionally downregulated (Takahashi et al., 2000; Wells et al., 2000; Ren et al., 2002; Cam et al., 2004). Although it has been demonstrated that E2F4 becomes largely cytoplasmic at later times in the cell cycle, the fate of DNA-bound E2F4 following cyclin D/cdk4-mediated phosphorylation of p130 has not been described. In this study, we provide evidence for the regulation of E2F4/DP1 interactions with DNA by cyclin D1/cdk4 resulting in loss of E2F4/DP1 DNA binding.
Materials and Methods
Cell culture and baculoviruses
Human C33A cells (obtained from the American Type Culture Collection) and mouse 3T3-L1 fibroblasts were grown in Dulbecco's modified Eagle media (DMEM) supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO). 3T3-L1 cells were serum starved by removing the medium from subconcluent cells and replacing it with DMEM without serum for 48 h. Cells were restimulated by replacing the media with DMEM containing 20% fetal bovine serum. SF9 cells, derived from Spodoptera fragiperda larvae, were cultured in Grace's insect cell media (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum, 0.07 g/L lactalbumin and 0.07 g/L yeastolate (Sigma, Gibco). Recombinant baculoviruses for E2F4-Ha, DP1, and E2F5 were generated using the Baculogold system (Pharmingen, San Diego, CA). Baculoviruses expressing cyclins D1, D2, and D3 and cdk4 were provided by Dr. Charles Sherr (Memphis, TN) and have been previously described (Kato et al., 1993).
Plasmids
The plasmids pBSkDP1, pCMVHADP1, pCMVHAE2F-4, pCMVHAE2F-5, and pBSKHAE2F-4 were generously provided by Dr. Kristian Helin (Milan, Italy). The cyclin expression plasmids Rc/cycE, Rc/cycD1 (Hinds et al., 1992) were provided by Dr. Robert Weinberg (Whitehead Institute) and pcmvCycE was provided by Dr. James Roberts (Seattle, Washington). The cdk-expressing plasmids pCMVcdk2 and pCMVcdk4 (van den Heuvel and Harlow, 1993) were provided by Dr. Ed Harlow (Massachusetts General Hospital). CMV p130 has been previously described (Li et al., 1993).
Plasmids expressing Ha-tagged E2F4 deletion mutants were constructed using polymerase chain reactions (PCR) to generate altered E2F4 cDNAs. To generate cDNAs encoding C-terminally truncated proteins, E2F4 was amplified using PCR from the plasmid pBSK HaE2F-4 using a common 5′ primer, T7 5′-TAATACGACTCACTATAGG-3′ and the following 3′ primers each containing a stop codon and Xba I restriction endonuclease site: mutant 382CT: 5′-GCTCTAGACTAACGAAGCAGAGGGGCA-3′; mutant 305CT: 5′-GCTCTAGACTACAGCAGGGCAGAAGAC-3′; mutant 202CT: 5′-GCTCTAGACTATGAGCTCCATGCCTCCTT-3′; mutant 176CT: 5′-GCTCTAGACTACTGCCCATTGAGACCCTC-3′. Amplified PCR products were digested with HindIII and XbaI and ligated into the HindIII/XbaI sites of the pcDNA3 expression vector.
E2F4 cDNAs encoding N-terminally truncated proteins were generated in a similar manner using a common 3′ primer: T3 5′-ATTAACCCTCACTAAAG-3′ and the following 5′ primers each containing a BamHI restriction endonuclease site:
mutant 44NT: 5′-CGCGGATCCCTGGCAGCTGACACCCTAGCT-3′; mutant 84NT: 5′-CGCGGATCCGGGCCTGGCTGCAATACCCGG-3′; mutant 128NT: 5′-CGCGGATCCACAGAGGACGTGCAGAACAGC-3′; mutant 179NT: 5′-CGCGGATCCCAGATTCACCTGAAGAGT-3′. The amplified PCR products were digested by BamHI and XbaI and ligated into the BamHI/XbaI sites of the pCMVHAE2F-4 vector. The BamHI digest of the vector retains sequences encoding N-terminal Ha epitope in frame with the newly added PCR product.
Plasmids expressing E2F4 with both C- and N-terminal truncations were made using a common 3′ primer: 5′-GCTCTAGACTAACGAAGCAGAGGGGCA-3′ and different 5′ primers: mutant 128NT\382CT: 5′-CGCGGATCCACAGAGGACGTGCAGAACAGC-3′; mutant 179NT\382CT: 5′-CGCGGATCCCAGATTCACCTGAAGAGT-3′. The PCR products were digested with BamHI and XbaI and ligated into the BamHI/XbaI-digested pCMVHAE2F-4 vector. The pCMVDP1 plasmid was produced by digesting pBSKDP1 by EcoRI and ligating into the EcoRI digested pcDNA3 vector.
Antibodies and immunoprecipitations
Polyclonal antibodies recognizing DP1 (K20), cyclin D3 (C-16), E2F4 (C20), p16 (M156), p130 (C20), and monoclonal E2F5 (MH-5) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The cyclin D1 (DCS-6) and cyclin D2 (DCS-3) monoclonal antibodies were a gift from Dr. Jiri Bartek (Copenhagen, Denmark). The cyclin D1 polyclonal employed in Western blots was purchased from Upstate Biotechnology (Lake Placid, NY). The anti-HA monoclonal antibody, 12CA5, was used for immunoprecipitations and the polyclonal antibody, Y-11 (Santa Cruz Biotechnology) was used for immunoblots. The antibody to alpha-tubulin was from Sigma. Horseradish-conjugated secondary antibodies were from Jackson Immunoresearch (West Grove, PA).
Metabolic labeling of SF9 cells with 35S-methionine/cysteine was as previously described (Lacy and Whyte, 1997). For mammalian cells, 35S-methionine/cysteine labeling and immunoprecipitations were as described (Harlow et al., 1986). Immunoblots were developed using Enhanced Chemiluminescence System (Amersham, Piscataway, NJ). Protein concentrations of clarified non-radioactive supernatants of lysed cells were determined by the Bio-Rad protein assay reagent and the supernatants were normalized for protein concentration (Bio-Rad Laboratories, Hercules, CA).
In vitro phosphorylation was assayed by resuspending protein A Sepharose containing immunoprecipitated complexes in 50 µl kinase buffer (50 mM Tris pH 7.4, 10 mM MgCl2, 5 mM MnCl2) with 5 µCi γ-32P-ATP and incubating at room temperature for 15 min. To re-immunoprecipitate specific proteins, the immune complex was disrupted by adding 100 µl of SDS release buffer (50 mM Hepes pH 7.0, 250 mM NaCl, 1% SDS, 0.5 mM DTT) and heating for 10 min at 100°C. The supernatant was removed following centrifugation to pellet the protein A Sepharose and diluted with 1.4 ml of E1A buffer and then used in a second round of immunoprecipitation.
Transient transfections
Transient transfections of mammalian cells employed the calcium phosphate precipitation technique (Graham and Van Der Eb, 1973). Ten microgram of plasmid DNA (unless stated otherwise) was transfected per 10 cm plate of mammalian cells. Fifteen hours post-transfection, the cells were rinsed twice with PBS and incubated with fresh medium for at least 24 h before harvesting.
Electrophoretic mobility shift assays (EMSA)
Double-stranded wild-type E2F oligonucleotide was made from the complementary base sequences 5′-ATTTAAGTTTCGCGCCCTTTCCAA-3′ and 5′-TTGGAAAGGGCGCGAAACTTAAAT-3′. The mutant double-stranded E2F oligonucleotide was made from the complementary base sequences 5′-ATTAAGTTTCGATCCCTTTCTCAA-3′ and 5′-TTGAGAAAGGGATCGAAACT TAAT-3′. The oligonucleotide was end-labeled with γ-32P-ATP using T4 polynucleotide kinase buffer (NEB), and purified by polyacrylamide gel electrophoresis. Subcellular fractionation of cells was performed using hypotonic lysis as previously described (Verona et al., 1997). The protein concentrations were determined using the Bio-Rad protein assay reagent.
Cell extracts were pre-incubated with the 32P-labeled E2F oligonucleotide at 4°C for 10 min. Each reaction contained the following: 2–10 µg nuclear or cytoplasmic extracts, 0.5–2 µg of poly (dI-dC) (Boehringer Mannheim, Indianapolis, IN), binding buffer (50 mM KCl, 20 mM Hepes pH 7.4, 1 mM MgCl2, 1 mM EDTA pH 8.0, 8.5% glycerol). Seventy-five ng of unlabeled double-stranded mutant E2F oligonucleotide was added to the reactions in Figure 4B and 8 to reduce background. To test for the presence of specific proteins, 1 µl of the appropriate antibody was added. To test for specificity of binding 75 ng of unlabeled wild-type double-stranded E2F oligonucleotide was added. After pre-incubation 1 µl of 32P-end-labeled double-stranded wild-type E2F oligonucleotide was added (representing an activity of at least 10,000 cpm) and incubated for a further 10 min. The samples were electrophoresed in 5% polyacrylamide gels buffered with 0.25 X TBE (22 mM Tris, 22 mM borate, 0.5 mM EDTA). The gel was washed for 10 min in water, dried, and exposed to film.
Chloramphenicol acetyltransferase (CAT) assays
C33A cells were transfected with 5 µg of the reporter plasmid E2F4Cat (Helin et al., 1993) containing four tandem E2F-binding sites from the E2A promoter to measure the E2F4-dependent transactivation. Two microgram of a luciferase expression plasmid pGL3-Control was transfected as an internal control to normalize for transfection efficiency. Five to ten microgram of the expression plasmids under study were transfected to each plate in any one experiment. When necessary pcDNA3 was added to adjust the total amount of transfected plasmid. Thirty-six hours after transfection, the cells were washed twice in PBS and then resuspended in extraction buffer (100 mM potassium phosphate, 1 mM DTT). Cells were freeze/thawed three times and the cellular debris was pelleted. Supernatants were assayed for CAT and luciferase activities as previously described (Sleigh, 1986; De Wet et al., 1987). After normalization of the CAT values the results for at least three different experiments were averaged and presented as a relative activity expressed with the standard error of the mean.
Results
Interaction of the D cyclins with E2F4, E2F5, and DP1 in Sf9 cells
To examine whether the D cyclins can bind directly to the E2F4, E2F-5, and DP1 proteins, recombinant baculoviruses expressing the E2F4-Ha, E2F5, and DP1 proteins were constructed. Baculovirus-directed expression of proteins corresponding to E2F4, E2F5, and DP1 were observed in the infected lysates when immunoprecipitated by an appropriate antibody (Fig. 1A, lanes 1,2,4,6). The expressed E2F4 protein contained a Ha (hemagglutinin) epitope facilitating its immunoprecipitation with the Ha monoclonal antibody 12CA5 (Fig. 1A, lane 1). No proteins were immunoprecipitated from the uninfected lysates indicating that the antibodies do not cross react with endogenous SF9 proteins (Fig. 1A, lanes 8–12). SF9 cells co-infected with E2F4-Ha, E2F5, or DP1 and a baculovirus expressing cyclin D1 were used to examine the ability of these proteins to interact with cyclin D1. Proteins from the co-infected lysates were immunoprecipitated with the indicated antibodies, blotted, and probed with a polyclonal cyclin D1 antibody (Fig. 1B). Cyclin D1 was detected as a co-immunoprecipitating protein with E2F4-Ha, E2F5, and DP1 suggesting that cyclin D1 can specifically interact with each of these proteins (Fig. 1B, lanes 6,9,12 compared with lanes 19,21,23). Cyclin D1 was also detected as a protein co-immunoprecipitating with E2F4, E2F5, and DP1 in 35S-methionine-labeled extracts using either antibodies to cyclin D1 or E2F4, E2F5, and DP1 (data not shown).
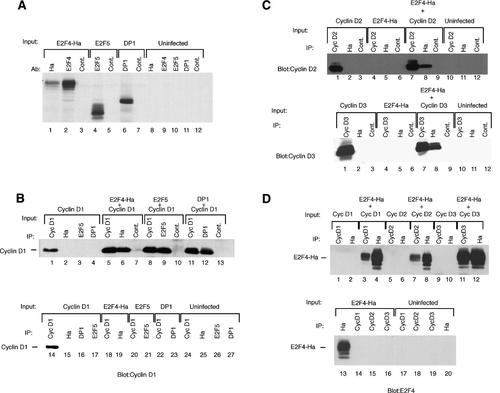
Cyclins D1, D2, and D3 interact with E2F4, E2F5, and DP1 in baculovirus-infected Sf9 cells. Sf9 cells were infected or co-infected with baculoviruses expressing the indicated proteins. A: Cells infected with baculoviruses expressing HA-tagged E2F4, E2F5, and DP1 and uninfected cells were metabolically labeled with 35S-met/cys and lysates were immunoprecipitated using the indicated antibodies. Immunoprecipitated proteins were separated on a 10% polyacrylamide gel and visualized by autoradiography. B: Cells were infected with baculoviruses (input) expressing cyclin D1, E2F4-Ha, E2F5, and DP1 or co-infected with baculoviruses expressing cyclin D1 plus E2F4-Ha (lanes 5–7), E2F5 (lanes 8–10), and DP1 (lanes 11–13). Following lysis, immunoprecipitations were conducted using antibodies to the indicated proteins (IP). The proteins were separated by SDS–PAGE, blotted to nitrocellulose and probed using an antibody to cyclin D1. C: Uninfected cells and cells infected with baculoviruses expressing cyclin D2, cyclin D3, and E2F4-Ha singly or in combination with cyclins D2 and D3 were lysed and immunoprecipitated using antibodies to the indicated protein. Following electrophoresis, the proteins were transferred to nitrocellulose and immunoblotted for cyclin D2 (upper part) or cyclin D3 (lower part). D: Uninfected cells and cells infected with baculoviruses expressing cyclins D1, D2, D3, and E2F4-Ha either alone or in combination with each of the D cyclins were lysed and immunoprecipitated using the indicated antibodies. Following electrophoresis and transfer to nitrocellulose, the blots were probed using an antibody to E2F4.
Because of the similarity of cyclins D1, D2, and D3, the possibility that cyclins D2 and D3 could interact with E2F4 was examined. SF9 cells were co-infected with recombinant baculoviruses expressing either cyclin D2 or cyclin D3 in combination with the E2F4-Ha expressing baculovirus virus and proteins from the infected lysates were immunoprecipitated using antibodies to cyclin D2, cyclin D3, and E2F4. Immunoblotting of the precipitated complexes using antibodies to cyclins D2 and D3 revealed the presence of cyclins D2 and D3 in the E2F4 immunoprecipitates (Fig. 1C, lanes 7–9 vs. lanes 4–6 in both upper and lower parts). Reciprocal experiments, blotting with an antibody to E2F4 were performed to confirm the interaction of the D-type cyclins with E2F4. Specifically, SF9 cells were infected with baculoviruses expressing cyclins D1, D2, or D3 in the presence or absence of a baculovirus-expressing E2F4. E2F4, cyclin D1, D2, and D3 were immunoprecipitated from the lysates and the precipitated proteins were blotted and probed for the presence of E2F4 (Fig. 1D). E2F4 was detected as a co-immunoprecipitating protein with each of the D-type cyclins (Fig. 1D, lanes 3,4,7,8,11,12). These results suggest that each of the D-type cyclins can interact with E2F4 and that at least cyclin D1 can interact with E2F5 and DP1.
Interaction of cyclin D1 with E2F4, E2F5, and DP1 in mammalian cells
To determine whether cyclin D1 could interact with E2F4, E2F5, and DP1 in mammalian cells, plasmids expressing cyclin D1 and Ha epitope-tagged E2F4, E2F5, and DP1 were transfected singly or in combination into C33A cells, a human cervical carcinoma cell line. E2F4, E2F5, and DP1 were immunoprecipitated, blotted, and probed with an antibody to cyclin D1. Cyclin D1 was detected as a protein co-immunoprecipitating with E2F4, E2F5, and DP1 (Fig. 2A, lanes 14,17,20). At the level of exposure shown, the endogenous cyclin D1 in C33A cells was not visible and did not contribute significantly in these assays (Fig. 2A, lanes 2,5,8). To verify the interactions, a reciprocal experiment was conducted in which the immunoprecipitated proteins were blotted and probed using an antibody to the Ha epitope tag. In this experiment, Ha-tagged versions of E2F4, E2F5, and DP1 were detected co-immunoprecipitating with cyclin D1 (Fig. 2B, lanes 13,16,19). These experiments and those in the previous Section indicate that cyclin D1 interacts specifically with E2F4, E2F5, and DP1.
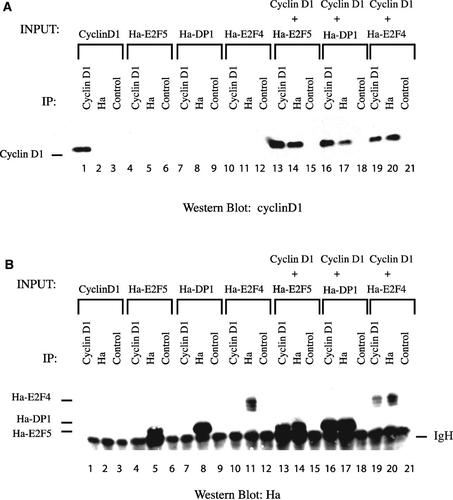
Cyclin D1 interacts with E2F4, E2F5, and DP1 in transfected C33A cells. Human C33A cervical carcinoma cells were transfected with plasmids expressing the indicated proteins. A: Antibodies to cyclin D1 and the Ha epitope were used to immunoprecipitate the proteins expressed from the transfected plasmids. Rabbit anti-mouse antibody was used as a non-reactive control antibody. After transferring to nitrocellulose, the blot was probed with an antibody to cyclin D1. B: Transfections and immunoprecipitations similar to (A) were conducted except that the blot was probed with an antibody to the Ha epitope tag. The positions of Ha-E2F4, Ha-E2F5, and Ha-DP1 are indicated on the left side of the blot. IgH is the immunoglobulin heavy chain.
Cyclin D1/cdk4 activity inhibits E2F4-mediated transactivation
As a step toward determining the functional basis for the interactions described above, we have focused on the interaction between cyclin D1 and E2F4. Previous work has demonstrated that E2F4 can facilitate transcription from a transfected reporter plasmid (Beijersbergen et al., 1994; Ginsberg et al., 1994). To help determine the role of interaction of cyclin D1 with E2F4, the effect of cyclin D1 and cdk4 on E2F4-mediated transactivation was examined. A reporter plasmid with a promoter containing four tandem E2F-binding sites fused to the chloramphenicol acetyl transferase (CAT) gene was used to determine the effect of cyclin D1/cdk4 activity on the ability of E2F4 to activate CAT expression. Transfection of E2F4 alone was sufficient to stimulate transcription from the reporter plasmid. When cyclin D1 and cdk4 were co-expressed with E2F4 in C33A cells, a significant reduction in CAT activity was observed (Fig. 3A). Co-transfection of cdk4 without cyclin D1 had only a modest effect which, presumably, was mediated through endogenous D cyclins. Co-expression of cyclin D1 and cdk4 reduced the relative amount of CAT activity by approximately 75% suggesting that cyclin D1 and cdk4 have an adverse effect on E2F4-mediated transcription. In contrast, cdk2 and cyclin E did not have a significant effect on the level of CAT activity suggesting that the effect of cyclin D1/cdk4 was specific. The role of cdk4 activity in suppressing E2F4 transactivation was examined further by co-transfecting constant amounts of the reporter plasmid and plasmids expressing E2F4, DP1, and cyclin D1 with increasing amounts of a plasmid-expressing cdk4 (Fig. 3B). The observed CAT activity diminished as the amount of cdk4 plasmid was increased indicating that the reduction in CAT activity was cdk4-dependent. These experiments suggest that the cyclin D1/cdk4 interaction with E2F4 functions to negatively regulate E2F4 transcriptional activity and that the effect is facilitated by cdk4.
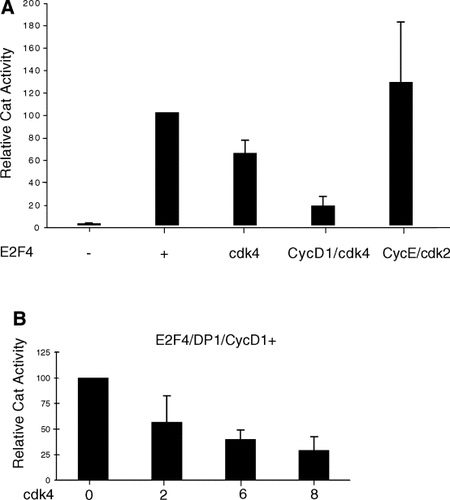
Co-expression of cyclin D1/cdk4 with E2F4 reduces E2F4-mediated transactivation. A: C33A cells were co-transfected with 2 µg of E2F4-CAT reporter plasmid and 2 µg pGL3-Control either alone or with 2 µg plasmid expressing E2F4-Ha and 5 µg of plasmids expressing cdk4, cyclin D1, cyclin E, and cdk2 as indicated. Each transfection used a total of 16 µg of plasmid and when necessary pcDNA3 was added to equalize the amount of DNA transfected. Forty-eight hours post-transfection, the cells were lysed and CAT activity was determined as described in Materials and Methods section. B: C33A cells were co-transfected with 2 µg of E2F4-CAT reporter plasmid, 2 µg pGL3-Control, 2 µg of plasmids expressing E2F4-Ha, DP1, cyclin D1, and the indicated amount of plasmid expressing cdk4. CAT activities were determined as for (A) except that activities are expressed as a percentage of the activity obtained in the absence of the cdk4-expressing plasmid. In both (A) and (B) the results are averages of three experiments with error bars representing the standard error of the mean.
Disruption of E2F4 DNA-binding activity by cyclin D1/cdk4
Previously, cyclin A/cdk2 was found to negatively regulate E2F1/DP1 by phosphorylating E2F1 and DP1 and disrupting DNA-binding capacity (Dynlacht et al., 1994; Krek et al., 1994; Xu et al., 1994; Kitagawa et al., 1995). Hence, it is possible that cyclin D1/cdk4 was regulating E2F4/DP1 DNA binding in a similar manner to the cyclin A/cdk2 regulation of E2F1. Consequently, the effect of cyclin D1/cdk4 on E2F4-Ha/DP1 DNA binding was assessed using nuclear extracts from transfected cells in electromobility shift assays (EMSA).
C33A cells were transfected with plasmids expressing E2F4-Ha and DP1 and nuclear extracts from the transfected cells were incubated with a 32P-labeled oligonucleotide probe containing a consensus E2F-binding site (Fig. 4A). A protein–DNA complex consistent with the formation of an E2F4-Ha/DP1 complex was apparent using extracts from the transfected cells but not when extracts from untransfected cells were used (Fig. 4A, lane 1 compared with lane 7). At the exposure level shown, the endogenous E2F species did not make a significant contribution to the banding pattern. The specificity of the E2F complex was confirmed by the addition of unlabeled E2F probe as a competitor resulting in a diminishment of the signal. In contrast, the addition of an unlabeled probe containing a mutated E2F site did not affect E2F complex formation (Fig. 4A, lane 2 compared with lane 3). The presence of E2F4 as a component of the DNA-binding complex was verified by disrupting the protein–DNA complex with an anti-E2F4 antibody, whereas, the addition of a non-specific antibody had no effect (Fig. 4A, lanes 4,6). These results confirm that the E2F4 and DP1 proteins expressed from the transfected plasmids form a DNA–protein complex that is readily detectable in nuclear extracts.
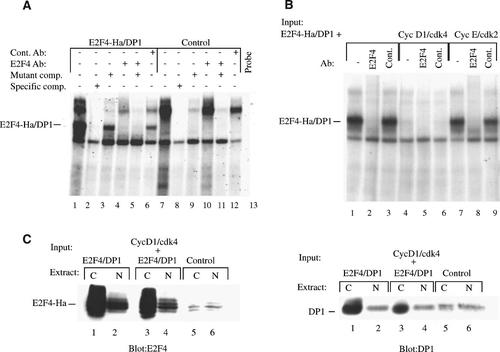
Cyclin D1/cdk4 disrupts the DNA-binding capacity of E2F4/DP1. A: Extracts from transfected (lanes 1–6) and untransfected (lanes 7–12) C33A cells were examined for the presence of E2F DNA-binding activity by electrophoretic mobility shift assays. Extracts were incubated with 32P-labeled oligonucleotide containing an E2F site then run on a 6% non-denaturing polyacrylamide gel and exposed to autoradiography. Unlabeled E2F oligonucleotide (75 ng) was added to the incubation mixture as a specific competitor (lanes 2,8) and an oligonucleotide with a mutated E2F site (75 ng) was used as a non-specific competitor (lanes 3,5,9,11). E2F4 antibody (lanes 4,5,10,11) or rabbit anti-mouse antibody (lanes 6,12) was added to the incubation to determine their effect on the complex formation. B: Nuclear extracts from cells transfected with plasmids expressing E2F4-Ha and DP-1 alone (lanes 1–3), or in combination with cyclin D1 and cdk4 (lanes 4–6), or cyclin E and cdk2 (lanes 7–9) were incubated with an E2F oligonucleotide probe and examined for complex formation as in (A). C: Nuclear and cytoplasmic extracts (N and C, respectively) from cells transfected in (B) were examined by immunoblotting for the presence of E2F4 and DP1. Proteins were separated by SDS–PAGE, transferred to nitrocellulose and probed with an antibody E2F4 (left part) or DP1 (right part).
The effect of cyclin D1/cdk4 on E2F4/DP1 binding was examined by co-transfecting plasmids expressing cyclin D1 and cdk4 in combination with plasmids expressing E2F4 and DP1. In the absence of cyclin D1/cdk4, an E2F4-Ha/DP1 complex was detected in nuclear extracts from cells transfected with E2F4-Ha/DP1 and the presence of E2F4 in the complex was confirmed by disruption of the complex using an E2F4 antibody (Fig. 4B, lanes 1–3). In contrast, the E2F4-Ha/DP1 complex was undetectable in nuclear extracts from cells that were co-transfected with cyclin D1 and cdk4 (Fig. 4B, lanes 4–6). This result suggests that cyclin D1/cdk4 activity disrupts the DNA-binding ability of E2F4/DP1. The effect of cyclin D1/cdk4 was specific for cyclin D1/cdk4 as cyclin E/cdk2 did not affect the E2F4/DNA complex formation (Fig. 4B, lanes 7–9). The effect of cyclin D1/cdk4 on DNA binding also was specific for nuclear E2F4. Cytoplasmic fractions from the same transfected cells contained both E2F4 and DP1 and the cytoplasmic E2F4/DP1 complex retained DNA-binding ability even in the cells co-transfected with cyclin D1 and cdk4 (data not shown). Because E2F4 is regulated, in part, by nuclear translocation, it was possible that cyclin D1/cdk4 affected the subcellular localization of E2F4. However, E2F4 and DP1 were detected by immunoblotting in both the nuclear and cytoplasmic fractions (Fig. 4C). The loss of DNA-binding activity in the extracts from cyclin D1/cdk4-transfected cells did not correspond to changes in either the level or localization of E2F4 or DP1.
Cyclin D1/cdk4 promotes E2F4 phosphorylation
One possible explanation for the preceding result was that cyclin D1/cdk4 targeted E2F4 and/or DP1 for phosphorylation resulting in a change in DNA-binding capacity. E2F4 exists in multiple phosphorylated forms that can be distinguished by mobility in SDS–polyacrylamide gels. The ability of cdk4 to alter the phosphorylation states of E2F4 was examined first by transfection experiments in C33A cells. Co-expression of E2F4 with cdk4 resulted in an increase in relative amount of the more slowly migrating forms of E2F4 suggesting an increase in the stoichiometry of phosphorylation (Fig. 5A, left part). Treatment of immunoprecipitated E2F4 with lambda phophatase resulted in loss of most of the more slowly migrating species indicating that slower migrating forms are generated by phosphorylation (Fig. 6B). For reasons that are not entirely clear, co-expression of cyclin D1 with cdk4 was slightly less efficient in producing the most slowly migrating form of E2F4 in these experiments and the experiments shown relied on co-expression of cdk4 only (data not shown). This effect may be related to the fact that C33A cells express relatively high amounts of INK4a/p16 and levels of p16-free cdk4 may be limiting. As a control for the activity of cyclin D1/cdk4, cells were transfected with plasmids expressing E2F4 and p130 and the effect of cyclin D1/cdk4 on the ability of E2F4 to interact with p130 was examined (Fig. 5A, right part). As expected, the interaction between p130 and E2F4 was easily detected by co-immunoprecipitation; however, when cyclin D1 and cdk4 were co-expressed, the interaction was undetectable and p130 had undergone a mobility shift consistent with phosphorylation. These results indicate that the expressed cyclin D1/cdk4 is catalytically active. The ability of cyclin D1/cdk4 to phosphorylate E2F4 in vitro in complexes immunoprecipitated from lysates of baculovirus-infected SF9 cells also was examined. Cells were infected with baculovirus-expressing E2F4-Ha in the presence or absence of viruses expressing cyclin D1 and cdk4 (Fig. 5B). Lysates of the infected cells were immunoprecipitated for cyclin D1 and subjected to an in vitro kinase reaction using 32P-ATP. Electrophoresis of these reactions revealed several 32P-labeled bands amid considerable background (data not shown). This was probably due to the relatively low in vitro kinase activity of cdk4 (compared to many other protein kinases) requiring longer exposure times. In order to minimize the presence of phosphorylated background proteins and to conclusively identify E2F4, following the in vitro kinase reaction the immunoprecipitates were incubated in SDS release buffer to disrupt protein–protein interactions and the contents were re-immunoprecipitated using the Ha antibody. In the presence of cyclin D1 and cdk4, phosphorylated E2F4-Ha could be detected (Fig. 5B). These results suggest that E2F4 can be phosphorylated by cyclin D1/cdk4. In these and other experiments, phosphorylation of DP1 was not detected suggesting that it is not a target of cyclin D1/cdk4.
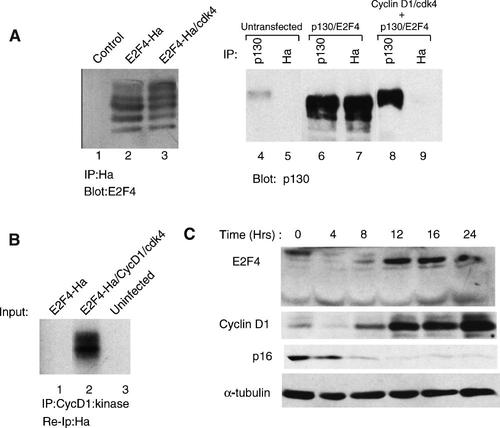
Cdk4 promotes phosphorylation of E2F4 in vitro and in vivo. A: C33A cells, untransfected (lane 1) and transfected with pCMV E2F4-Ha alone (lane 2) and together with pCMV cdk4 (lane 3), were lysed and immunoprecipitated using an antibody to the Ha epitope tag. Proteins were separated on a 7.5% SDS–polyacrylamide gel and then immunoblotted with an antibody to E2F4. In the right part, C33A cells that were untransfected, transfected with plasmids expressing E2F-Ha and p130, and transfected with plasmids expressing E2F4-Ha, p130, cyclin D1, and cdk4 were lysed and immunoprecipitations were conducted using antibodies to Ha and p130. Following separation by SDS–PAGE, the proteins were immunoblotted with an antibody to p130. B: Sf9 cells, uninfected (lane 3) or infected with baculoviruses expressing E2F4-Ha alone (lane 1) or E2F4-Ha, cyclin D1, and cdk4 (lane 2) were lysed and immunoprecipitated with an antibody to cyclin D1. Immunoprecipitates were subjected to an in vitro kinase reaction with 32P ATP. The proteins were dissolved in SDS release buffer and E2F4-Ha was immunoprecipitated with an anti-Ha antibody. Immunoprecipitated proteins were resolved by SDS–PAGE and visualized by autoradiography. C: Mouse 3T3-L1 cells were serum starved for 48 h and then restimulated by adding fresh medium containing 20% fetal bovine serum. At the indicated times, aliquots of cells were lysed and 100 µg of whole cell lysate from each time point was separated by SDS–PAGE, transferred to PVDF, and then probed with the indicated antibodies.
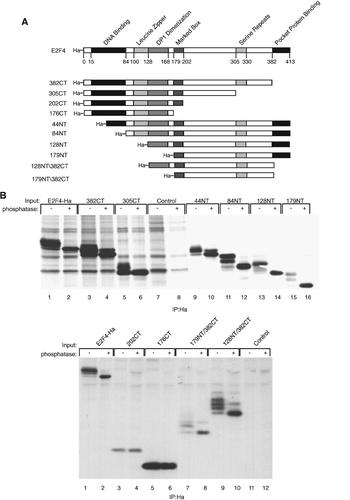
Construction and expression of E2F4 deletion mutants. A: Full length and mutant E2F4 proteins are schematically represented with structural and functional domains highlighted. Numbers refer to amino acid position. Ha is the influenza hemagglutinin tag present at the amino terminus. Mutants are named for the amino acid position at the deletion endpoint. B: Plasmids expressing E2F4-Ha and each of the mutants shown in (A) were transfected into C33A cells and metabolically labeled with 35S-methionine. Proteins were immunoprecipitated from the cell lysates using an antibody to the Ha tag and then incubated with or without phosphatase prior to SDS–PAGE.
To determine whether E2F4 undergoes cell cycle-dependent changes in its phosphorylation pattern, mouse 3T3-L1 cells were serum starved into quiescence and then stimulated with serum to re-enter the cell cycle. Whole cell lysates were prepared at various times after stimulation and immunoblotted for E2F4, cyclin D1, p16 and, as a control, α-tubulin. In the serum-starved cells, the predominant form E2F4 appeared as a slow migrating species suggesting a high level of phosphorylation or some other form of post-translational modification (Fig. 5C). Following serum stimulation, the slowest migrating form of E2F4 was lost and E2F4 became more diffuse before it began to refocus as a species with intermediate mobility. The emergence of the intermediate form of E2F4, starting at 8 h post-stimulation, correlated well with synthesis of cyclin D1 and the loss of p16. The mobility changes in E2F4 are consistent with the notion that cyclin D1/cdk4 is among the kinases that phosphorylate E2F4.
Cyclin D1 interacts with two regions of E2F4
Deletion mutants for E2F4 were generated (shown schematically in Fig. 6A) and used to localize the cyclin D1-binding domain. The E2F4 mutants each encoded an N-terminal Ha epitope to facilitate detection. Expression of truncated E2F4 proteins from each of the deletion mutants was detected following transfection of expression plasmids into C33A cells (Fig. 6B). Treatment of the immunoprecipitated proteins with lambda phosphatase was used to determine which of the multiple bands observed were due to phosphorylation (Fig. 6B). In each case, the phosphatase-treated proteins migrated as one predominant band corresponding to the fastest migrating band of the untreated immunoprecipitated protein. This suggests that the banding pattern was due to largely phosphorylation and not due to breakdown products.
To examine the ability of the mutant E2F4 proteins to interact with cyclin D1, plasmids encoding the N-terminal deletions were transiently transfected with, and without, a plasmid expressing cyclin D1 (Fig. 7A). Immunoprecipitations followed by immunoblotting for cyclin D1 resulted in detection of cyclin D1 in each of the lysates co-expressing the N-terminally truncated mutants suggesting that a region of E2F4 between amino acids 179 and the C-terminus could interact with cyclin D1 (Fig. 7A, lanes 6,8,20,22,24). Each of the C-terminally truncated mutants also was able to bind to cyclin D1 suggesting a binding site within the first 176 amino acids of E2F4 (Fig. 7B, lanes 12,14,16,18). The cyclin D1 interaction with the shortest N-terminal and the shortest C-terminal mutant, 179NT and 176CT, respectively, implies that there are two different binding sites for cyclin D1 on E2F4 (Fig. 7A, lane 22 and Fig. 7B, lane 18). A mutant producing an E2F4 protein truncated at both ends 128NT/382CT was able to bind to cyclin D1 whereas 179NT/382CT failed to interact (Fig. 7C, lane 12 compared with lane 14). These results suggest that a region identical to or overlapping with the DP1 dimerization domain and a region near the C-terminus of E2F4 each can mediate the interaction with cyclin D1. The interaction of cyclin D1 at the DP1 dimerization domain may occur indirectly through DP1.
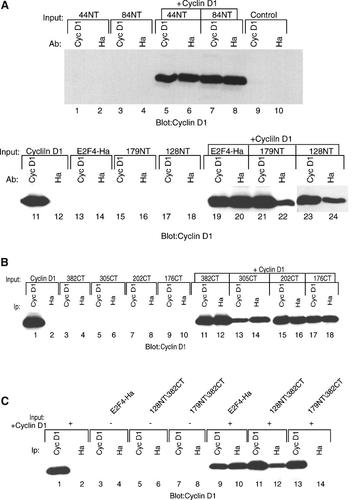
Cyclin D1 interacts with E2F4 through two distinct sites. A: C33A cells were transfected with 5 µg of plasmids expressing E2F4-Ha and each of the N-terminal deletion mutants of E2F4 (described in Fig. 6) with and without 5 µg of a cyclin D1 expressing plasmid. Proteins were immunoprecipitated using the indicated antibodies, separated by SDS–PAGE and immunoblotted with an antibody to cyclin D1. B: C33A cells were transfected as described above except that plasmids expressing C-terminal deletions of E2F4 were used. Proteins were immunoprecipitated as in (A) and immunoblotted using an antibody to cyclin D1. C: C33A cells were transfected with plasmids expressing N-terminal/C-terminal double mutants of E2F4 and cell lysates were immunoprecipitated with antibodies to cyclin D1 and Ha and then immunoblotted with an antibody to cyclin D1 similarly to (A).
The DNA-binding activity of 382CT is unaffected by co-expression of cyclin D1 and cdk4
To determine whether the cyclin D1 interaction with the C-terminal region of E2F4 contributes to the loss of E2F4 DNA-binding potential, the 382CT protein was expressed in C33A cells and examined for its ability to interact with DNA in electrophoretic mobility shift assays. Using nuclear extracts from transfected cells, when co-expressed with DP1, both wild-type E2F4 and the 382CT protein were capable of binding to the 32P-labeled E2F oligonucleotide (Fig. 8). The specificity of the DNA complex was confirmed by competing with an unlabelled oligonucleotide but not with an oligonucleotide containing a mutant E2F site. Nuclear extracts from cells transfected with cyclin D1 and cdk4 in combination with wild-type E2F4 and DP1 or 382CT and DP1 revealed that the DNA-binding complex formed by wild-type E2F4/DP1 was disrupted by cyclin D1/cdk4 (Fig. 8, lanes 12–16) while DNA binding by 382CT/DP1 was unaffected by the presence of cyclin D1/cdk4 (Fig. 8, lanes 22–26). The differential effect of cyclin D1/cdk4 on DNA binding by E2F4 and the 382CT indicates that the cyclin D1 interaction site located at the C-terminus of E2F4 is critical for disruption of E2F4 DNA-binding activity by cyclin D1/cdk4. The importance of the N-terminal cyclin D1-binding site of E2F4 could not be examined in these studies because of the requirement for the DP1 dimerization to efficiently bind DNA.
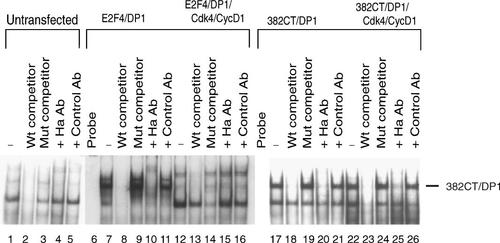
The DNA-binding activity of 382CT/DP1 is not affected by cyclin D1/cdk4. C33A cells were transfected with combinations of expression plasmids for E2F4-Ha/DP1 and 382CT/DP1 alone and in combination with plasmids expressing cyclin D1/cdk4. Nuclear extracts were prepared from the transfected cells and also from untransfected cells. The extracts were incubated with a 32P-labeled E2F oligonucleotide as described in Figure 4. Specific and non-specific competitor oligonucleotides and antibodies (Ab) to the indicated proteins were added to the incubation reaction to probe for the presence of specific proteins as described for Figure 4. Protein–DNA complexes were resolved on a 5% non-denaturing gel.
Discussion
Activation of cyclin D/cdk4 or cyclin D/cdk6 kinase complexes during mid-G1 is thought to be critical for the transition of quiescent cells into the cycling state but targets of the cyclin D/cdk enzymes have remained elusive. The role of the D-type cyclins in targeting pRb, p107, and p130 for phosphorylation has been well studied (Sherr and Roberts, 2004; Cobrinik, 2005). Phosphorylation of the pRb family of proteins is thought to disrupt their interactions with the E2F transcription factors, although some studies have suggested that cdk-mediated phosphorylation of the RB family also can positively influence E2F interactions (Ezhevsky et al., 2001; Calbo et al., 2002). The D cyclins directly target several other transcription factors thereby altering their transcriptional activities through both cdk-dependent and cdk-independent mechanisms (Neuman and Ladha, 1997; Ganter et al., 1998; Inoue and Sherr, 1998; Zwijsen et al., 1998; Knudsen, 1999; Voit et al., 1999; Bienvenue et al., 2001; Lin and Gelman, 2002; Lamb et al., 2003; Matsuura et al., 2004). The results of our study identify E2F4, E2F5, and DP1 as additional potential targets of the D cyclins. The interaction of cyclin D1/cdk4 with E2F4 potentially could disrupt the DNA-binding capacity of E2F4/DP1 in a cdk-dependent manner. This interaction may function to terminate DNA binding by E2F4 at the time of cyclin D1/cdk4 activation in mid G1.
Presently, the individual roles of the E2F and DP proteins in the differential usage of E2F sites are not entirely understood but growing evidence supports a model in which different E2F proteins can fulfill different roles in gene regulation. Evidence from a number of studies supports a role for E2F4 in G0 and early G1 (Attwooll et al., 2004; Dimova and Dyson, 2005). E2F4 is the predominant E2F family member in G0 and, with DP1, it forms complexes preferentially with p130 (Cobrinik et al., 1993; Ginsberg et al., 1994; Moberg et al., 1996; Smith et al., 1996; Lindeman et al., 1997; Muller et al., 1997; Verona et al., 1997). The appearance of cyclin D/cdk4 activity during progression through G1 is thought to result in disruption of interactions between E2F4 and p130 due to phosphorylation of p130 (Johnson, 1995; Mayol et al., 1995; Dong et al., 1998; Hansen et al., 2001; Calbo et al., 2002; Farkas et al., 2002). Our results demonstrating the ability of E2F4 to disrupt the DNA-binding ability by cyclin D1/cdk4 suggest that E2F4 is cleared from promoters at the same time that the RB-related proteins are phosphorylated by cyclin D/cdk4. This model is consistent with the loss of E2F4 interactions with specific E2F sites during mid-G1 in the promoters of several E2F-regulated genes (Zwicker et al., 1996; Tommasi and Pfeifer, 1997; LeCam et al., 1999; Takahashi et al., 2000). In some cases, mutation of these E2F sites results in derepression of the genes during G0 and early G1 without affecting expression at later times in the cell cycle suggesting that a repressor complex is released from the DNA during G1 progression (reviewed in Dyson, 1998; Nevins, 1998). In the absence of a mechanism for releasing E2F4 from the DNA, E2F4 might remain bound to the promoter potentially influencing the pattern of transcription.
Using chromatin precipitation techniques to study promoter occupation in T98G cells, one group observed co-ordinate loss of both p130 and E2F4 from the promoters of several known E2F-regulated genes as cells progressed from quiescence through G1 (Takahashi et al., 2000). Somewhat different observations were made in a similar study using NIH 3T3 cells in which E2F4 was detected bound to promoters during both G1 and S phase for the promoters examined (Wells et al., 2000). Thus, E2F4 binding to promoter sites may be influenced through additional mechanisms in a promoter-specific and/or cell-type-specific manner. Examination of E2F4 promoter occupation during the G1 stage of continuously cycling T98G cells indicated that distinct classes of genes are regulated by p130/E2F4 and p107/E2F4, as well as, by E2F4 in a manner that appeared to be independent of the RB-related proteins (Balciunaite et al., 2005). Association of E2F4 with p107 and pRb following serum or mitogen stimulation of quiescent cells also has been reported, although gene targets were not identified in these studies (Moberg et al., 1996; Calbo et al., 2002). The mechanisms that distinguish E2F4 association of p130 and p107 or that allow it to function independently of these proteins have not yet been determined. Our results indicate that E2F4 is phosphorylated at multiple sites and that the pattern of E2F4 species changes as cell enters into the cell cycle. Interestingly, the slowest migrating form of E2F4 appears in serum-starved cells when E2F4 is associated with p130 and bound to chromatin. The extent to which post-translational modification of E2F4 regulates differential association with p130, RB, and p107 as well as binding to DNA will need to be addressed in future studies.
Many E2F-regulated promoters contain more than one E2F site and it is unlikely that all E2F sites function identically. For a given E2F site, there are different possible outcomes to the elimination of E2F4 by cyclin D/cdk4. One possibility is the elimination of E2F4 at the promoter element without replacement by another E2F family member. Evidence supporting this model has come from in vivo footprinting experiments examining E2F sites in the promoters of the cyclin E, B-myb, and cdc2 genes (Tommasi and Pfeifer, 1995; Zwicker et al., 1996; LeCam et al., 1999). E2F sites within the cyclin E, B-myb, and cdc2 promoters are occupied during G0 and early in G1 but remain unoccupied at later stages of the cell cycle when the genes are transcriptionally active. A p130/E2F4 complex is thought to occupy E2F sites in these promoters and repress transcription during G0 and G1. Elimination of E2F4 DNA binding at these promoters in mid-G1 is consistent with the timing of cyclin D/cdk4 activity. Studies on the human E2F1 promoter indicated that the E2F promoter site is derepressed by cyclin D1/cdk4 activity (Johnson et al., 1994; Johnson, 1995; Smith et al., 1996; Araki et al., 2003). In these studies derepression correlated with the removal of a G0 E2F4/p130 complex. Mutation of the distal E2F-binding site within the promoter sequences relieved negative control of the E2F1 promoter in early G1 and a mutant E2F protein that was incapable of transactivation competed for the E2F site leading to gene activation. These results imply that mid-G1 derepression of the E2F1 promoter occurs when the distal E2F DNA site is no longer bound.
A second possible outcome of activated cyclin D1/cdk4 is the substitution of E2F4 by other E2Fs during early G1. This model is supported by studies indicating that temporal control of the same promoter site by different E2Fs may occur during cell cycle progression (Ohtani et al., 1995, 1996; Karlseder et al., 1996; Sears et al., 1997; Hiyama et al., 1998; Watanabe et al., 1998). For example, an E2F-binding site is present in the murine thymidine kinase promoter and in vivo footprinting detected the presence of constitutively bound E2F activity (Karlseder et al., 1996; Tommasi and Pfeifer, 1997). As the predominant member during the early stage of the cell cycle, E2F4 is thought to occupy this promoter site when gene expression is repressed. However, transcriptional activation of the thymidine kinase gene from this promoter site requires SP1 to act in synergy with E2F1 not E2F4. These experiments suggest that E2F4 must be replaced by E2F1 before activation of expression can take place. A more direct demonstration of E2F switching in a cell cycle-dependent manner used chromatin immunoprecipitation with antibodies to different E2F proteins (Takahashi et al., 2000). This study was able to detect specific E2F proteins bound to the promoters of selected genes although many of the promoters examined contained more than one E2F site. Remarkably, p130 and E2F4 were bound to each of the promoters examined during G0 and G1 but both were undetectable after mid-G1. One or more of E2F1, E2F2, and E2F3 were detected bound to the same promoter region at the time that E2F4 binding was lost. Thus, elimination of E2F4 binding may be required for loading of E2F1, E2F2, or E2F3. This model raises the possibility that the p130/E2F4 species not only represses transcription but also prevents the binding of E2F1, E2F2, or E2F3, which presumably are involved in activation of transcription.
Acknowledgements
We thank Drs. Ali Fattaey, Kristian Helin, Ed Harlow, John Hassell, Jim Roberts, Charles Sherr, and Robert Weinberg for providing reagents. We thank Drs. John Capone and Michael Rudnicki as well as members of the Whyte lab for their helpful discussions. This work was supported by a grant from the National Cancer Institute of Canada with funds made available by the Canadian Cancer Society.




