Osterix is a key target for mechanical signals in human thoracic ligament flavum cells
Abstract
Mechanical stress is considered to be an important factor in the progression of thoracic ossification of the ligament flavum (TOLF). To elucidate the mechanism underlying mechanical stress-induced TOLF, we investigated the effect of stretching on cultured flavum ligament cells derived from TOLF and non-TOLF patients. We found that the mRNA expression of alkaline phosphatase (ALP), osteocalcin, Runx2, and osterix, but not that of Dlx5 and Msx2, was significantly increased by stretching in TOLF cells. In addition, the effect seems to be finely tuned by stretching-triggered activation of distinct mitogen-activated protein kinase cascades. Specifically, a p38 specific inhibitor, SB203580, significantly inhibited stretching-induced osterix expression as well as ALP activity, whereas a specific inhibitor of ERK1/2, U0126, prevented stretching-induced Runx2 expression. We showed that overexpression of osterix resulted in a significant increase of ALP activity in TOLF cells, and osterix-specific RNAi completely abrogated the stretching-induced ALP activity, indicating that osterix plays a key role in stretching-stimulated osteogenic effect in TOLF cells. These results suggest that mechanical stress plays important roles in the progression of TOLF through induction of osteogenic differentiation of TOLF cells, and our findings support that osterix functions as a molecular link between mechanostressing and osteogenic differentiation. J. Cell. Physiol. 211: 577–584, 2007. © 2007 Wiley-Liss, Inc.
Thoracic ossification of ligamentum flavum (TOLF) of the spine is characterized by a heterotopic bone formation in the thoracic flavum ligament that is normally composed of fibrous tissues. In some cases ossification enlarges in the spinal canal and compresses the spinal cord, resulting in severe neurological damage (Ben Hamouda et al., 2003). Previous studies have suggested factors such as genetic background, dietary habits, and production of cytokines or growth factors in the thoracic ligamentum flavum as causations of TOLF, but the etiology of this disease is still largely unknown (al-Orainy and Kolawole, 1998; Pascal-Moussellard et al., 2005).
Clinically, mechanical stress is believed to play an important role in the progression of TOLF (Shiokawa et al., 2001; Vasudevan and Knuckey, 2002; Li et al., 2006). It has been reported that OLF occurred most frequently in the thoracolumbar junction, and the size of ossification depended on the orientation of the zygapophyseal joints (Maigne et al., 1992); the ossification in T1 and T2 is typically small in size, and largest size of ossification is usually observed in T11 and T12. Evidence has also accumulated to suggest the important function of Dlx5, Msx2, osterix, and Runx2 during cell osteogenic differentiation. It is believed that these transcription factors exert their osteogenic activity through regulating the expression of osteoblast markers such as ALP, osteopontin, and osteocalcin (Morsczeck, 2006). It has also been documented that signaling through mitogen-activated protein kinases (MAPKs) are not only essential for the early stages of osteoblast differentiation, but also involved in the mechanostressing signal pathway (Kletsas et al., 2002; Jadlowiec et al., 2004). Whether transcription factors response to mechanical stress stimulation remains an interesting question, and elucidation of the specific transcriptional factors that operate in mechanotranscription circuitry will contribute to a better understanding of mechanical stress-induced TOLF progression which may ultimately set the basis for pharmacological intervention of TOLF patients.
In current study, we hypothesized that mechanical stress may transmit signals to induce osteogenic differentiation of thoracic ligament cells in TOLF through induction of the expression of osteogenic transcriptional factors. With biaxial stretching as a mechanical stimulation, we investigated the mRNA expression of ALP, osteocalcin, Dlx5, Msx2, osterix, and Runx2 and ALP activity. Both gain-of-function and loss-of-function experiments demonstrated that osterix mediated the biochemical effect of mechanical stress on the osteogenic differentiation of TOLF cells.
Materials and Methods
Clinical diagnosis and spinal ligament samples
The diagnosis of TOLF or non-TOLF (i.e., other thoracic diseases) was confirmed by X-ray, computerized tomography, and magnetic resonance imaging of the whole spine preoperatively to avoid any other non-thoracic OLF patients. The clinical diagnoses and the spinal ligament tissues used in this study are shown in Table 1. Ligaments were aseptically harvested from patients during surgery and rinsed with PBS. Surrounding tissue was carefully removed under a dissecting microscope. In all cases, the ligaments were extirpated carefully from non-ossified sites to avoid any possible contamination of osteogenic cells. This study was approved by the Ethics Committee of Peking University Health Science Center.
| Code | Diagnosis | Sex/age | Tissue |
|---|---|---|---|
| TOLF | |||
| O-1 | TOLF | M/51 | TLF |
| O-2 | TOLF | M/52 | TLF |
| O-3 | TOLF | M/60 | TLF |
| O-4 | TOLF | M/68 | TLF |
| O-5 | TOLF | M/69 | TLF |
| O-6 | TOLF | F/48 | TLF |
| O-7 | TOLF | F/53 | TLF |
| O-8 | TOLF | F/64 | TLF |
| O-9 | TOLF | F/65 | TLF |
| O-10 | TOLF | F/66 | TLF |
| O-11 | TOLF | F/60 | TLF |
| Non-TOLF | |||
| N-1 | TT | M/46 | TLF |
| N-2 | ST | M/48 | TLF |
| N-3 | TT | M/47 | TLF |
| N-4 | TT | M/56 | TLF |
| N-5 | TSS | M/57 | TLF |
| N-6 | TT | F/43 | TLF |
| N-7 | TT | F/57 | TLF |
| N-8 | TT | F/65 | TLF |
| N-9 | TF | F/66 | TLF |
| N-10 | TT | F/73 | TLF |
| N-11 | TT | F/32 | TLF |
- TOLF, thoracic ossification of the ligamentum flavum; TLF, thoracic ligamentum flavum; TT, thoracic trauma; ST, spinal tuberculosis; TSS, thoracolumber spinal stenosis; TF, thoracic fibroneuroma; M, male; F, female.
Antibodies and reagents
Polyclonal anti-osterix, polyclonal anti-ERK1/2, polyclonal anti-p38MAPK, polyclonal anti-JNK, and monoclonal anti-phosphor-ERK1/2, anti-phosphor-p38MAPK, and anti-phosphor-JNK were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). SB203580 were obtained from Promega and U0126 were from Calbiochem (La Jolla, CA).
Cell cultures
Collected ligaments were minced into about 0.5 mm3 pieces and washed twice with PBS, then plated in 6 cm culture dishes, and maintained in DMEM (10% FBS, 1% L-glutamine, 100 units/ml of penicillin G sodium, 100 µg of streptomycin sulfate) in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The cells derived from explants were removed from the dish with 0.02% EDTA/0.05% trypsin for passage (Specchia et al., 2001).
Mineralization assay
Cells from TOLF patients (TOLF cells) and non-TOLF patients (non-TOLF cells) were plated at 500,000 cells/chamber and maintained in DMEM supplemented with 10% FBS. Upon 95% confluence, designated Day 0, cells were then exposed to medium containing DMEM supplemented with 10% FBS, 50 µg/ml of ascorbic acid, and 10 mM β-glycerophosphate. The medium was replaced every 3–4 days. Samples were processed 4 weeks and alizarin red assay (Sigma Chemical, St. Louis, MO) was performed to determine mineralization. Briefly, cells were washed with PBS and fixed with ice-cold 100% ethanol for 60 min at 4°C. Fixed cultures were incubated with 1% alizarin red for 30 min and washed with distilled water for several times. Extracellular matrix mineral-bound stain was photographed under microscopy (Gregory et al., 2004).
Stretch apparatus
Cells (second to fifth passages) were trypsinized and seeded onto a silicone membrane within an equi-biaxial stretch chamber (Zhou et al., 2005) at the concentration of 500,000 cells/chamber. After cultures reached confluence, the medium was replaced with medium containing 1% FBS prior to stretching. Cells were then subjected to 0%, 6 %, 9%, and 12% stretch in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Alkaline phosphatase activity assay
The ALP activity was measured after rinsing the cells twice with ice-cold PBS (pH 7.4) followed by trypsinization then solubilization of cells in Tris/glycine/Triton buffer (pH 8.5, 50 mM Tris, 100 mM glycine, and 0.1% Triton X-100) and sonication with 0.6 sec/35 W pulses on ice. Cellular mixture was centrifuged at 5,000g for 15 min at 4°C, and the supernatant was collected. One hundred milliliter of freshly prepared p-nitrophenyl-phosphate (PNPP) substrate (1.5 mg/ml) was added to 200 µl of the supernatant and the mixture was incubated at 37°C for 30 min. For the generation of a standard curve, serial dilutions of a p-nitrophenol standard solution were prepared and 100 µl of each concentration was included in each tube. The enzymatic reaction was terminated with 0.3 ml of ice-cold 0.1 M NaOH solution. The absorbance was read at 405 nm. Concentrations of protein were determined with the Bradford protein assay with BSA as the standard. Results were expressed as nmoles of p-nitrophenol per micrograms of cellular protein per minute (Lin et al., 2005).
RNA preparation and real-time RT-PCR
Total RNA was extracted from the cell monolayers using TRIzol reagents (Invitrogen, Carlsbad, CA). Any potential DNA contamination was removed by RNase-free DNase treatment. mRNA expression for various genes was determined by reverse transcription-polymerase chain reaction (RT-PCR). Two micrograms of total RNA were reverse-transcribed using the Superscript first-strand synthesis system (Invitrogen). Relative transcript levels were measured by real time PCR in 50 µl reaction volume on 96-well plate using ABI PRISM 7300 sequence detection system and SYBR-Green master mix (Applied Biosystems) with the expression of β-actin as the controls. The primers used for amplification are described in Table 2.
| Gene | Accession number | Forward primer | Reverse primer |
|---|---|---|---|
| Osteocalcin | NM_199173 | 5′-AGGGCAGCGAGGTAGTGA-3′ | 5′-CCTGAAAGCCGATGTGGT-3′ |
| ALP | NM_000478 | 5′-CTGATGTGGAGTATGA-3′ | 5′-TGTATCTCGGTTTGAA-3′ |
| Osterix | NM_152860 | 5′-CCCAGGCAACACTCCTACTC-3′ | 5′-GGCTGGATTAAGGGGAGCAAA-3′ |
| Runx2 | NM_001024630 | 5′-GAGTAGGTGTCCCGCCTCAGAACCC-3′ | 5′-TCTGAAGCACCTGCCTGGCTCTTCT-3′ |
| Dlx5 | NM_005221 | 5′-CCAACCAGCCAGAGAAAGAA-3′ | 5′-GCAAGGCGAGGTACTGAGTC-3′ |
| Msx2 | NM_002449 | 5′-GATGGATGCTTGTTTCA-3′ | 5′-CAGCTATGTCGTGTGGC-3′ |
| β-actin | NM_001101 | 5′-CAAGAGATGGCCACGGCTGCT-3 | 5′-TCCTTCTGCATCCTGTCGGCA-3′ |
| Integrin* | NM_002211 | 5′-TTGACCTCTACTACCTTATGGACCTG-3′ | 5′-ATGGCATCGAAACCACCT-3′ |
- * Mean primers only for RT-PCR.
Western blotting analysis
For immunodetection of ERK, p38, JNK, and β-actin proteins, the cells were washed twice with ice-cold PBS. Cells were lysed with lysis buffer (50 mM Tris (pH7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 10% glycerol) and the supernatant was obtained by a centrifugation for 10 min at 10,000g and subjected to SDS–PAGE. Proteins were electrotransferred to nitrocellulose membranes (NC) (Millipore, Billerica, MA) at 100 V for 1.5 h. They were blocked using 5% milk in TBS (20 mM Tris at pH 7.5, 500 mM NaCl, and 0.05% Tween 20) and then hybridized for 1 h with anti-ERK, p38, JNK and its phosphorylation antibody (Santa Cruz) and anti-β-actin monoclonal antibody (Sigma Chemical) followed by incubation for 1 h with horseradish peroxidase conjugated secondary IgG. Signals were visualized with diaminobenzidine (DAB) on a LAS3000 Lumi-Imager (Fuji Photo Film Co., Ltd). Densitometry was analyzed using the AlphaImager 2200 (Alpha Innotech Corporation, San Leandro, CA).
Plasmid construction
The coding region of osterix was amplified by RT-PCR with primers 5′-ccgaattcacccgttgcctgcactctccc-3′ (forward), and 5′-cgcgggatcctcagatctccagcaagttgc-3′ (reverse). The PCR products were digested with EcoR I and BamH I and then ligated into the pcDNA3.1 vector (Invitrogen) to generate plasmid named pcDNA3.1-osterix.
RNA interference
Five target sequences (see below) for human osterix (sp7) mRNA were chosen according to Ambion's siRNA online design tool. The specificity of all sequences was confirmed by BLAST search. Plasmids were constructed by inserting a synthesized 64-mer oligonucleotide containing the target sequences into pSUPER vector (Brummelkamp et al., 2002). The negative control (scrambled siRNA) siRNA was purchased from Ambion. The vector was then transfected into cells with the LipofectAMINE 2000 Reagent (Invitrogen). siRNA1 (133–153): Sense: GUUCACUAUGGCUCCAGUCTT, Antisense: GACUGGAGCCAUAGUGAACTT; siRNA2 (314–334): Sense: GCACUAAUGGGCUCCUUU CTT, Antisense: GAAAGGAGCCCAUUAGUGCTT; siRNA3 (795–815): Sense: ACCCAAGGCAGUGGGAAAUTT, Antisense: AUUUCCCACUGCCUUGGGUTT; siRNA4 (1104–1124): Sense: GAGGUUCACUCGUUCGGAUTT, Antisense: AUCCGAACGAGUGAACCUCTT; siRNA5 (1475–1495): Sense: UCACUCUCUUUACCCCAUGTT, Antisense: CAUGGGGUAAAGAGAGUGATT.
Statistical analysis
Data were analyzed for statistical significance by one-way ANOVA using the Dunnett's test with the SPSS software.
Results
Effect of stretching on osteogenic differentiation of TOLF and non-TOLF cells
The effect of stretching on TOLF and non-TOLF cell osteogenic differentiation was examined first in our experimental systems. In these experiments, TOLF and non-TOLF cells were treated with different levels of stretch for different periods of time. Cell osteogenic differentiation was assessed by real-time RT-PCR, ALP activity assays, and mineralization assays. ALP activity is considered as an early marker of osteogenic differentiation (Meury et al., 2006). As shown in Figure 1, 9% stretch stimulated the ALP activity in TOLF cells but not 6% and 12%, whereas non-TOLF cells exhibited no increase in ALP activity with stretch stimulation. Stretch stimulation resulted in significant increases in the mRNA expression of ALP and osteocalcin in a time-dependent manner in TOLF cells, whereas non-TOLF cells exhibited no increase with stimulation (Fig. 2A,B). In addition, stretch stimulation was also associated with upregulated osteogenic transcription factors osterix and Runx2 but not Dlx5 and Msx2 (Fig. 3). Moreover, although both TOLF cells and non-TOLF cells exhibited a fibroblast-like and spindle-shaped appearance, only TOLF cell matrix began to mineralize and crystals appeared at 4 weeks after stretch stimulation (Figs. 2C,D). Collectively, these experiments indicated that stretch stimulation resulted in osteogenic differentiation of TOLF cells but not non-TOLF cells, suggesting that stretching as a stimulatory factor for TOLF.
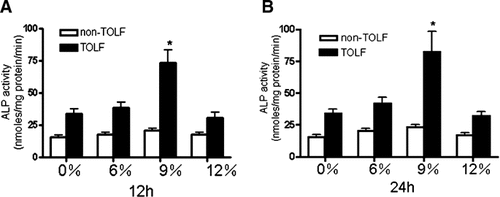
The effect of mechanical strains on ALP activity in TOLF and non-TOLF cells. Mechanical strain was applied in human TOLF and non-TOLF cells cultures at 6–12% for different periods of time (from 12 to 24 h). ALP activity was measured as described under Materials and Methods section. A magnitude of 9% strain induced highest ALP activity in TOLF cells. Data were combined from 11 experiments and shown as mean ± SEM (*P < 0.05).
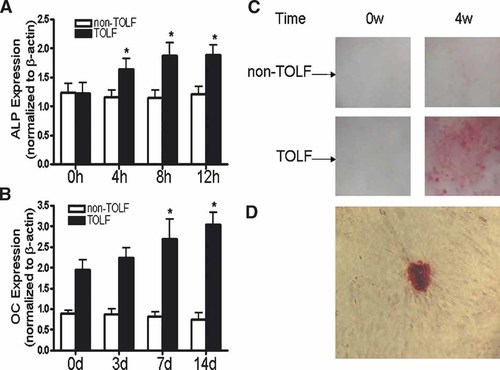
The expression of ALP (A) and osteocalcin (B) in TOLF and non-TOLF cells exposed to mechanical strain. The fold increases of mRNAs are averages from 11 different experiments. C: Formation of mineralized nodules in TOLF and non-TOLF cells. Cells were incubated in DMEM supplemented with 10% FBS. After cultures reached confluence, cells were maintained in DMEM medium containing 10% FBS and 10 mM β-glycerophosphate, and 9% mechanical strain was applied for the indicated times and then stained with alizarin red. D: A microscopic view of TOLF cells at 4 weeks (×40). [Color figure can be viewed in the online issue, which is available at <URL>www.interscience.wiley.com.</URL>].
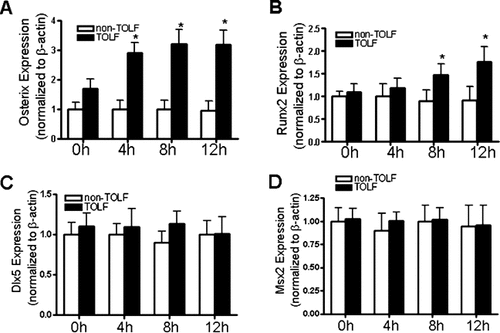
Effects of mechanical strain on mRNA expression of Dlx5, Msx2, osterix, and Runx2 after mechanical strain stimulation in TOLF and non-TOLF cells. A, B, C, and D show cells subjected to 9% mechanical strain for different periods of time (0–12 h). The mRNA level of each gene was measured by real-time RT–PCR and was normalized to β-actin transcript level. The fold increases of these mRNAs to control are shown from eight different experiments (*P < 0.05).
Stretch-induced activation of ERK-p38 signaling pathway but not JNK signaling pathway in osteogenic differentiation of TOLF cells
It is believed that stretch exerts biological effect through integrin β1-mediated signaling pathway (Kippenberger et al., 2000; Aikawa et al., 2002) which involves activation of ERK, p38, and JNK. To gain insight into the molecular mechanism underlying the cell osteogenic effect of stretch, we first examined the expression of integrin β1 subunit in non-TOLF cells and TOLF cells. These cells were grown in normal media for 3d and harvested for total RNA extraction. The expression of integrin β1 subunit was examined using RT-PCR. As shown in Figure 4, the integrin β1 subunit was detected in both non-TOLF cells and TOLF cells. We next examined the changes in phosphorylation of ERK, p38 and JNK after the treatment of stretch in TOLF cells. Cellular proteins were extracted from TOLF cells that were exposed to stretch for various periods of time and were then immunoblotted with an antibody against phosphor-ERK1/2, phosphor-p38, or phosphor-JNK. These experiments showed that phosphorylation of ERK1/2 and p38 but not JNK were stimulated by stretch and the phosphorylation occurred in a time-dependent fashion (Fig. 5).
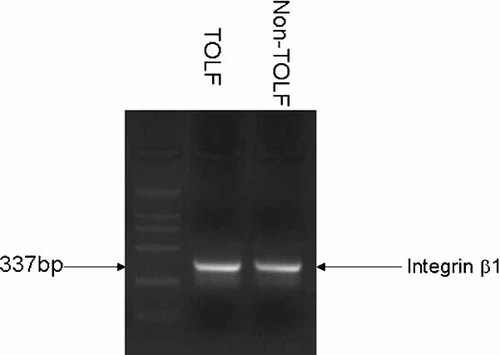
Expression of integrin β1 mRNA in TOLF cells and non-TOLF cells. Total RNA was extracted from TOLF cells and non-TOLF cells and analyzed by RT-PCR.
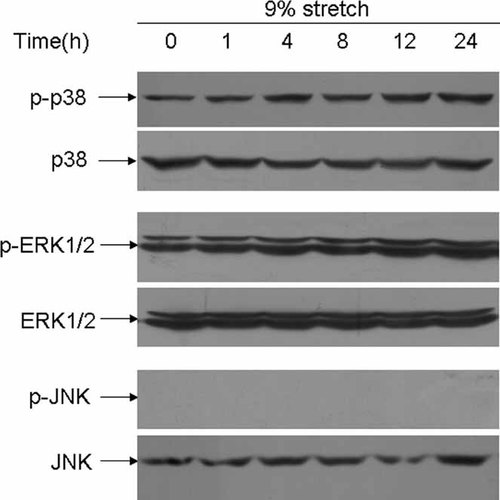
Activation of ERK, p38, and JNK in TOLF cells by mechanical strain. TOLF cells were treated with 9% mechanical strain for the time indicated, and cellular lysates were immunoblotted with specific antibodies against total or phosphorylated form of ERK, p38, or JNK.
Stretch-induced osterix expression through p38 signaling pathway in the early osteogenic differentiation of TOLF cells
To investigate whether the ERK1/2 and p38 phosphorylation was linked to the cell osteogenic effect of stretch, we used specific inhibitors to block ERK1/2 and p38 phosphorylation, and the cell osteogenic differentiation was then measured under the treatment of stretch by real-time PCR and ALP activity assay. In these experiments, TOLF cells were stimulated with stretch in the presence or absence of ERK 1/2 specific inhibitor U0126 or p38 specific inhibitor SB203580. As shown in Figure 6, ERK1/2 and p38 phosphorylation were inhibited by their specific inhibitors, respectively. While SB203580 significantly inhibited stretch-induced osterix expression and ALP activity, U0126 completely prevented stretch-induced Runx2 expression and partially decreased ALP activity.
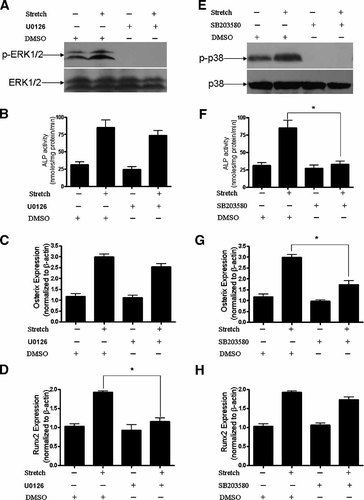
Effects of SB203580 (p38 inhibitor) and U0126 (ERK inhibitor) on osteogenic stimulation of TOLF cells by mechanical strain. TOLF cells were treated with 9% of mechanical strain or left untreated for 12 h prior to treatment with U0126 or SB203580. A: Cellular lysates were prepared from TOLF cells pretreated with U0126 for 12 h and immunoblotted with specific antibodies against total or phosphorylated form of ERK. B, C, and D show effects of mechanical strain and U0126 on the ALP activity and mRNA expression of osterix and Runx2 in TOLF cells. E: Cellular lysates were prepared from TOLF cells pretreated with SB203580 for 12 h and immunoblotted with specific antibodies against total or phosphorylated form of p38. F, G, and H show effects of mechanical strain and U0126 on the ALP activity and mRNA expression of osterix and Runx2 in TOLF cells. The ALP activity and fold increases of mRNAs are averages from eight different experiments (*P < 0.05).
Effect of osterix on stretch-induced ALP activity in TOLF cells
The results described above indicate that osterix has an important role in the regulation of ALP activity induced by mechanical stress. To further explore the potential role of osterix in stretch-stimulated early osteogenic effect in TOLF cells, osterix gain-of-function and loss-of-function experiments were performed. As shown in Figure 7, overexpression of osterix resulted in a significant increase of the ALP activity in TOLF cells even without stretch. On the other hand, knockdown the expression of osterix with siRNA led to a complete inhibition of ALP activity even under mechanical stress in TOLF cells. These experiments clearly indicated that stretch-induced ALP activity in TOLF cells was mediated by osterix.
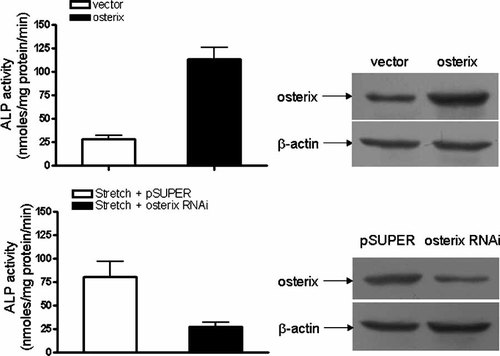
The effect of osterix overexpression and knockdown on stretch-induced ALP activation in TOLF cells. A: Overexpression of osterix strongly enhanced ALP activity in TOLF cells. TOLF cells were transfected with an osterix expression plasmid for 48 h. Total proteins were extracted and Western blottings were performed using antibodies against osterix. B: Stretch-induced ALP activation in TOLF cells with osterix RNAi (siRNA1104–1124 led to remarkable suppression of osterix expression in five siRNA against different target sites of osterix by Western blot, while no change was observed after scrambled siRNA transfection, data not shown). TOLF cells were transfected with pSUPER vectors carrying specific small interfering RNAs against the mRNAs of osterix, and stimulated with 9% stretch for 48 h. ALP activity was measured from sextuplet experiments (*P < 0.05).
Discussion
Recently, mechanical stress has been reported to stimulate the osteogenesis in various cell types. It can stimulate not only osteoprogenitor cells, but also the pluripotent stem cell that can give rise to chondroblastic lineage. There have been numerous clinical reports indicating that mechanical stress plays an important role in TOLF progression (Shiokawa et al., 2001; Ben Hamouda et al., 2003; Fong and Wong, 2004; Liao et al., 2005; Li et al., 2006). Epidemiological studies have also suggested a correlation between mechanical stress and the occurrence of TOLF (Ohtsuka et al., 1986; Miyakoshi et al., 2003). Thus, understanding of the molecular mechanism involved in mechanical stress-induced signal transduction is important in TOLF prevention and management. The equi-biaxial stretch chamber enables application of two-dimensional mechanical strains at 4–16% to ligament fibroblast. This stretch apparatus has been successfully used to mimic the stretching forces applied to the OLF tissue in the spine joint (Lee et al., 1996, 1999; Hsieh et al., 2000, 2002; O'Connor et al., 2004; Zhou et al., 2005). In the present study, we showed that the mRNA expressions of ALP and osteocalcin as well as ALP activity were significantly increased by biaxial stretching in TOLF cells. These observations suggest that TOLF cells have a greater potential to differentiate into osteogenic cells in response to stretching than non-TOLF cells.
Our data indicated that TOLF cells manifest phenotypic characteristics of osteoblasts, while non-TOLF cells are fibroblastic. In our experiments, mechanical stretching induced mineralization, the expression and activation of ALP and the expression of osteocalcin in TOLF cells. It is conceivable that the metaplasia of TOLF cells into osteogenic cells had already occurred in TOLF. Consistent with other studies on ossification of flavum ligament pathology (Yusof and Pratap, 1990; Vasudevan and Knuckey, 2002), our experiments may explain why different responsiveness to mechanical stretching was observed between TOLF cells and non-TOLF cells.
Recent evidence has accumulated to indicate that osteoblastic transcription factors such as Dlx5, Msx2, osterix, and Runx2 play key roles during cell osteogenic differentiation (Komori, 2006). However, whether these osteoblastic transcription factors are involved in the signaling pathway that converts mechanical stress into osteogenic response in TOLF cells are still not clear. Our experiments showed that mechanical stress strongly induced mRNA expression of osterix and also upregulated Runx2, but failed induced the expression of Dlx5 and Msx2, suggesting that osterix and Runx2 are critical players in mechanical stress-induced cell osteogenic differentiation.
ERK1/2, p38 and JNK proteins, members of MAPKs, have been shown to be important mediators of mammalian cell differentiation. At the molecular level, ERK1/2, p38, and JNK act to regulate a number of transcription factors that are critically involved in cell differentiation (Afzal et al., 2005; Ambrosino et al., 2006). In this report we showed that stretching stimuli activated ERK1/2 and p38 pathway, but not JNK pathway in TOLF cells. In addition, SB203580, a p38 specific inhibitor, significant inhibited stretching-induced osterix expression and ALP activation, whereas U0126, a specific inhibitor of ERK1/2, only slightly attenuated ALP activity but completely inhibited stretching-induced Runx2 expression, suggesting differential activation of MAPKs in cell osteogenic differentiation.
Osterix is a key transcription factor that regulates cell osteogenic differentiation. In osterix null mice, osteoblast differentiation is impaired and bone formation is absent (Nakashima et al., 2002). We showed in this report that mechanical stress induced osterix expression in TOLF cells. Moreover, we demonstrated that overexpression of osterix significantly enhanced the ALP activity, whereas osterix knockdown completely suppressed stretch-induced ALP activation. These data indicate that stretching stimulated ALP activation in TOLF cells by a mechanism involving upregulating the expression of osterix, thus identifying osterix as a mechanical signal target. However, the upregulation of osterix by mechanical stress was not completely inhibited by SB203580, implying that other signal pathways in addition to p38 may be involved in its regulation. Further studies are required to delineate other signal pathways involved in regulation of osterix expression under mechanical stress.
In conclusion, our experiments indicated that mechanical stress plays important roles in the progression of TOLF, at least in part through the induction of osteogenic differentiation in thoracic ligament flavum cells. Our experiments showed that osterix is a critical molecular link between mechanostressing and cell osteogenic differentiation.
Acknowledgements
We thank Dr. Paul Sung (University of California, San Diego) for providing the stretch chamber.




