Adenosine receptors in colon carcinoma tissues and colon tumoral cell lines: Focus on the A3 adenosine subtype
Abstract
Adenosine may affect several pathophysiological processes, including cellular proliferation, through interaction with A1, A2A, A2B, and A3 receptors. In this study we characterized adenosine receptors in human colon cancer tissues and in colon cancer cell lines Caco2, DLD1, HT29. mRNA of all adenosine subtypes was detected in cancer tissues and cell lines. At a protein levels low amount of A1, A2A, and A2B receptors were detected, whilst the A3 was the most abundant subtype in both cancer tissues and cells, with a pharmacological profile typical of the A3 subtype. All the receptors were coupled to stimulation/inhibition of adenylyl-cyclase in cancer cells, with the exception of A1 subtype. Adenosine increased cell proliferation with an EC50 of 3–12 µM in cancer cells. This effect was not essentially reduced by adenosine receptor antagonists. However dypiridamol, an adenosine transport inhibitor, increased the stimulatory effect induced by adenosine, suggesting an action at the cell surface. Addition of adenosine deaminase makes the A3 agonist 2-chloro-N6-(3-iodobenzyl)-N-methyl-5′-carbamoyladenosine (Cl-IB-MECA) able to stimulate cell proliferation with an EC50 of 0.5–0.9 nM in cancer cells, suggesting a tonic proliferative effect induced by endogenous adenosine. This effect was antagonized by 5-N-(4-methoxyphenyl-carbamoyl)amino-8-propyl-2(2furyl)-pyrazolo-[4,3e]-1,2,4-triazolo [1,5-c] pyrimidine (MRE 3008F20) 10 nM. Cl-IB-MECA-stimulated cell proliferation involved extracellular-signal-regulated-kinases (ERK1/2) pathway, as demonstrated by reduction of proliferation with 1,4-diamino-2,3-dicyano-1,4-bis-[2-amino-phenylthio]-butadiene (U0126) and by ERK1/2 phosphorylation. In conclusion this study indicates for the first time that in colon cancer cell lines endogenous adenosine, through the interaction with A3 receptors, mediates a tonic proliferative effect. J. Cell. Physiol. 211: 826–836, 2007. © 2007 Wiley-Liss, Inc.
Adenosine, which is released from metabolically active cells or is generated extracellularly by degradation of released ATP, regulates a wide variety of physiological processes interacting with one or more of four known cell-surface receptors. The different subtypes, named A1, A2A, A2B, and A3, can be distinguished pharmacologically and differ also in their coupling to second messenger systems (Fredholm et al., 2001). In particular, A1 and A3 inhibit, through Gi proteins, adenylyl cyclase activity, whereas A2A and A2B stimulate, via Gs proteins, this enzyme (Fredholm, 2003). The activation of additional effector systems like PLC or K+ channels has been shown for A1, A2B, and A3 adenosine receptors (Yaar et al., 2005) and recently it has been demonstrated that all adenosine subtypes mediate phosphorylation of extracellular-regulated kinase 1/2 (ERK1/2; Schulte and Fredholm, 2000). Depending on the extracellular concentration, expression of different adenosine receptor subtypes and the signal transduction mechanisms activated following the binding of specific agonists, adenosine has been shown to modulate cell proliferation, differentiation and apoptosis in tumoral cells (Fishman et al., 2000; Merighi et al., 2002; Mujoomdar et al., 2003, 2004). A large body of literature attributed pro or anti mitogenic effects to A1 and A2A adenosine receptors (Merighi et al., 2003a). However, the development of potent A3 agonists and selective antagonists revealed that the A3 subtype plays a pivotal role in the adenosine-induced modulation of tumor cell proliferation (Bar-Yehuda et al., 2001; Merighi et al., 2005a). Indeed, contrasting results have been reported about the effects mediated through the A3 receptors activation; it seems that it profoundly affects cell survival, by promoting cell protection or cell death depending upon the cell type and/or agonist concentration (Jacobson, 1998; Merighi et al., 2003a). In support of the A3 receptor involvement in tumors, it has initially been shown that A3 receptors appeared highly expressed on the cell surface of tumor cells (Gessi et al., 2001, 2002; Merighi et al., 2001; Suh et al., 2001) and recently it has been reported that the overexpression is confirmed also in human colon tumor tissues (Gessi et al., 2004; Madi et al., 2004). The effects of adenosine in epithelial colon cell proliferation have been investigated in the past with controversial results and without considering the presence of A3 subtype (Lelievre et al., 1998a,b; Barry and Lind, 2000; Mujoomdar et al., 2003; Fishman et al., 2004). However we think that after the introduction of more selective ligands as new tools to identify adenosine receptors (Baraldi and Borea, 2000; Jacobson and Gao, 2006), several actions of adenosine should be reconsidered. Recently, we have demonstrated that A3 receptors are overexpressed in colon cancer tissues (Gessi et al., 2004). However, comparative studies of all adenosine receptors performed in both tumor tissues and derived cell lines are not currently available. Therefore the aims of our study were twofold. First we evaluated the expression of all adenosine receptor subtypes, by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and radioligand binding studies in colon cancer tissues and different colon cancer cell lines. Second, taking advantage of the availability of selective antagonists, we set out to clarify the effect of adenosine and adenosine receptors ligands on colon cancer cell proliferation.
Materials and Methods
Materials
5-N-(4-methoxyphenyl-carbamoyl)amino-8-propyl-2(2furyl)-pyrazolo-[4,3e]-1,2,4-triazolo [1,5-c] pyrimidine ([3H]MRE 3008F20, specific activity 67 Ci mmol−1), N-benzo[1,3]dioxol-5-yl-2-[5-(1,3-dipropyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)-1-methyl-1H-pyrazol-3-yl-oxy]-acetamide ([3H]MRE 2029F20, specific activity 123 Ci mmol−1) were obtained from Amersham International (Buckinghamshire, UK), (4-(2-[7-amino-2-(2-furyl)-[1,2,4]triazolo-[2,32][1,3,6]triazinyl-amino] ethyl)-phenol) ([3H]ZM 241385, specific activity 20 Ci mmol−1) was purchased from Tocris (Boston, MA) and 1,3-dipropyl-8-cyclopentyl-xanthine ([3H]DPCPX, specific activity 120 Ci mmol−1) was derived from NEN Research Products (Boston, MA). The Anti-ACTIVE® mitogen-activated protein kinase (MAPK) antibody was purchased by Promega (Bergamo, Italy). The antibodies for A1, A2A, A2B, and A3 adenosine receptor subtypes were purchased from Alpha Diagnostic (S. Antonio, TX). 7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]-pyrimidine (SCH 58261), 5-[[(4-Clorophenyl)amino]carbamoyl]amino-8-butyl-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (MRE 3105-F20), 5-[[(4-trifluoromethyl-phenyl)amino]carbamoyl]amino-8-butyl-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (MRE 3101-F20), 5-[[(3-methossy-phenyl)amino]carbamoyl]amino-8-butyl-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (MRE 3103-F20), were synthesized by Prof. P.G. Baraldi (Department of Pharmaceutical Sciences, University of Ferrara, Italy). All other reagents were of analytical grade and obtained from commercial sources.
Cell culture conditions
Caco 2, DLD1, and HT29 cells were grown adherently and maintained in Eagle's Minimum Essential medium in Earle's BSS plus sodium pyruvate (1 mM), RPMI and McCoys medium respectively, supplemented with l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml) and 10% fetal calf serum, at 37°C in humidified air with 5% carbon dioxide.
Patients and tissues
Tissues were obtained from 10 patients with moderate differentiated colorectal adenocarcinomas (TNM stage II and III) who underwent surgical resection. The protocol was approved by the local Ethics Committee and informed consent was obtained from each patient.
Real-time RT-PCR experiments
Total cytoplasmic RNA was extracted by the acid guanidinium thiocyanate phenol method. Quantitative real-time RT-PCR assay (Higuchi et al., 1993) of adenosine receptor mRNAs was carried out using gene-specific fluorescently labelled TaqMan MGB probe (minor groove binder) in a ABI Prism 7700 Sequence Detection System (Applied Biosystems, Warrington Cheshire, UK). For the real-time RT-PCR of A1, A2A, A2B and A3 adenosine subtypes the assays-on- demand™ Gene expression Products NM 000674, NM 000675, NM 000676, and NM 000677 were used respectively. Moreover curves of adenosine receptors cDNA plasmid standards with a range spanning at least six orders of magnitude (10−11–10−16 g/µl) were generated. These standard curves displayed a linear relationship between Ct values and the logarithm of plasmid amount (Gessi et al., 2005). Quantification of adenosine receptor messages in cancer tissues and cells was made by interpolation from standard curve of Ct values generated from the plasmid dilution series. For the real-time RT-PCR of the reference gene the endogenous control human β-actin kits was used, and the probe was fluorescent-labeled with VIC™ (Applied Biosystems, Monza, Italy).
Membrane preparation
Colon cancer cells and human colon specimens were homogenized respectively in hypotonic buffer and PBS, with a Polytron (Luzern, Switzerland), (Kinematica) and centrifuged for 30 min at 48,000g as described previously (Gessi et al., 2004). The protein concentration was determined according to a Bio-Rad method (Bradford, 1976) with bovine albumin as a standard reference.
Radioligand binding assays
Binding assays were carried out according to Gessi et al. (2004). Saturation experiments of [3H]MRE 3008F20 to colon cancer tissues were carried out in a total volume of 250 µl containing 50 mM Tris HCl buffer, 10 mM MgCl2, 1 mM EDTA, pH 7.4, by adding 100 µl of membrane homogenate (80 µg of protein assay −1) in duplicate with 10–12 different concentrations of [3H]MRE 3008F20 in the range 0.8–80 nM. In colon cancer cells saturation studies of [3H]MRE 3008F20 binding were performed in the range 0.4–40 nM. Incubation time was 120 min at 4°C to allow equilibrium to be reached. In competition experiments, 4 nM [3H]MRE 3008F20 was incubated in duplicate with at least 12–14 different concentrations of each of the agonists or antagonists examined. Incubation time was 120 min at 4°C to allow equilibrium to be reached. This temperature was chosen in consideration of the fact that A3 antagonist binding is prevalently enthalpy-driven (Varani et al., 2000). Non-specific binding, defined as binding in the presence of 1 µM MRE 3008F20, at the KD value for the radioligand was ≈30–34% of total binding. As for the other adenosine subtypes in saturation experiments, 100 µl of membrane homogenate (80–100 µg of protein assay−1) were incubated in duplicate, in a final volume of 250 µl in test tubes containing 50 mM Tris HCl buffer (10 mM MgCl2 for A2A and 10 mM MgCl2, 1 mM EDTA, 0.1 mM benzamidine for A2B) pH 7.4, with 10–12 different concentrations of [3H]DPCPX (0.2–20 nM), [3H]ZM 241385 (0.3–30 nM) and [3H]MRE 2029F20 (0.2–20 nM) to label A1, A2A, and A2B adenosine receptors, respectively. Non-specific binding, defined as binding in the presence of 1 µM DPCPX, 1 µM SCH 58261, 1 µM MRE 2029F20 for A1, A2A, and A2B adenosine receptors, respectively, at the KD value for each radioligand was ≈35–40% of total binding. Bound and free radioactivity were separated, after an incubation time of 120 min at 4°C, by filtering the assay mixture through Whatman GF/B glass-fiber filters using a cell harvester (Packard Instrument Company, Meriden, CT). The filter bound radioactivity was counted on Top Count Microplate Scintillation Counter (efficiency 57%) with Micro-Scint 20.
Measurement of cAMP levels
Colon cells (5 × 106/assay) were suspended in 0.5 ml of incubation mixture (150 mM NaCl, 2.7 mM KCl, 0.37 mM NaH2PO4, 1 mM MgSO4, 1 mM CaCl2, 5 mM glucose, 1 mM Hepes, 10 mM MgCl2, pH 7.4 37°C), 0.5 mM 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (Ro 20–1724), 2.0 IU ml−1 adenosine deaminase (ADA) and incubated with drugs at 37°C. The reaction was terminated by the addition of cold 6% trichloroacetic acid (TCA). The final aqueous solution was tested for cyclic AMP levels by a competition protein binding assay carried out essentially as described previously (Varani et al., 2000). Samples of cyclic AMP standards (0–10 pmol) were added to each test tube containing Trizma base 0.1 M; aminophylline 8.0 mM; 2 mercaptoethanol 6.0 mM; pH 7.4 and [3H]-cyclic AMP in a total volume of 0.5 ml. The binding protein, previously prepared from bovine adrenal glands, was added to the samples and incubated at 4°C for 150 min. After the addition of charcoal, samples were centrifuged at 2,000g for 10 min. The clear supernatant (0.2 ml) was mixed with 4 ml of Atomlight (Packard BioScience, Meriden, CT) and counted in a LS-1800 Beckman scintillation counter (Beckman Coulter, Fullerton, CA).
MTT
The activity of living cells was determined evaluating the mitochondrial dehydrogenase activity by using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) that is converted in formazan product in living cells (Carmichael et al., 1988). The cells were incubated with drugs for 24 h, and then 550 µl of an acid propanol solution (0.1 N HCl in isopropyl alcohol) were added to each well to dissolve the formazan. The optical density of each well was read on a spectrophotometer at 570 nm.
Trypan blue
Colon cell proliferation was monitored by cell counting. 1 × 105 cells/ml were cultured in 24-well plates, in the presence of drugs. Cells were collected after 24 h and stained with 0.4% of trypan blue for 5 min at room temperature before being examined under the microscope. The number of viable cells was determined by trypan blue exclusion.
Proliferation assay
Cells were plated, 1 × 105 cells in 1 ml of culture medium, starved for 24 h and challenged with 10% serum and drugs in the presence of [3H]thymidine (1 µCi/ml). After 24 h the cells were harvested, using vacuum aspiration on Whatman GF/C glass-fiber filters using a Micro-Mate 196 cell harvester (Packard Instrument Company). The filter-bound radioactivity was counted on a Top Count Microplate Scintillation Counter (efficiency 57%) with Micro-Scint 20.
Flow cytometry analysis
Cells were plated, 5 × 105 cells in 10 ml of culture medium, starved for 24 h and challenged with 10% serum and drugs. Then they were trypsinized, mixed with floating cells, washed with PBS and permeabilized in 70% (v/v) ethanol/PBS solution at 4°C for at least 24 h. The cells were washed with PBS and the DNA was stained with a PBS solution, containing 20 µg/ml propidium iodide and 100 µg/ml RNAse, at room temperature for 30 min. Cells were analyzed with an EPICS XL flow cytometer (Beckman Coulter, Miami, FL) and the content of DNA was evaluated. Cell distribution among cell cycle phases was evaluated as previously described (Merighi et al., 2002). Briefly, the cell cycle distribution is shown as the percentage of cells containing 2n (Go/G1 phases), 4n (G2 and M phases), 4n > x > 2n DNA amounts (S phase) judged by propidium iodide staining. Apoptotic population is the percentage of cells with DNA content lower than 2n.
Western blotting
The presence of adenosine receptor subtypes was evaluated by using specific antibodies towards human A1, A2A, A2B and A3 receptors in CHO cells transfected with human A1, A2A, A3 receptors, in HEK 293 cells transfected with the human A2B subtype and in colon cancer cell lines. The protein concentration was determined using a BCA protein assay kit (Pierce, Rockford, IL). Equivalent amounts of protein (40 µg) were subjected to electrophoresis on 10% sodium dodecyl sulfate-acrylamide gel. The gel was then electroblotted onto a nitrocellulose membrane. The membranes were probed with anti-A1, A2A, A2B, and A3 antibodies. The phosphorylation and activation of ERK-1 and ERK-2 was detected using the Anti-ACTIVE® MAPK pAb, as previously described (Merighi et al., 2002). Briefly, colon cells were starved for 24 h and then incubated with drugs for 5, 15, and 60 min. Cells were harvested and washed with ice-cold PBS containing 1 mM sodium orthovanadate, 104 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 0.08 mM aprotinin, 2 mM leupeptin, 4 mM bestatin, 1.5 mM pepstatin A, 1.4 mM E-64. Cells were then lysed in Triton lysis buffer. The membranes were probed with phosphorylated (Tyr183/Tyr185) or total ERK-1/ERK-2 MAPK pAb (1:5,000) in TBST (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween-20)/0.5% BSA for 1 h at room temperature. Specific reactions were revealed with the Enhanced Chemiluminescence Western blotting detection reagent (Amersham Corp., Arlington Heights, IL).
Data analysis
Binding studies were analyzed with the program LIGAND (Munson and Rodbard, 1980). EC50 and IC50 values in the cyclic AMP assay and cell proliferation studies were calculated with the nonlinear least-squares curve fitting program Prism (GraphPAD, San Diego, CA). Statistical analysis was performed by means of analysis of variance (ANOVA) and the Dunnett's test. P<0.05 was considered significant.
Results
Expression of adenosine receptors in cancer tissues and colon cancer cells
The expression level of adenosine receptor subtypes was examined, through real-time RT-PCR experiments, in colon cancer tissues. All adenosine subtype mRNAs were detected in tumors investigated, with the following rank order: A2B > A3 > A2A > A1 as shown in Figure 1A. Then, expression of adenosine receptors was evaluated in colon cancer cells. Our results indicate the presence of low levels of the A3 adenosine receptor in Caco 2, DLD1, and HT29 cells. As for the transcripts of the other adenosine subtypes, low amount of A1, with the exception of Caco2 cells, and high level of A2B subtypes in the cell lines investigated were detected (Fig. 1A). Evaluation of adenosine receptors message was made by interpolation from standard curve of Ct values generated from the plasmid dilution series. Analogue results were obtained when the expression level of adenosine receptors was normalized to the expression level of β-actin.
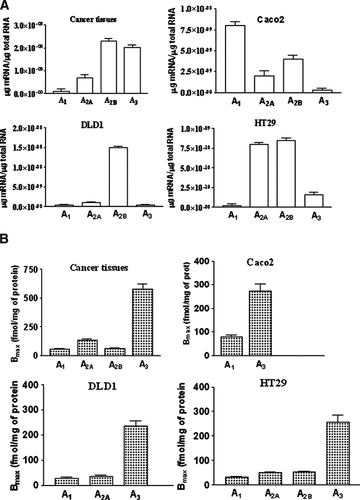
mRNA expression of adenosine receptors in colon cancer tissues and cell lines. Bar graph showing µg mRNA/µg total RNA (A) and Bmax values (fmol/mg of protein) (B) of human A1, A2A, A2B, and A3 adenosine receptors in colon cancer tissues, Caco2, DLD1 and HT29 tumoral cell lines. Experiments were performed as described in Experimental Procedures. Values are the means and vertical lines SE of the mean of four separate experiments performed in triplicate.
Binding to adenosine receptors in cancer tissues and colon cancer cells
The presence of adenosine receptor was investigated in colon cancer tissues obtained from 10 patients undergoing surgery. [3H]DPCPX, [3H]ZM 241385, [3H]MRE 2029F20 and [3H]MRE 3008F20 antagonist radioligands were used to evaluate binding to A1, A2A, A2B, and A3 receptors, respectively (Fig. 1B). As for A1, A2A, and A2B receptors, KD values were of 1.8 ± 0.3, 3.3 ± 0.2, 2.2 ± 0.1 nM and Bmax values were 53 ± 5, 132 ± 9, 60 ± 7 fmol/mg of protein, respectively. Cancer tissues expressed the A3 receptor with KD value of 8.22 ± 0.65 and Bmax value of 578 ± 45 fmol/mg of protein revealing that the A3 receptor was the most expressed subtype in cancer tissues. Similar experiments were performed in colon carcinoma cells (Fig. 1B). The saturation curves of [3H]DPCPX showed KD values of 1.32 ± 0.19, 1.70 ± 0.55, 1.81 ± 0.23, and Bmax values of 79 ± 2, 28 ± 2, 32 ± 4 fmol/mg of protein in Caco2, DLD1 and HT29 cells. [3H]ZM 241385 bound A2A receptors with KD values of 1.90 ± 0.20, 1.30 ± 0.20, and Bmax values of 36 ± 2, 49 ± 4, fmol/mg of protein in DLD1 and HT29 cells. In contrast any specific binding was observed in Caco2 cells. By using the selective antagonist [3H]MRE 2029F20 we could reveal A2B receptors only in HT29 cells with a KD value of 1.60 ± 0.30, and a Bmax value of 52 ± 4 fmol/mg of protein. [3H]MRE 3008F20 recognized A3 receptors with KD values of 4.73 ± 0.27, 4.30 ± 0.60, 3.97 ± 0.39, and Bmax values of 266 ± 15, 236 ± 17, 257 ± 22 fmol/mg of protein in Caco2, DLD1, and HT29 cells, respectively. Figure 2A shows the saturation curve and related scatchard plot to A3 receptors in DLD1 cells. These data demonstrate that the A3 subtype, is the most represented adenosine receptor in both colon cancer tissues and cell lines. Therefore we investigated the pharmacological profile of [3H]MRE 3008F20 binding in DLD1 cells by using a series of typical adenosine ligands. Antagonist competition curves for [3H]MRE 3008F20 binding showed a rank order of potency of MRE3105F20>MRE3101F20>MRE3008F20>MRE 3103F20>ZM241385>DPCPX each of which exhibited Hill slopes near to unity, SCH 58261 showed a Ki value >10 µM (Fig. 2B). The competition curves of [3H]MRE 3008F20 binding by 2-chloro-N6-(3-iodobenzyl)-N-methyl-5′-carbamoyladenosine (Cl-IBMECA) and 5′-N-ethyl-carboxamidoadenosine (NECA) showed Hill coefficients less than unity (0.58 and 0.55, respectively) and were best described by the existence of one high-affinity (KH of 0.7 ± 0.1 and 24 ± 5 nM, respectively) and one low-affinity (KL of 76 ± 8 and 1550 ± 165 nM, respectively) agonist-receptor binding state. Similar results were obtained in Caco2 and HT29 cells (Table 1).
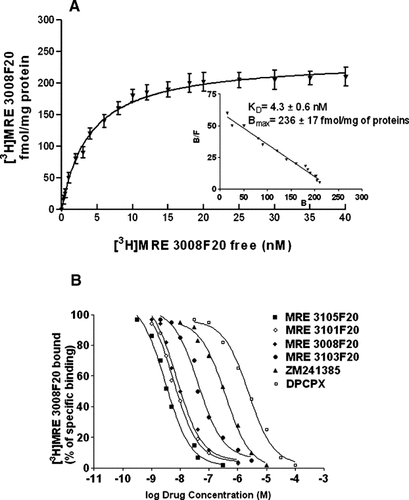
Binding experiments to A3 adenosine receptors in DLD1 cells. Saturation curve and related scatchard plot (inset) of [3H]-MRE 3008-F20 binding to A3 adenosine receptors in DLD1 cells (A). Competition curves of specific [3H]-MRE 2029-F20 binding to human A3 adenosine receptors in DLD1 cells by adenosine antagonists (B). Curves are representative of a single experiment from a series of four independent experiments. Experiments were perfomed as described in Experimental Procedures. Values are the means and vertical lines SE of the mean of four separate experiments performed in triplicate.
| Compounds | Caco-2 [3H]MRE 3008F20 binding KH, KL, Ki (nM) | DLD1 [3H]MRE 3008F20 binding KH, KL, Ki (nM) | HT29 [3H]MRE 3008F20 binding KH, KL, Ki (nM) |
|---|---|---|---|
| Agonists | |||
| CL-IB-MECA | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| 72 ± 11 | 76 ± 8 | 70 ± 7 | |
| NECA | 22 ± 4 | 24 ± 5 | 20 ± 3 |
| 1,540 ± 156 | 1,550 ± 165 | 1,520 ± 150 | |
| Antagonists | |||
| MRE 3008F20 | 4.3 ± 0.3 | 4.0 ± 0.4 | 4.5 ± 0.5 |
| MRE 3101F20 | 3.4 ± 0.3 | 3.5 ± 0.4 | 4.0 ± 0.3 |
| MRE 3105F20 | 2.2 ± 0.2 | 2.5 ± 0.2 | 3.5 ± 0.3 |
| MRE 3103F20 | 27 ± 2 | 23 ± 2 | 30 ± 3 |
| ZM 241385 | 245 ± 23 | 240 ± 25 | 250 ± 22 |
| DPCPX | 1,500 ± 130 | 1,650 ± 150 | 1,900 ± 140 |
| SCH 58261 | >10,000 | >10,000 | >10,000 |
- KH and KL are the Ki values of the high- and low-affinity states for agonists, respectively.
Detection of adenosine receptors by means of Western blotting analysis
The presence of adenosine receptor subtypes at the level of the protein was also assessed by means of Western blotting experiments in colon cancer cell lines. The antibodies specific for each adenosine subtype were used in CHO cells transfected with human A1, A2A, A3 receptors, in HEK 293 cells transfected with the human A2B subtype and in Caco2, DLD1, and HT29 colon cancer cell lines. As shown in Figure 3, HT29 cells that in binding studies showed the presence of all adenosine subtypes also reveal immunoreactivity for A1, A2A, A2B, and A3 antibodies in Western blotting experiments. As transfected cells expressed a density of each adenosine subtype of about 300 fmol/mg of protein we can conclude that the A3 receptor appears the most expressed adenosine subtype in HT29 cells. Also in DLD1 and Caco2 cells immunoblot data confirm the results obtained by means of binding experiments (data not shown).
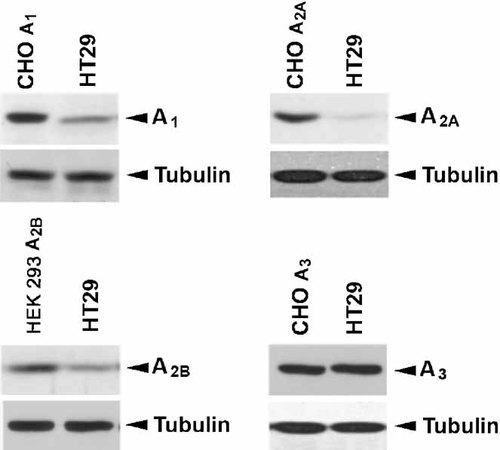
Adenosine receptors detection by Western blot analysis. Immunoreactivity of A1, A2A, A2B, and A3 antibodies was evaluated in CHO cells transfected with A1, A2A, and A3 receptors, in HEK 293 cells transfected with the A2B subtype and in HT29 cells. Cellular extracts were prepared and subjected to immunoblot assay using anti-A1, A2A, A2B, and A3 antibodies. Tubulin shows equal loading protein.
Modulation of cAMP levels by adenosine receptor activation
To evaluate the functional coupling of adenosine receptors in colon cancer cells, we determined the potency of typical agonists in cAMP determinations. As for the adenosine subtypes coupled with the inhibition of cAMP levels, we evaluated the effect of the A1 compound 2-chloro-N6-cyclopentyladenosine (CCPA) and of the A3 agonist Cl-IB-MECA. CCPA, in the range of concentrations 0.1–100 nM, was not able to inhibit forskolin-stimulated cAMP levels in any of the cell lines investigated. Instead Cl-IB-MECA inhibited forskolin-stimulated cAMP levels in DLD1 cells with an IC50 value of 12 ± 0.3 nM (Fig. 4A). The selective A3 antagonist MRE 3008F20 1 µM fully antagonized the effect of Cl-IB-MECA (100 nM) suggesting the involvement of the A3 adenosine subtype (Fig. 4B). In order to investigate the functional coupling with the stimulatory subtypes A2A and A2B, we carried out dose-response curves by using the nonselective agonist NECA. This compound, increased cyclic AMP production, with an EC50 value of 141 ± 19 and 163 ± 17 nM in HT29 and DLD1 cells, respectively. In DLD1 cells the selective A2A antagonist SCH 58261 (1 µM) blocked the rise in cAMP levels due to NECA (100 nM) suggesting that the stimulatory effect was essentially A2A-mediated (Fig. 4C). Instead in HT29 cells, SCH 58261 (1 µM) did not reverted completely the NECA-induced cAMP increase (Fig. 4C) suggesting the involvement of another adenosine subtype. Therefore, the dose-response curve of NECA-induced cyclic AMP accumulation in the presence of SCH 58261 1 µM, a concentration that produced maximal blockade of A2A receptors while leaving A2B receptors free, was repeated. In this condition we found a shift in the NECA EC50 value (2,270 ± 230 nM) indicating the presence of functional A2B receptors in HT29 cells (Fig. 4D). Finally, in Caco2 cells it was not possible to reveal nor functional adenylyl cyclase-coupled A2A or A2B receptors, in agreement with binding assays.
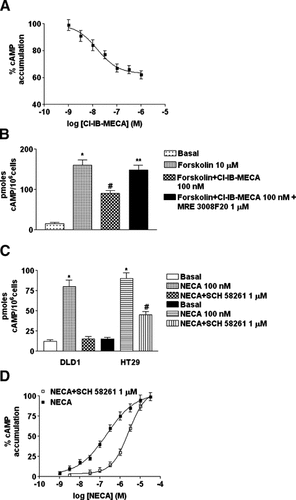
Effect of adenosine receptors on cAMP levels in colon cancer cell lines. Effect of increasing concentrations of Cl-IB-MECA on cAMP accumulation in DLD1 cells (IC50 = 12 ± 0.3 nM) (A). Antagonism of Cl-IB-MECA-inhibited cAMP levels by MRE 3008F20 1 µM (B) *P < 0.01 with respect to basal, #P < 0.05 with respect to forskolin, **P < 0.05 with respect to Cl-IB-MECA and forskolin. Antagonism of NECA-stimulated cAMP levels by SCH 58261 1 µM in DLD1 and HT29 cells (C) *P < 0.01 with respect to basal, #P < 0.05 with respect to NECA 100 nM. Dose-response curves of NECA for cAMP accumulation in the absence (▪) and in the presence (□) of a fixed concentration of SCH 58261 (1 µM) (EC50 = 141 ± 19 and 2,270 ± 230 nM, respectively) in HT29 cells (D). Results are presented as the mean ± SEM of three independent experiments.
Cl-IB-MECA at variance with Adenosine stimulates proliferation of colon cancer cells through A3 receptor activation
By using trypan blue exclusion, cell counts and MTT assay we observed that adenosine did not induce cell death effects in any of the cell lines analyzed (data not shown). Instead, performing [3H]thymidine incorporation experiments, we found that adenosine, after 24 h of treatment, exerted a proliferative effect. The addition of EHNA, an ADA inhibitor, did not revert the adenosine proliferative effect suggesting that adenosine, and not its metabolic product, was responsable for the cell growth effect observed. Interestingly, EHNA slightly increased by about 20% the unstimulated [3H]-thimidine incorporation in Caco2, DLD1, and HT29 cells suggesting a proliferative effect induced by endogenous levels of adenosine (Fig. 5). The EC50 of adenosine to stimulate cell proliferation was 9.6 ± 0.9, 12 ± 1.0, 3.4 ± 0.5 µM in Caco2, DLD1 and HT29 colon cancer cells, respectively (mean ± SE, N = 10, Fig. 6A). Adenosine induced a biphasic effect on cell proliferation with a decrease at millimolar concentrations. To verify if the nucleoside was acting through membrane-receptor interaction, the adenosine curve was repeated in the presence of selective adenosine antagonists. As shown in Figure 6A, in Caco2, DLD1, and HT29 cells, 100 nM MRE 3008F20 antagonized adenosine's proliferative effect, suggesting that the A3 receptor stimulation may be responsible for cell proliferation. As A1 receptors are present in all the three cell lines we investigated the effect of DPCPX (10 nM) and we did not found a significant inhibition in any of the cell lines investigated; DLD1 cells were also treated with SCH 58261 (100 nM) in order to block the A2A effect; HT29 cells, which express A2A and A2B subtypes were incubated with both SCH 58261 and MRE 2029F20 (100 nM) to evaluate the A2A and A2B involvement. Only in HT29 cells SCH 58261 and MRE 2029F20 induced a slight inhibitory effect (23 ± 5 and 18 ± 4% of inhibition versus adenosine 100 µM, respectively) on adenosine-mediated cell proliferation, leading us to attribute essentially to A3 receptors the observed effects. To further investigate the role of A3 receptors in the adenosine-mediated cell proliferation, increasing concentrations (0.1–1,000 nM) of Cl-IB-MECA were tested in the incorporation of [3H]-thymidine; these experiments were performed in the presence of ADA in order to avoid competition between endogenous adenosine and Cl-IB-MECA. The addition of ADA (3 U/ml) alone reduced cell proliferation by about 15–20% in all the three cell lines suggesting again a tonic stimulation of cell proliferation by endogenous adenosine. By adding ADA we could detect a significant stimulatory effect by Cl-IB-MECA with an EC50 of 0.7 ± 00.6, 0.9 ± 0.08, and 0.6 ± 0.07 nM in Caco2, DLD1, and HT29 cells respectively; also in this case the response was biphasic with a decrease at micromolar doses of the A3 agonist, with the exception of HT29 cells. The Cl-IB-MECA effect was blocked by the addition of 100 nM MRE 3008F20, suggesting the involvement of A3 receptors (Fig. 6B). However the lack of stimulation of cell proliferation induced by Cl-IB-MECA in the absence of ADA at variance with the proliferative effect triggered by exogenous adenosine in colon cancer cells suggest the possibility that exogenous adenosine was not acting through the A3 subtype. Therefore we performed experiments in the presence of 10 nM MRE 3008F20, a dose lower than 100 nM but sufficient to block A3 receptors, and we found that in this condition the adenosine effect was not significantly reduced in contrast to the antagonism of Cl-IB-MECA effect (Fig. 6A,B). These results may indicate that the A3 subtype is responsible of a basal stimulatory effect induced by endogenous adenosine levels whilst exogenous adenosine probably mediates its proliferative effect through a mechanism independent by A3 receptor activation.
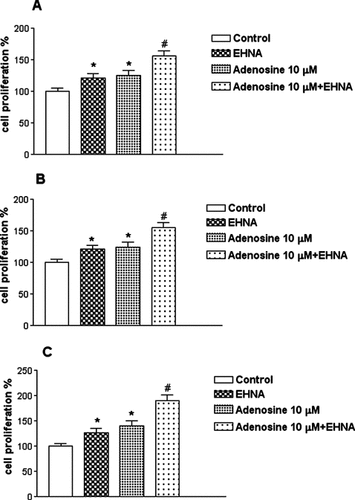
Effect of EHNA on cell proliferation induced by adenosine in Caco2 (A), DLD1 (B) and HT29 (C) cell lines. Proliferation rate is reported in ordinate as percentage of [3H]-thymidine incorporation of untreated cells. Values are mean ± SEM of five independent experiments, *P < 0.05 with respect to untreated cells, #P < 0.05 with respect to EHNA alone, analysis was by Student's t test.
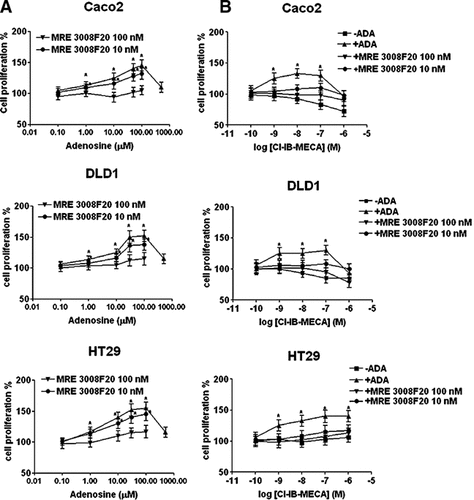
Adenosine (A) and Cl-IB-MECA (B) proliferative effect in Caco2, DLD1 and HT29 cells, and antagonism by MRE 3008F20, measured by [3H]-thymidine incorporation assay. Starved cells were challenged with new medium containing 10% serum and increasing concentrations of drugs for 24 h. Proliferation rate is reported in ordinate as percentage of [3H]-thymidine incorporation of untreated cells. Values are mean ± SEM of ten independent experiments. *P < 0.05 with respect to untreated cells, analysis was by ANOVA followed by Dunnett's test.
Cell cycle analysis
Data from [3H]thymidine incorporation experiments were confirmed using cell cycle analysis. Incubation of DLD1 cells with 10 µM adenosine induced a significant increase in the proportion of cells in S phase from 16 ± 1 to 20 ± 1% (mean ± SEM, four independent experiments; Table 2). Interestingly, in the presence of dypiridamol, an adenosine transport inhibitor, the stimulatory effect induced by adenosine 10 µM on the cells in S phase, was increased of 25% suggesting that prevention of the nucleoside transport into the cells potentiated its effect at cell surface (Table 2). Addition of 10 nM DPCPX, 100 nM SCH 58261, 10 nM MRE 3008F20, and 100 nM MRE 2029F20 did not significantly affected DNA synthesis induced by adenosine (data not shown). After treatment with ADA a major number of cells versus control were blocked into G1/G0 phase (80 ± 2 vs. 72 ± 2%, respectively) and following the addition of Cl-IB-MECA 100 nM the percentage of cells in S phase increased from 11 ± 1 to 15 ± 1% corresponding to an increase of 36%, (significant increase, P < 0.05). In this case MRE 3008F20 10 nM blocked the agonist effect suggesting a mechanism mediated by A3 adenosine receptors (Table 2).
| G1/G0 | S | G2/M | |
|---|---|---|---|
| Control | 72 ± 2 | 16 ± 1 | 12 ± 1 |
| Adenosine 10 µM | 65 ± 2* | 20 ± 1* | 15 ± 1 |
| Adenosine 10 µM + Dypiridamole 5 µM | 58 ± 2* | 25 ± 1* | 17 ± 1* |
| ADA | 80 ± 2* | 11 ± 1* | 9 ± 1 |
| Cl-IB-MECA 100 nM + ADA | 73 ± 1** | 15 ± 1** | 12 ± 1 |
| Cl-IB-MECA 100 nM + MRE 3008 F20 10 nM + ADA | 79 ± 2 | 12 ± 1 | 9 ± 1 |
- Cells were treated as in Figure 6A,B and flow cytometry assay was performed at the indicated times. Values are the percentages of cells in G1/G0, S and G2/M phases of the cell cycle obtained by flow cytometric analysis of 10,000 events. Values are mean ± SEM of four independent experiments.
- * P < 0.05 with respect to control.
- ** P < 0.05 with respect to ADA, analysis was by ANOVA followed by Dunnett's test.
Cl-IB-MECA mediates cell proliferation through involvement of ERK1/2 kinases
To evaluate whether the transduction pathway of CL-IBMECA-induced cell proliferation involved ERK1/2 kinases, we investigated the effect of Cl-IB-MECA 100 nM in the presence of a specific mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) inhibitor 1,4-diamino-2,3-dicyano-1,4 bis[2-aminophenylthio]butadiene (U0126) 10 µM in colon cancer cell lines. Measurement of [3H]thymidine incorporation revealed a strong reduction of Cl-IB-MECA-induced cell growth in all the three cell lines investigated (Fig. 7). Similar results were obtained by cell cycle analysis (data not shown).
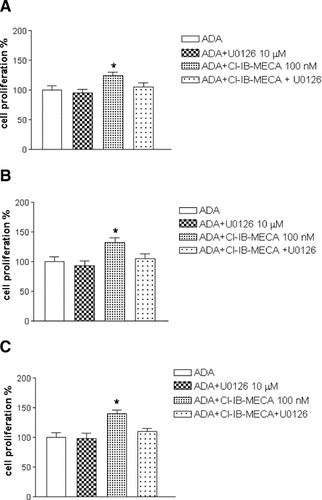
Effect of U0126 on cell proliferation induced by Cl-IB-MECA in Caco2 (A), DLD1 (B) and HT29 (C) cell lines. Starved cells were challenged with new medium containing 10% serum, ADA and drugs for 24 h. Proliferation rate of cells treated with Cl-IB-MECA is reported in ordinate as percentage of [3H]-thymidine incorporation of cells treated with ADA alone. Values are mean ± SEM of five independent experiments, *P < 0.05 with respect to cells treated with ADA alone, analysis was by ANOVA followed by Dunnett's test.
ERK1/2 phosphorylation
The changes in ERK1/2 phosphorylation were investigated in colon cancer cell lines by Western blotting studies. Cl-IB-MECA activated ERK1/2 in a time-dependent manner. The A3 agonist stimulated ERK1/2 phosphorylation within 5–15 min and returned to control in 60 min in Caco2, HT29 and DLD1 cells (data not shown). Immunoblotting indicated that both ERK1 and ERK2 were activated, because both bands were labeled by anti-phospho-specific ERK in colon cancer cells. Moreover the Cl-IB-MECA-induced ERK1/2 phosphorylation was blocked by MRE 3008F20 100 nM (Fig. 8). Similar results were obtained in the presence of MRE 3008F20 10 nM (data not shown).
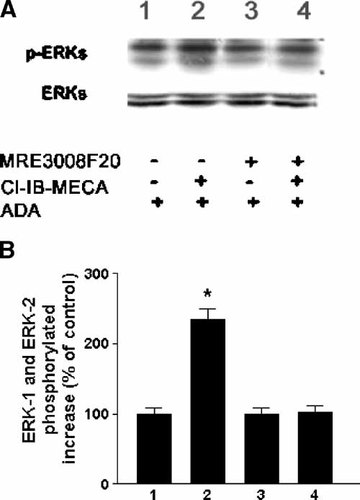
ERK-1 and ERK-2 activated isoforms detection by Western blot. DLD1 cells were treated with ADA in the absence and in the presence of Cl-IB-MECA 100 nM and exposed to MRE 3008F20 100 nM. The detection of ERK-1 and ERK-2 phosphorylated isoforms is shown. The blot was then stripped and used to determine total ERK1/2 expression using an anti- ERK 1/2 antibody (pAb) (A). The immunoblot signals were quantified using Molecular Analyst/PC densitometry software (Bio-Rad). Densitometric analysis of ERK1/2 phosphorylated isoforms is reported. 100% indicated the unstimulated level. Values are mean ± SEM of five independent experiments (B). *P < 0.05 with respect to cells treated with ADA alone, analysis was by ANOVA followed by Dunnett's test.
Discussion
The possibility that A3 adenosine receptor plays a role in the development of cancer has aroused considerable interest in recent years (Merighi et al., 2003a). In particular we have demonstrated an overexpression of the A3 subtype in colon cancer tissues obtained from patients undergoing surgery in comparison to normal mucosa (Gessi et al., 2004), in agreement to Madi et al. (2004). The present study was undertaken to investigate the presence of the A3 adenosine receptor on human colon cancer cells and to evaluate the functional effect of this receptor on colon cancer cell biology. However as adenosine receptors are often coexpressed on a single cell (Fredholm et al., 2001) we also considered it important to investigate the presence of the other adenosine subtypes. This is relevant in view of the evaluation of adenosine-mediated effects, and to see whether colon cancer cells reflect a similar pattern of expression of human tumors. The presence of all adenosine receptors mRNAs in both tissues and colon cancer cells was detected, by means of real time RT-PCR studies, with the A2B being the more express in comparison to A1, A2A, and A3 subtypes. However as this result is not predictive of the presence of adenosine receptors in the membrane surface due to posttranscriptional events, to quantify exactly the density of A1, A2A, A2B, and A3 adenosine receptor protein, saturation studies by using selective antagonist radioligands were performed. Our results showed that the density of A1, A2A, and A2B was quite low if compared with that of the A3 subtypes. Similar results were obtained in colon cells suggesting for the first time that the pattern of expression of adenosine receptors is very similar in colon cancer tissues and colon carcinoma cell lines and that the A3 subtype is the most abundant adenosine receptor present in both. Due to the discrepance between mRNA and binding data, protein levels were also evaluated by Western blotting experiments obtaining analogous results. This supports the emerging evidence that mRNA expression patterns are necessary but are by themselves insufficient for the quantitative description of biological systems. This evidence includes discoveries of posttranscriptional mechanisms controlling the protein translation rate or the half-lives of specific proteins or mRNAs (Gygi et al., 1999; Audic and Hartley, 2004; Weinzierl et al., 2007).
Because adenylyl cyclase is a common effector, negatively coupled to A1 and A3 and positively linked to A2A and A2B receptors, we investigated the functional activation of adenosine receptors in colon cancer cell lines. The A1 agonist CCPA did not inhibit cAMP levels in any colon cancer cell line, but this was not surprising due to the low receptor expression detected by binding studies. In DLD1 cells, chosen as paradigmatic of colon cancer cells for their intermediate differentiated phenotype, cAMP levels were inhibited by Cl-IB-MECA and MRE 3008F20 was able to antagonize this effect. Conversely, the non selective agonist NECA induced a stimulation of cAMP levels in both DLD1 and HT29 cells, that was inhibited by SCH 58261. In addition the presence of functional A2B receptors was revealed by using NECA in the presence of a highly selective A2A antagonist to selectively stimulate A2B receptors in HT29 cells (Feoktistov and Biaggioni, 1998; Gessi et al., 2005). Starting from the observation that adenosine could be detected in the interstitial fluid surrounding a carcinoma (Blay et al., 1997), a growing body of literature indicates that, depending upon the experimental conditions, adenosine may be either toxic and inhibit cell growth or alternatively stimulate cellular proliferation (Merighi et al., 2003a). Therefore in order to ascertain the potential effects of this nucleoside on colon carcinoma, we choose human colon cancer cell lines at different degrees of differentiation such as Caco2, DLD1 and HT29 showing a well, intermediate and low differentiated aspect, respectively. Interestingly, binding experiments performed in this work, revealed a differential expression of adenosine receptors in these cell lines (A1 and A3 in Caco 2 cells; A1, A2A, and A3 in DLD1 cells and A1, A2A, A2B, and A3 in HT29 cells) rending this panel of cells an interesting model to investigate the contribution of each adenosine subtype in cell growth. The results of this study indicate that adenosine induces a dose dependent stimulatory effect in Caco2, DLD1 and HT29 cells with an EC50 in the range 3–12 µM. The proliferative curve showed biphasic effects with a reduction of cell proliferation from its maximum at millimolar concentrations. Other authors found that high adenosine levels may be responsible for apoptosis or necrosis in various cell types, as a consequence of either activation of extracellular adenosine receptors as well as intracellular action, depending upon the cell type (Hashemi et al., 2005). It is possible that a balance between proliferative and apoptotic effects take place at millimolar doses of adenosine, however in our experimental conditions we did not reveal any induction of cell death in the cell lines investigated.
The proliferative effect was due to stimulation by adenosine and not by its metabolic product as demonstrated in the presence of EHNA in agreement with previous results (Mujoomdar et al., 2003, 2004). Interestingly, addition of EHNA alone was responsible of a slight significant cell growth effect suggesting a tonic proliferative effect induced by endogenous adenosine. Accordingly with finding reporting that adenosine breakdown in solid tumors is regulated essentially by ADA (Blay et al., 1997) and similarly to what found in colon cancer cell lines (Lelievre et al., 1998a), we observed that addition of ADA mediated an inhibitory effect on cell proliferation, indicating again a tonic proliferative role played by the endogenous nucleoside. The adenosine-mediated stimulatory effect was reduced by MRE 3008F20 100 nM suggesting the involvement of the A3 subtype in Caco2, DLD1, and HT29 cells. Therefore to corroborate this finding we tested the enhancing effects of Cl-IB-MECA on colon cancer cell growth in the absence and in the presence of ADA. Only after removal of endogenous adenosine it was detected an A3-dependent stimulatory effect of colon carcinoma cells proliferation, in line with a previously reported tonic cytoprotective role attributed to a basal activation of A3 receptors (Yao et al., 1997; Jacobson, 1998; Gao et al., 2001). The lack of efficacy of Cl-IB-MECA to stimulate proliferation without ADA at variance with the effect of exogenous adenosine may be explained by different mechanisms. The first is that a stable analogue may produce very different effects than adenosine on the same cell type, even under the same assay conditions, as suggested by Blay and colleagues (Mujoomdar et al., 2003). Alternatively, it is also possible that adenosine acts at the cell surface to modulate proliferation through an extracellular site, as suggested by our flow cytometry experiments in the presence of dypiridamol, or an unconventional receptor, as previously underlined by Blay and other authors (Tan et al., 2004); in this case exogenous adenosine and Cl-IB-MECA in colon cancer cells would act through different sites. In order to shed light on this issue novel experiments in the presence of a lower dose of the A3 antagonist (10 nM) were performed. In our condition MRE 3008F20 10 nM inhibited the proliferative effect induced by Cl-IB-MECA but not that induced by exogenous adenosine, indicating that in colon cancer cells there was a tonic activation of the A3 subtype by endogenous adenosine that was mimicked by Cl-IB-MECA and blocked by MRE 3008F20, whilst the exogenous nucleoside was acting probably not through the involvement of the A3 receptor. Even though MRE 3008F20 100 nM shows high selectivity for A3 subtype in binding and cAMP assays, in the case of cell proliferation it may affect mechanisms also independent by A3 receptor activation (Merighi et al., 2003b).
As for Cl-IB-MECA in the high micromolar range we observed an inhibitory effect on cell proliferation that was in agreement with literature reports. In this respect several hypotheses may be raised: (a) a recent paper by our group demonstrated that Cl-IB-MECA at micromolar doses inhibit cell proliferation and this effect was reduced by silencing the receptor, supporting a role for the A3 subtype (Merighi et al., 2005a); (b) it has been previously reported that IB-MECA at micromolar doses in breast cancer cells inhibit cell proliferation through interaction with receptors different from adenosine subtypes such as estrogen receptor α (Lu et al., 2003, 63: 6413–6423); (c) at micromolar doses Cl-IB-MECA loses its selectivity for A3 receptors and the complicating presence of other adenosine subtypes might be involved in the final response; (d) the difference between the effects induced by low and high doses of Cl-IB-MECA could be attributed to the receptor desensitization of A3 receptors that has been demonstrated by other authors in various cell systems (Trincavelli et al., 2002).
In addition to the traditional effector systems like adenylyl cyclase and phospholipase C, recently it has been reported that A3 adenosine receptors can also activate MAPK kinases. The MAPK family consists of three main subgroups, the extracellular signal-regulated kinases ERK1/2, the c-jun N terminal kinases and the stress-activated protein kinase p38. In particular ERK1/2 play an important role in cell proliferation of cancer cells and it has been demonstrated that all adenosine receptors mediate the phosphorylation of ERK1/2 into CHO cells (Schulte and Fredholm, 2000). A3 receptors-mediated ERK1/2 activation involved pertussis toxin-sensitive Gi/G0 proteins, phosphatidylinositol 3-kinase and tyrosine kinase activation, whilst it was not dependent by protein chinase C (Graham et al., 2001). PI3K pathway was also activated by A3 subtype in rat basophilic leukemia cells (Gao et al., 2001). Further studies in CHO cells demonstrated that A3 receptor stimulation trigger a signalling involving βγ release from Gi/G0, PI3K, Ras, and MEK to induce ERK 1/2 phosphorylation, (Schulte and Fredholm, 2002). Growing evidence suggest that ERK1/2 pathway plays an important role in the pathogenesis, progression and oncogenic behavior of human colorectal cancer (Fang and Richardson, 2005). Therefore we evaluated the A3-mediated stimulation of ERK/MAPK pathway in colon cancer cell lines. The involvement of ERK1/2 kinases on Cl-IB-MECA-induced cell proliferation was investigated by using a specific inhibitor of MEK, which is the upstream kinase for ERK1/2. Pretreatment of colon cancer cells with increasing concentrations of U0126 reduced the DNA synthesis induced by CL-IB-MECA, as measured by thymidine incorporation and cell cycle analysis. ERK1/2 activation was also confirmed by immunoblot analysis revealing MAPK phosphorylation. This effect was rapid and transient as it returned almost to the control levels within 1 h of treatment, according to which found in transfected cells (Schulte and Fredholm, 2000, 2002). Therefore this is the first study reporting the physiological role of A3 receptors-mediated activation of ERK1/2 pathway in the regulation of colon cancer cell proliferation. In conclusion, in line with literature data reporting that adenosine may promote cancer cell proliferation (Mujoomdar et al., 2003, 2004), stimulate angiogenesis (Montesinos et al., 2004), HIF activation (Merighi et al., 2005b), and inhibit anti-tumor immune response (MacKenzie et al., 1994), our current data suggest that endogenous adenosine in colon cancer cells behaves like a stimulator of tumor growth, through the involvement of the A3 adenosine subtype and ERK1/2 phosphorylation and that in colon cancer cell lines there exist a tonic stimulatory effect on cell proliferation that is mediated by A3 receptor activation.




