The Helicobacter pylori's protein VacA has direct effects on the regulation of cell cycle and apoptosis in gastric epithelial cells
Abstract
In this study, we have evaluated the effects on cell cycle regulation of VacA alone and in combination with other two Helicobacter pylori proteins, cytotoxin-associated protein (CagA) and HspB, using the human gastric epithelial cells (AGS). Our results indicate that VacA alone was able to inhibit the G1 to S progression of the cell cycle. The VacA capacity of inhibiting cell progression from G1 to S phase was also observed when cells were co-transfected with CagA or HspB. Moreover, VacA over-expression caused apoptosis in AGS cells through activation of caspase 8 and even more of caspase 9, thus indicating an involvement of both the receptor-mediated and the mitochondrial pathways of apoptosis. Indeed, the two pathways probably can co-operate to execute cell death with a prevalence of the mitochondrial pathways. Our data taken together provide additional information to further enhance our understanding of the molecular mechanism by which H. pylori proteins alter the growth status of human gastric epithelial cells. J. Cell. Physiol. 214: 582–587, 2008. © 2007 Wiley-Liss, Inc.
One of the most important breakthroughs in gastroenterology in the latter part of this century was the finding that a bacterial infection causes ulcer diseases (Graham, 1994).
At the end of 1983, several groups almost simultaneously reported the presence of spiral bacteria in patients with chronic gastritis and peptic ulceration. Now it is recognized and accepted that Helicobacter pylori infection is the most frequent cause of gastritis around the world. Its infection affects millions of people in the world, with peaks of 90% of the population infected in countries with poor sanitary conditions and a low socio-economical level (Montecucco et al., 1999; De Luca et al., 2004).
The association between H. pylori infection and peptic ulcer disease has been well established, and although most infected individuals only develop a chronic inflammation of the stomach, some patients progress to chronic gastritis, duodenal ulceration, or gastric cancer (Nomura et al., 1991; Blaser, 1993; Gerhard et al., 2002; De Luca et al., 2004).
The mechanism by which H. pylori causes disease in humans can be described as a multi-step process in which the organism first has to pass the gastric acid barrier and enter the mucous layer (colonization) and then adapt and multiply under the environmental conditions of the gastric mucus (persistence). All these events are due to the ability of the bacterium to produce a number of protein products including urease, cytotoxin, flagellin, VacA, cytotoxin-associated protein (CagA), heat shock proteins, and adherence factors that contribute to its ability to colonize, avoid host defenses, and inflict damage to the host (Rieder et al., 2005).
However, it has been demonstrated that only some H. pylori strains causes neoplasia, and enhanced cancer risk may be considered as the summation of the polymorphic nature of the bacterial population in the host, the host genotype, and environmental exposures (Peek and Blaser, 2002; De Luca et al., 2004). It has been demonstrated that approximately 50% of H. pylori strains express the vacuolating cytotoxin protein VacA (Montecucco et al., 2001), a secreted exotoxin encoded by the vacA gene (De Luca and Iaquinto, 2004; De Luca et al., 2004). This toxin inserts itself into the epithelial cell membrane allowing bicarbonate and organic anions release and facilitating the formation of transmembrane pores which permeabilize the gastric epithelium to urea (Szabo et al., 1999; Tombola et al., 1999a; Jungblut et al., 2000) and probably providing the bacterium with nutrients (De Luca et al., 2004). The vacA gene was cloned by several groups using degenerate oligonucleotides that were designed based on partial amino acid sequence data. DNA sequence analyses identified a single open reading frame that corresponds to a protein of 140 kDa in size, which is processed to yield a mature 87 kDa protein. The structure has similarity with several secreted bacterial serine protease suggesting that VacA can have in the cells a proteolytic activity. The active VacA form is able to interact with several networks of family proteins, such as G proteins, receptor-like protein tyrosine phosphatase (Yahiro et al., 2004), and fibronectin (Hennig et al., 2005). H. pylori VacA-secreting strains are more common among patients with distal gastric cancer than among patients with gastritis alone (Miehlke et al., 2000; De Luca et al., 2004).
H. pylori strains can be divided into two classes, type I and type II, based on the presence or the absence of the cag pathogenicity island (PAI), a 40-kb region that encodes over 40 putative bacterial proteins (Censini et al., 1996; Alm and Trust, 1999; Tombola et al., 1999b). The terminal gene in the island is the cagA encoding CagA. Several studies have demonstrated that infection with cagA-positive H. pylori strains significantly increases the risk of developing severe gastritis, atrophic gastritis, peptic ulcer disease, and distal gastric cancer (Cover et al., 1991; Blaser, 1995; Kuipers et al., 1995; Peek et al., 1995; Parsonnet et al., 1997; De Luca et al., 2003, 2004). The explanation is that the genes present in the pathogenicity island require CagA to be able to induce the expression of cytokines, especially IL-8, by epithelial gastric cells (Yuan et al., 2004). In addition, it has also been shown that H. pylori strains expressing CagA protein induce a proto-oncogene activation that may represent an important step in the mechanism of H. pylori-induced neoplasia (Higashi et al., 2002). Moreover, it has been recently demonstrated that CagA protein when translocates from the bacteria to the gastric cells is able to form a physical complex with SHP-2 tyrosine phosphatase, inducing a growth factor-like response in gastric epithelial cells (Higashi et al., 2002).
A few years ago, another protein HspB (Dunn et al., 1992; Macchia et al., 1993) was correlated to a major risk to develop a gastric carcinoma (Iaquinto et al., 2000; De Luca et al., 2003). Several strains of H. pylori produce this 58 kDa protein that has been cloned, characterized, and named upon its homology with the family of the heat shock proteins: HspB (Macchia et al., 1993; Dunn et al., 1997). This protein is secreted by the bacterium and it has been shown on the mucosa surface and within epithelial cells (Dunn et al., 1997; Engstrand et al., 1997; Cao et al., 1998; Kamiya et al., 1998). HspB is able to stimulate a strong immune response in patients with gastritis and to increase the risk of gastric carcinoma in infected patients (Pérez-Pérez et al., 1996; Iaquinto et al., 2000).
Drawing from this background, to better investigate the mechanisms and relationships between H. pylori's proteins and their effects on gastric epithelial cells, we examined the influence of three H. pylori's proteins (VacA, CagA, and HspB) on cell kinetics of human gastric epithelial (AGS) cell lines. Taken together, our data provide additional information to further enhance our understanding on the molecular mechanisms by which H. pylori proteins are able to alter the growth status of the host cell.
Materials and Methods
Cell culture and transfection
AGS human gastric epithelial cells (American Type Culture Collection, Manassas, VA) were grown in Ham's F-12 medium supplemented with 10% FBS and 50 µg/ml penicillin-streptomycin in an atmosphere of 5% CO2 at 37°C. Plasmid expression construct for VacA was prepared as previously described in our article (De Luca et al., 2003). AGS cells were transiently transfected with mammalian expression vector for CagA, HspB, and VacA using Lipofectamine (Invitrogen, Carlsbad, CA) as previously described in our article (De Luca et al., 2003) and according to the manufacturer's instruction. Briefly, the cells were plated in 60 mm dishes (1 × 106 cells/dish) 24 h before transfection and the same amount of total DNA was used. We typically transfected between 50 and 70% of cells as judged using a control pcDNA3 vector expressing EGFP protein. In the experiments, we used synchronized cells by withdrawing the serum 24 h before the transfection and tested with flow cytometry to prove the synchronization. Cells were collected at 48 and 72 h after transfections and were prepared for flow cytometry analysis or prepared for protein extracts.
Flow cytometry for detection of cell cycle and apoptosis
Unsynchronized and synchronized cells after 48 and 72 h from the transfection were collected by mild trypsination, washed in PBS, and fixed in 70% ethanol at 4°C. Cells were collected and re-suspended in 500 µl of a hypotonic buffer (0.1%TritonX-100, 0.1% sodium citrate, 50 µg/ml propidium iodide, and 100 µg/ml RNase). Then the cells were analyzed using a Becton Dickinson FACSCalibur flow cytometer and the percentages of G1, S, G2/M, and sub-G1 (apoptotic cells) populations were calculated, respectively. All of the experiments were performed three times. Cells used for caspase activity assay (Altucci et al., 2005) were collected 72 h after transfection and the pellets were lysed in 50 ul of ice-cold lysis buffer. After incubation for 10 min in ice, samples were centrifuged for 1 min at 10,000g. After measuring protein levels (Bio-Rad, Hercules, CA), the activities of the apical caspases 8 and 9 and effector caspase 3 were determined using colorimetric assays according to the supplier's instructions (R&D; Alexis, San Diego, CA). All the experiments were performed three times.
Immunoblotting
Plates of 70–80% confluent AGS cells were transiently transfected with the plasmids by the Lipofectamine protocol. After 72 h, the cells were collected. Briefly, the cells were lysed in 100 ul lysis buffer (50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 250 mM NaCl, 50 mM NaF, 0.1% Triton X-100, 0.1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and 10 ug/ml leupeptin) for 30 min in ice. Lysates were centrifuged at 14,000g for 10 min at 4°C. Total cell protein extracts were normalized for concentration by the Bradford assay (Bio-Rad) and 30 ug of proteins were separated by SDS–PAGE and transferred to polyvinylidene difluoride membrane (Millipore's Corporate, Billerica, MA). Membranes were incubated with rabbit polyclonal cleaved caspase 3 (Cell Signaling Technology, Danvers, MA) and with rabbit polyclonal antibodies against Rb/XZ55 able to recognize the p68/Rb fragment (Ping Dou and Bing, 1998). Primary antibodies were detected using anti-rabbit horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Inc., Piscataway, NJ) and visualized by the ECL detection system (Amersham Biosciences, Inc.) according to the manufacturer's instructions. Each membrane was probed with the Hsp70 antibody (heat shock proteins) (Santa-Cruz, Biotechnology, Santa Cruz, CA) to estimate equal protein loading. The expression levels of the protein transfected were detected using the monoclonal antibody against TAG (data not shown) (Novagen, Darmstadt, Germany) as previously described (De Luca et al., 2003).
Results
H. pylori protein VacA induced growth arrest both alone and in combination with CagA and HspB
In a previous work, we have demonstrated that efficient co-expression of CagA and HspB is able to influence human gastric epithelial (AGS) cell growth by inducing cell proliferation through an increase in the S-G2-M phase of the cell cycle (De Luca et al., 2003). In order to better investigate the interplay among the three pathogenic proteins of H. pylori, we have performed a co-transfection experiments using VacA in several combination with CagA and HspB in AGS cells. Vector alone was used as negative control. Equal amount of exogenous proteins were transfected, as determined by immunoblotting for TAG (data not shown).
The cells were collected 48 h after transfection and analyzed by flow cytometry (Fig. 1). We showed that the presence of VacA together with HspB or CagA was able to suppress the cell proliferation observed by co-transfection with HspB and GagA (as showed previously), increasing the percentage of cells in G0/G1 phase of the cell cycle and decreasing the percentage of cells in G2 phase.
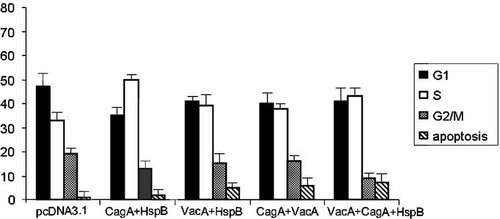
FACS analysis of AGS cells transfected with empty vector, VacA, CagA, and HspB, alone and in different combination among them and collected at 48 h.
At the same time, apart from inhibiting the cell growth, the presence of VacA was also able to induce an increase in the percentage of apoptotic cells. This effect was more pronounced when the three H. pylori's proteins were simultaneously in the same cell (Fig. 1).
VacA over-expression induced an increase of apoptotic AGS cells
In order to better clarify the potential involvement of VacA cell cycle regulation and apoptosis triggering, AGS cells were transiently transfected with VacA and vector alone (as negative control), and collected at 48 and 72 h. To mimic the in vivo physiological condition of gastric mucosa, we used unsynchronized and synchronized cells as described in the Materials and Methods section.
Cell cycle analysis revealed that VacA induced inhibition of cell growth and time-dependent induction of apoptosis (Fig. 2). In particular, we showed that VacA over-expression caused a decrease in the G2/M phase in both unsynchronized and synchronized AGS cells. Moreover, we observed that transient transfection of VacA increased the percentage of apoptotic cells with a strong different result among unsynchronized and synchronized cells. Specifically, at 48 h after transfection the percentage of apoptotic cells in synchronized cells transfected with VacA increased about 12-folds compared to the control, whereas in the unsynchronized cells the increase of apoptotic cells was of fivefolds compared to the control. At 72 h after transfection, the percentage of apoptosis in the synchronized cells remained constant, while in the unsynchronized cells the apoptotic rate increased 20-folds compared to the sample at 48 h (Fig. 2).
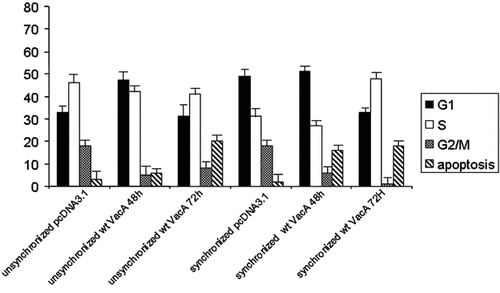
FACS analysis of unsynchronized and synchronized AGS cells transfected with the empty vector and VacA. The cells were collected at 48 and 72 h.
VacA transfection in AGS cells induces caspase activation
In order to deeply investigate the apoptotic pathways activated by VacA transfection, we monitored the enzymatic activity of the caspases 8 and 9 and of the effector caspase 3 using flow cytometry. In both synchronized and unsynchronized cells, we showed that, at 72 h, caspases 8 and 9 were activated (Fig. 3). This suggests that both apoptotic pathways are activated in AGS cells in the presence of VacA with a higher activation of caspase 9 representing the mitochondrial pathway compared to caspase 8.
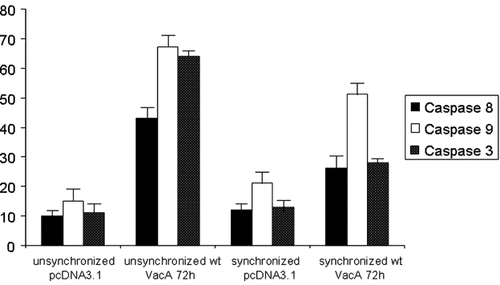
Flow cytometry analysis of unsynchronized and synchronized AGS cells transfected with the empty vector and VacA, by caspases 8, 9, and 3.
Next, we performed immunoblot analysis using caspase 3. Caspase 3 is present primarily as a molecule of approximately 32 kDa, instead the active-cleaved form runs around a 20 kDa. We used an antibody able to recognize the active form of caspase 3. Unsynchronized (lanes 1 and 2) and synchronized cells (lanes 3 and 4) were transfected with empty vector (lanes 1 and 3) and VacA (lanes 2 and 4) and were collected at 72 h. The active form of caspase 3 was observed after VacA transfection both in unsynchronized and synchronized cells but the protein level was high in the unsynchronized cell compared with synchronized cells (lanes 2 and 4 of Fig. 4), accordingly to the flow cytometry data. The membrane was also probed with the Hsp70 antibody to estimate equal protein loading as shown in the upper part of Figure 4.
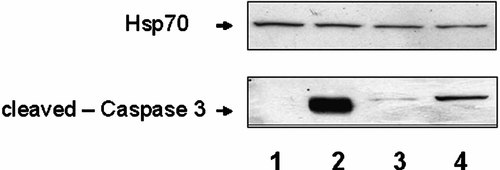
Immunoblotting analysis of unsynchronized and synchronized AGS cells transfected with the empty vector and with VacA. The cells were collected at 72 h. Western blotting was performed using anti-cleaved caspase 3 antibody. Lanes 1 and 2: unsynchronized cells transfected with empty vector and VacA, respectively; lanes 3 and 4: synchronized cells transfected with empty vector and VacA, respectively. Upper part showed immunoblotting assay with Hsp70 antibody to estimate equal protein loading.
VacA transfection in AGS cells induces Rb cleavage during apoptosis
It has been shown that the cleavage of pRb into p68 and p48 fragments, operated through caspase activity, is involved in the apoptotic progression in AGS cells (Ping Dou and Bing, 1998; Jin et al., 2005). Therefore, we decided to examine the degradation of Rb protein in the unsynchronized (lanes 1 and 2) and synchronized AGS cells (lanes 3 and 4) transfected with empty vector (lanes 1 and 3) and VacA (lanes 2 and 4) and collected at 72 h after. We performed immunoblot analysis using Rb antibody XZ55 that reacts with p120/Rb, p115/Rb, and with p68/Rb.
The protein lysates were splitted in two equal parts. One part was loaded on the 6% SDS–PAGE showing the phosphorilated Rb form of 120 and 115 kDa molecular weight; the second part was loaded on the 10% SDS–PAGE showing the cleaved Rb fragment of about 68 kDa molecular weight.
In the unsynchronized cells transfected with VacA, the protein level of the p68/Rb fragment was high (lane 2, low part of Fig. 5) while the p120/Rb and p115/Rb levels decreased (lane 2, upper part of Fig. 5).
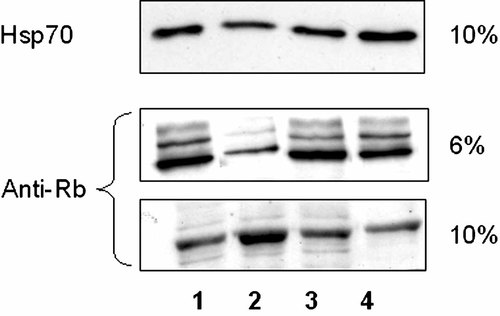
Immunoblotting analysis of unsynchronized and synchronized AGS cells transfected with the empty vector and with VacA. The cells were collected at 72 h. Western blotting was performed using anti-Rb XZ55 antibody. Upper part shows, in a 6% gel, the phosphorilated Rb form and low part shows, in a 10% gel, the cleaved Rb fragment. Lanes 1 and 2: unsynchronized cells transfected with empty vector and VacA, respectively; lanes 3 and 4: synchronized cells transfected with empty vector and VacA, respectively. Upper part showed immunoblotting assay with Hsp70 antibody to estimate equal protein loading.
In the unsynchronized and synchronized control and in the synchronized cells transfected with VacA, the p68/RB, p120/Rb, and p115/RB proteins levels remained unchanged (lanes 1, 3, and 4 of Fig. 5). Finally, the membrane was also probed with the Hsp70 antibody to estimate equal protein loading as shown in the upper part of Figure 5.
Discussion
Balance between proliferation and apoptosis is the essential element in maintaining the integrity of gastric mucosa. Proliferation is tightly controlled in the late G1 phase of the cell cycle through a process that involves cyclins, cyclin-dependent kinase, CDK inhibitors, retinoblastoma, and other regulatory proteins (MacLachlan et al., 1995). The disturbance of this balance could result in either cell loss with mucosal damage and ulcer formation or cell accumulation leading to cancer development. The molecular mechanisms by which H. pylori causes disease in humans remain unclear. It has been proposed that the development of cancer is due to a non-specific accumulation of random mutations mainly caused by the synthesis of reactive oxygen species following the infection (Baik et al., 1996), or to an induction of hyper-gastrinemia caused by the host response to H. pylori infection (Hocker et al., 1997). However, several published data suggest that this bacterium is able to alter the cell cycle regulation of infected gastric cells by hyper-proliferative effects specifically due to some proteins produced by the pathogenic strains of H. pylori (De Luca et al., 2004). Indeed, it has been shown that H. pylori strains expressing the CagA induce a proto-oncogene activation that may represent an important step in the mechanism of H. pylori-induced neoplasia (Meyer-ter-Vehn et al., 2000). Moreover, it has recently been demonstrated that CagA protein secreted in the gastric cells is able to form a physical complex with SHP-2 tyrosine phosphatase, inducing a growth factor-like response in gastric epithelial cells (Higashi et al., 2002). Our research group has recently found that efficient co-expression of CagA and HspB is able to influence AGS cell growth by inducing cell cycle proliferation through an increase in the S/G2-M phase of the cell cycle. The effects of H. pylori protein products do not seem less important on apoptosis. It has been demonstrated that H. pylori-associated chronic gastritis involves apoptotic cell death, and in fact a markedly elevated number of apoptotic cells is identified in surface epithelium, antral pyloric glands, and lamina propria in 83% of biopsies from patients with H. pylori-associated gastritis (Moss et al., 1996). Furthermore, it has been proposed that H. pylori may induce hyper-proliferation through increasing apoptosis (Peek et al., 1997). Drawing from this background, we decided to look at the effects on cell cycle regulation and apoptosis of the over-expression of VacA alone and in combination with the other two H. pylori proteins, CagA and HspB proteins in AGS cells, independently from any pathogenetic mechanism induced by the infection.
First of all, we have shown that VacA over-expression was able, independently by the presence of the other two H. pylori proteins CagA and HspB to induce a stop in G1 of the cell cycle in AGS cells and an increase in the percentage of apoptotic cells. In order to mimic more closely the physiological conditions of the gastric mucosa, we set up two different conditions of cell treatments, synchronized and unsynchronized cells. In fact, the gastric mucosa is formed by cells (human gastric epithelial cells that are proliferating) that have a mitotic turnover, localized in the isthmus and neck of the gastric glands (Penta et al., 2005). These epithelial cells first proliferating and then migrating upward from these regions, replace superficial cells (human gastric epithelial cells that are not proliferating) that are exfoliated into the lumen after a superficial injury. Our results suggest that the epithelial cells of the glands localized in the bottom of the stomach were more sensitive to the damage from H. pylori than the cells localized in the outer layers of gastric mucosa. Specifically, we showed that the percentage of apoptotic cells was different in synchronized and unsynchronized cells, with the percentage of apoptotic cells higher in the unsynchronized cells. Interestingly, this difference was time dependent. Indeed, serum starvation enhanced the sensitivity of gastric epithelial cells so increasing the ability of VacA to induce apoptosis only for a limited time. Initially, the synchronized cells were more sensitive to apoptosis induced by VacA but then they acquired resistance. Intriguingly, on the unsynchronized cells, the VacA effect was slower.
A central mechanism in the apoptotic cell death process is the activation of caspases, a family of cysteine proteases. There are two apoptotic pathways: the receptor-mediated pathway, formed by Fas ligand (FasL), Fas and caspase 8, the mitochondrial pathway, mediated by the Bcl-2 family, Apaf-1 and caspase 9. Activation of caspase 8 occurs as a consequence of surface receptor binding to its ligand (Ashkenazi and Dixit, 1998). Our results on synchronized and unsynchronized cells show that caspase 8 was indeed activated but the major pathway was independent of caspase 8 activation and that VacA is capable of inducing apoptosis mainly through the activation of caspase 9, that is, through the mitochondrial pathway.
However, we also have to mention that outer membrane vesicles are constantly shed by the bacteria and can provide an additional mechanism for pathogenicity by releasing non-secretable factors which can then interact with epithelial cells. Ayala et al. showed that external membrane vesicles are able to induce apoptosis not mediated by mitochondrial pathway in gastric (AGS) epithelial cells, as demonstrated by the lack of cytochrome c release with an activation of caspases 8 and 3. Apoptosis induced by these vesicles does not require a classic VacA+ phenotype, as a negative strain with a truncated and therefore non-secretable form of this protein can also induce cell death (Ayala et al., 2006).
Finally, we have to remember that the deregulation of cell cycle progression is essential for the initiation of apoptosis. Retinoblastoma protein, Rb, is an important tumor suppressor and a cell cycle regulator. Different studies suggest that Rb also plays a regulatory role in the process of apoptosis; the hyper-phosphorylated form of Rb (p120Rb) is converted to a hypo-phosphorylated form (p115Rb) and this new form is immediately cleaved by a protease that has properties of the caspase family (Ping Dou and Bing, 1998). Hypophosphorylated form is cleaved into p68 and p48 fragments and the interior cleavage of Rb is tightly associated with the initiation of apoptotic execution. Indeed, our experimental results demonstrate that there was an increase in the processing of pRB in the wt-VacA unsynchronized cells with respect to the control, thus suggesting an involvement of pRb cleavage by caspase in the regulation of the apoptotic process.
In conclusion, we report here that the deregulation of epithelial proliferation and apoptosis observed in chronic H. pylori infection can be specifically stimulated in AGS cells by some of the bacterial protein products. Our experimental strategy based on the transfection of these proteins, indeed, eliminated all the insults to the cells eventually caused by the infection of the culture with H. pylori. To the best of our knowledge, this is the first report showing a specific apoptotic effect on gastric epithelial cells caused by the action of the VacA bacterial protein, independently from any effect due to the mechanism of infection.
Finally, some limitations of this study should be acknowledged. First of all, this experimental system looks only at the effects of over-expression of some bacterial proteins in the gastric cells. Therefore, it ignores possible effects of VacA, CagA, or HspB on the other elements present in the stomach, such as the fibroblasts of the gastric lamina propria, that might also be targets of their action. Secondly, the possibility cannot be excluded that with this transfection model, intracellular levels of bacterial proteins are higher than those actually seen in H. pylori infection. Then, the functional effects analyzed can only be the consequence of an artificial situation that is not present in the gastric cells of the stomach infected with H. pylori. Finally, AGS cells are derived from a tumor and this might partially affect the results, since these cells are already transformed. Moreover, another limitation is the Caucasian origin of the AGS cells, which may explain the molecular mechanisms by which H. pylori proteins are implicated in this tumor only in the Western world. Further experiment should be performed using also cell lines KATO-III, YCC from Asia where gastric cancer is more frequent.
Nevertheless, the major contribution of this work, that is, the demonstration of a direct effect of the H. pylori protein VacA on the cell cycle regulation and apoptosis of a gastric carcinoma cell line, is not weakened by these limitations.
In conclusion, this study further contributes to the elucidation of the molecular mechanisms involved in the effects of H. pylori on cell cycle control and provides insights into the roles played by the organism's proteins in gastric carcinogenesis. Nevertheless, it indicates possible molecular targets of diagnosis and therapy for this kind of neoplasm.
Acknowledgements
We thank Dr. Pia Furno for editing the article. This work was supported in part by grants from Second University of Naples and AIRC (A.D.L.), by Ministero della Salute (G.C.), and by FUTURA-onlus (A.B.). L.M is supported by “Dottorato in Microbiologia ambientale ed ecosistema cutaneo.” We thank the I.S.S.C.O. (president H.E. Kaiser) for the continuous support.




