A co-clustering model involving α5β1 integrin for the biological effects of GPI-anchored human carcinoembryonic antigen (CEA)
Abstract
CEA functions as an intercellular adhesion molecule and is up-regulated in a wide variety of human cancers, including colon, breast and lung. Its over-expression inhibits cellular differentiation, blocks cell polarization, distorts tissue architecture, and inhibits anoikis of many different cell types. Here we report results concerning the molecular mechanism involved in these biological effects, where relatively rapid molecular changes not requiring alterations in gene expression were emphasized. Confocal microscopy experiments showed that antibody-mediated clustering of a deletion mutant of CEA (ΔNCEA), normally incapable of self binding and clustering, led to the co-localization of integrin α5β1 with patches of ΔNCEA on the cell surface. Activation of α5, as defined by an anti-α5 mAb-sensitive increase in cell adhesion to immobilized fibronectin, and an increased binding of soluble fibronectin to cells, was also observed. This was accompanied by the recruitment of integrin-linked kinase (ILK), protein kinase B (PKB/Akt), and the mitogen-activated protein kinase (MAPK) to membrane microdomains and the phosphorylation of Akt and MAPK. Inhibition of PI3-K and ILK, but not MAPK, prevented the α5β1 integrin activation. Conversely, anti-α5 antibody inhibited the PI3-K-mediated activation of Akt, implying the involvement of outside-in and inside-out signaling in integrin activation. Therefore we propose that CEA-mediated signaling involves clustering of CEA and co-clustering and activation of the α5β1 and associated specific signaling elements on the internal surfaces of membrane microdomains. These changes may represent a molecular mechanism for the biological effects of CEA. J. Cell. Physiol. 211: 791–802, 2007. © 2007 Wiley-Liss, Inc.
The glycophosphatidyl-inositol (GPI) anchored cell surface glycoproteins CEA and CEACAM6 belong to the CEA subfamily within the immunoglobulin superfamily and are over-expressed in a variety of human carcinomas, including colon, breast and lung (Chevinsky, 1991; Hammarstrom, 1999). CEA family members function in vitro, at least, as homotypic intercellular adhesion molecules (Benchimol et al., 1989; Oikawa et al., 1989; Stanners and Fuks, 1998). In a variety of in vivo and in vitro model systems, our laboratory has demonstrated that de-regulated over-expression of CEA and CEACAM6 leads to inhibition of cellular differentiation (Eidelman et al., 1993; Screaton et al., 1997; Taheri et al., 2003) and disruption of cell polarization and tissue architecture (Ilantzis et al., 2002). Anoikis, an apoptotic process initiated by the absence of cell-ECM contacts, is also inhibited by CEA and CEACAM6 over-expression in multiple cell types, including human colorectal carcinoma cells (Ordonez et al., 2000; Soeth et al., 2001), and human pancreatic carcinoma cells (Duxbury et al., 2004a, b).
What molecular events initiated by CEA/CEACAM6 expression underlie these major changes in cellular state? GPI-anchored proteins are often concentrated in small membrane microdomains in the plasma membrane (membrane rafts; Simons and Ikonen, 1997; Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998; Sharma et al., 2004) where cytoplasmic elements implicated in signal transduction are co-localized (Stefanova et al., 1991; Brown, 1993; Rodgers et al., 1994). Localized signaling has been observed when GPI-anchored proteins present in membrane rafts are clustered with antibodies or by physiologically relevant ligands (Janes et al., 1999; Kramer et al., 1999; Stuermer et al., 2001; Suzuki et al., 2001; Duxbury et al., 2004a).
Previous studies showed that the critical structural features of CEA necessary for its differentiation-blocking activity are external domains capable of self-binding linked to a processed carboxy terminal domain that determines a specific GPI anchor (Screaton et al., 2000). The importance of self-binding of the extracellular domains was shown by the expression of a CEA mutant lacking a major portion of the N-domain (ΔNCEA), including the subdomains required for self-binding. This mutant was completely ineffective in blocking myogenic differentiation but, by cross-linking with specific anti-CEA antibodies, regained this activity (Taheri et al., 2003). Based on these results we have proposed the following model: cell surface CEA–CEA binding can activate signaling by clustering CEA molecules and thereby the membrane microdomains they inhabit (Screaton et al., 2000); cytoplasmic signaling molecules associated with these microdomains are thus clustered, facilitating their activation. This activation leads to perturbations in cell–ECM interactions leading to changes in cell and tissue state, including differentiation, architecture and anoikis.
In certain cell systems, association of GPI-linked proteins with cell surface integrin receptors is involved in the activation of intracellular signaling (Ossowski and Aguirre-Ghiso, 2000; Ahmed et al., 2003; Chaurasia et al., 2006; Tarui et al., 2006). Such signaling can lead to changes in cell proliferation, differentiation and survival (Giancotti and Ruoslahti, 1999). Previous evidence supports the role of α5β1 integrin, the main receptor for the ECM component, fibronectin, in the CEA-mediated inhibition of differentiation and anoikis in vitro (Ordonez et al., 2007). The latter work demonstrated that CEA/CEACAM6 over-expression on the cell surface leads to aberrant cell–ECM interactions through increased binding of integrin α5β1 to fibronectin. Blockage of the α5β1-fibronectin interaction using specific mAbs against either α5β1 or fibronectin reversed CEA/CEACAM6-mediated inhibition of cell differentiation and anoikis (Ordonez et al., 2007), indicating a central role for this particular integrin-ligand combination.
Integrins can assume various affinity states that can be regulated by bidirectional signaling (Hynes, 2002; Qin et al., 2004), that is, either by “outside-in” signaling, induced by extracellular factors, or “inside-out” signaling, where intracellular events involving the cytoplasmic domains of α and β integrin subunits lead to extracellular conformational changes that determine the “affinity” for ligands (Shimaoka et al., 2002; Humphries et al., 2003; Campbell and Ginsberg, 2004; Ginsberg et al., 2005) and/or clustering of integrin receptors on the cell surface, termed “valency” changes (Hato et al., 1998; Carman and Springer, 2003; Cluzel et al., 2005). This is essentially a positive feedback system that can ensure robust activation and subsequent downstream signaling.
In the case of CEA-mediated signaling, presented in the current study, we focused on very early events, occurring within minutes after antibody-mediated clustering of CEA, on the assumption that such events are more likely to reflect direct consequences of CEA action which mimic the aggregation of CEA on the surface of CEA-expressing tumor cells. Rapid events would mainly involve changes in phosphorylation state and intracellular localization. We report here that cross-linking of the adhesion-incapacitated ΔNCEA expressed in several cell types induced a rapid activation of the α5β1 integrin receptor, consistent with previous results.
Our choice of certain signaling elements, amongst the plethora of elements that can affect cellular state, for candidates as downstream targets of α5β1 activation was governed by the assumption that early changes in cell surface-associated elements (the localization of CEA) would most likely be linked to the initiation of signaling activation determining cell state. In particular, surface-localized elements that have previously been shown to be functionally linked with integrin α5β1 were carefully examined. These include integrin-linked kinase (ILK), a ser/thr kinase that binds to and phosphorylates the cytoplasmic tail of the β1 integrin subunit (Hannigan et al., 1996), a modification known to cause a functional activation of the α5β1 integrin due to increased cell surface integrin clustering (Wu et al., 1998). Over-expression of ILK in rat intestinal epithelial cells thereby increases fibronectin matrix assembly (Wu et al., 1998) and inhibits anoikis (Attwell et al., 2000) and, in C2C12 mouse skeletal myoblasts, leads to the inhibition of myogenic differentiation (Huang et al., 2000), resembling quite closely the phenotype of CEA/CEACAM6-expressing L6 myoblasts. We report here that ILK shows rapid modifications after ΔNCEA cross-linking that are required for α5β1 activation.
Since ILK is modulated by the phosphoinositide 3-kinase (PI3-K) (Delcommenne et al., 1998), the PI3-K/Akt signaling cascade was also investigated. These elements also showed rapid changes that were necessary for integrin activation. Finally, the mitogen-activated protein kinase (MAPK) pathway was studied and shown to be activated by ΔNCEA cross-linking via the increase in integrin α5 activity.
Thus, our data supports a model whereby GPI-anchored CEA clustering leads to α5β1 integrin activation, which involves the activation the PI3-K/ILK/Akt and MAPK signaling pathways which, in turn, may mediate CEA inhibitory effects on cell differentiation and anoikis.
MATERIALS AND METHODS
Antibodies and inhibitors
The following antibodies were used: anti-integrin α2 monoclonal antibody (H-293), and anti-integrin α5 mAbs, HMα5-1, (partial blocking Ab which recognizes rat integrin α5 subunit) and H-104 (reacts with integrin α5 of mouse, rat, and human); polyclonal goat anti-Fibronectin Ab, C-20, polyclonal goat anti-ILK Ab, C-9, and mouse anti-NCAM mAb, 123C3, all purchased from Santa Cruz Biotechnologies, Santa Cruz, CA. Integrin function-blocking human anti-integrin α5 mAb, 11A1, anti-integrin α2 mAb, P1E6, and rat anti-integrin α2 mAb, Hα1/29, were purchased from BD Pharmingen, Mississauga, ON. Mouse anti-ILK mAb (clone 3) which recognizes human, rat and mouse ILK, was obtained from BD Transduction laboratories. Rabbit anti-Akt polyclonal antibody (detects total, phosphorylation-state independent Akt1, Akt2, and Akt3 proteins), anti-phospho Ser 473 Akt polyclonal Ab (detects endogenous levels of Akt1 only when phosphorylated at Ser473 and also Akt2 and Akt3 when phosphorylated at the corresponding residues), anti-p44/42 MAPK (Erk1/Erk2; detects total levels of endogenous p44/42 MAPK kinase), and anti-phospho-p44/42 MAPK (Erk1/Erk2; Thr183/Tyr185; detects endogenous levels of p42 and p44 MAPK only when activated by phosphorylation at Thr183 and Tyr185 of rat Erk2), were purchased from Cell Signaling, New England Biolabs, Ipswich, MA. The mouse-anti CEA mAbs, J22, and D13, were generated in the laboratory of Dr. A. Fuks (McGill University, Montreal, PQ, Canada; Zhou et al., 1993). J22 binds to internal domains of CEA (Zhou et al., 1993), while the epitope of D13 is in the N domain of CEA (Taheri et al., 2000). The pharmacological inhibitors PD098059 (MAPK specific inhibitor) and LY294002 (PI3-K specific inhibitor) were purchased from Sigma, St. Louis, MI.
Cell lines and culture conditions
L6 rat myoblasts (Yaffe, 1968), the Caco-2 human colorectal carcinoma cell line (both obtained from ATCC, Rockville, MD) and CHO-derived LR-73 (LR) fibroblasts (Pollard and Stanners, 1979), were grown in monolayer cultures in DMEM (L6) or α-MEM (Caco-2 and LR-73) containing 10% fetal bovine serum (FBS, Invitrogen, Burlington, ON) supplemented with 100 µg/ml streptomycin and 100 U/ml penicillin (Invitrogen; growth medium: GM), at 37°C in a humidified atmosphere with 5% CO2. Cultures of subconfluent proliferating cells were used for all experiments.
Transfectant cells
L6, LR-73, and Caco-2 cells were transfected by the calcium phosphate-mediated co-precipitation method (Screaton et al., 2000; Taheri et al., 2003). Briefly, stable G-418 resistant colonies of L6 and LR-73 were obtained after co-transfection with the p91023B expression vector (Screaton et al., 2000) containing cDNA coding for the CEA deletion mutant ΔNCEA [lacking the last 75 amino acids of the N domain (Oikawa et al., 1991)] and the pSV2neo plasmid as a dominant selectable marker (L6 and LR cells) (Taheri et al., 2003). The LR-73(ΔNCEA) transfectants represented a pool of such colonies selected by FACS for higher cell surface expression whereas the L6(ΔNCEA) transfectant was a single clone. Double LR and L6 transfectants (ΔNCEA + NCAM) were obtained by co-transfection of both NCAM, a GPI-anchored variant with a muscle-specific domain (Peck and Walsh, 1993) and ΔNCEA cDNA with pSV2(neo) (LR cells); L6(ΔNCEA) transfectant cells were supertransfected with NCAM cDNA and 0.5 µg of pBabe (puro). Resistant clones were pooled after selection with 400–600 µg/ml neomycin (G418; GIBCO BRL, Burlington, ON) or 1 µg/ml puromycin (Sigma), respectively. Pooled Caco-2(ΔNCEA) stable transfectant cells were obtained (Ilantzis and Stanners, unpublished results) using the PML1 Zn2+-inducible episomal expression vector containing the mouse metallothionein promoter (mMT1) and the hygromycin-B resistance gene as a selectable marker (Lukashev et al., 1994). Cell surface expression of ΔNCEA in LR-73, L6, and Caco-2 was assessed by FACS analysis; all cells were labeled with monoclonal anti-CEA antibody (J22), giving FACS mean values of 450, 732, and 823, respectively. LR-73 and L6(ΔNCEA + NCAM) double transfectants showed FACS mean values of 383, 530 for ΔNCEA, and 295, 160 for NCAM, respectively.
ΔNCEA cross-linking and cell pretreatment
Cultures of ΔNCEA transfectants and ΔNCEA+NCAM double-transfectant cells were incubated for 5 min in serum-free medium at 37°C with 30 µg/ml mouse anti-CEA mAb (J22) or mouse anti-NCAM antibody, followed by 5 min with secondary donkey anti-mouse IgG antibody (minimal cross-reaction with other species; Jackson, Burlington, ON ImmunoResearch Laboratories, Inc.). For the soluble fibronectin cell-binding assay, cells were incubated with only the primary anti-CEA mAb. As controls, in all experiments, ΔNCEA and double-transfectant (ΔNCEA + NCAM) cells were incubated with isotype-matched mouse IgG anti-CEA mAb D13, whose N domain epitope is deleted in ΔNCEA, followed by secondary donkey anti-mouse IgG. For integrin inhibition experiments, L6(ΔNCEA) cells were pre-incubated with 5 µg/ml of functional blocking mouse anti-integrin α5 (HM1α-5) or α2 (Hα1/29) mAbs, for 30 min at 37°C in serum-free medium. Caco-2(ΔNCEA) cells were pre-treated with human anti-integrin α5 (11A1) or α2 (P1E6) mAbs.
For kinase inhibition experiments, cells were treated in serum-free medium at 37°C with either PD098059 (MAPK specific inhibitor) at 30 µM or LY294002 (PI3-K specific inhibitor) at 50 µM for 30 min prior as well as during antibody-mediated ΔNCEA cross-linking. Control cells were treated with media containing DMSO vehicle alone (kinase inhibitor solvent used at 0.1%). Cell viability following incubation with kinase inhibitors was evaluated using trypan blue dye exclusion, with greater than 90% viable cells.
Confocal microscopy experiments
On day 0.7 × 103 L6(ΔNCEA) myoblast transfectants were seeded per well in 8-well Lab-Tek Permanox Chamber Slide plates (Nalge Nunc, Inc., Naperville, IL). After growth in GM for 48 h, the cells were processed for visualization of membrane proteins. Cells were rinsed twice with PBSF (PBS + 2%FBS) and labeled with 5 µg/ml of monoclonal mouse antibody specific for ΔNCEA (J22) together with anti-α5 (HMα5-1) or anti-α2 (Hα1/29) integrin mAbs for 20 min at 37°C. After unbound antibody was rinsed off, primary antibodies were clustered with secondary antibodies coupled to fluorescent dyes: rhodamine-conjugated goat anti-Armenian hamster IgG (minimal cross-reaction to bovine, human, mouse, rabbit, and rat serum proteins) and FITC-coupled goat anti-mouse IgG antibodies (Jackson) for 20 min at 37°C. After washing with PBSF the cells were fixed by 5 min incubation with 4% paraformaldehyde (PFA) at room temperature (RT). Control cells were fixed with 4% PFA before incubation with primary and secondary antibodies, or after primary antibody incubation. Negative controls were performed by incubation of cells with primary anti-mouse or anti-hamster mAbs followed by secondary anti-hamster or anti-mouse Abs, respectively. Also, cross-reactivity among secondary mAbs, as well as staining specificity was assessed using appropriate controls. Images were captured using a Zeiss Pascal 5 microscope with a Plan-Apochromat 63×/1.4 oil DIC objective. Image analysis was done using Zeiss LSM image browser.
ILK gene silencing with small interfering RNA (siRNA)
2 × 105 LR-73(ΔNCEA) transfectant cells were seeded in 100 mm tissue culture dishes and co-transfected 24 h later, by calcium phosphate-mediated co-precipitation, with the pSilencer 2.1-U6 siRNA expression vector (Ambion, Austin, TX) containing a human U6 RNA pol III promoter (Kunkel and Pederson, 1989; Myslinski et al., 2001) a puromycin resistance gene, and a hairpin ILK siRNA. The human ILK gene sequence specifically targeting the integrin binding domain (ILK-A; 5′-GACGCTCAGCAGACATGTGGA-3′) was used (Tan et al., 2004). Human and mouse ILK-A amino acid sequences shared 100% sequence homology. A universal scrambled siRNA control sequence with no significant homology to mouse, rat or human gene sequences provided by the Silencer siRNA construction kit was used as a negative control. LR-73 transfectants were isolated by selection with puromycin (2 µg/ml, Sigma). After 10–14 days, resistant clones were evaluated for ILK expression levels. Densitometric analysis of western blots as detailed before, with the ImageJ program (http://rsb.info.nih.gov/ij/) showed that 3 out of 50 clones tested expressed about 70%, 50%, and 33% of control siRNA ILK protein levels (after ILK expression was normalized to β-actin expression levels).
Isolation of membrane raft-containing fractions
Raft membrane microdomains were isolated by isopycnic sucrose density gradient centrifugation at 4°C. 30 × 106 L6(ΔNCEA) cells were collected from T180 flasks with non enzymatic detachment using PBS-citrate (Screaton et al., 2000) at 37°C and then suspended in 1% Brij 98—containing lysis buffer (20 mM Tris-HCl pH 8, 140 mM NaCl, 2 mM EDTA pH 8; protease inhibitors, aprotinin and leupeptin at 10 µg/ml each, PMSF at l00 µg/ml; and phosphatase inhibitors, Na3VO4 at 1 mM plus 10 mM NaF) for 30 min at 4°C. The cell lysates, syringed through a 27-gauge needle to ensure proper homogenization, were adjusted to 40% sucrose using cold 80% sucrose buffer (10 mM Tris-HCl pH 8, 140 mM NaCl and 2 mM EDTA pH 8). A sucrose step gradient was prepared in a polyallomer tube (11 mm × 60 mm, Beckman, Mississauga, ON) by successive addition of 2 ml of the 40% sucrose cell lysate, 2 ml of 35% sucrose and 1 ml of 5% sucrose all in sucrose buffer. The gradient was centrifuged at 45,000 rpm for 18 h at 4°C. Fractions (350 µl each) were sequentially harvested from the top. Sucrose concentrations were determined by an Abbes' refractometer. Membrane fractions corresponding to the 5–35% sucrose interface (visually detected as a band in the density gradient) were assessed by dot blot for the presence of ganglioside GM1, using cholera toxin B subunit peroxidase conjugate (Sigma), to confirm the proper identification of the membrane fractions.
Cell binding of soluble fibronectin
On day 0, L6(ΔNCEA) cells were seeded at 104 cells/cm2 in a 96-well tissue culture plate. Two days later, the cells were washed with PBS + % FBS (PBSF), and then cross-linked with anti-CEA J22 antibody for 5, 15, 30, and 60 min in serum-free DMEM in the presence of 10 µg/ml of human fibronectin (Fn; BD Biosciences, Bedford, MA) at 37°C. As a control L6(ΔNCEA) cells were simultaneously treated with isotype-matched IgG mouse anti-CEA D13 antibody in the presence of soluble fibronectin for the same lengths of time. Fn bound to cells was evaluated by ELISA as follows: cells were washed three times with PBSF, then fixed with 4% formaldehyde; to prevent antibody non-specific interactions, cells were incubated for 1 h at RT with 3% BSA (Sigma) in PBS (PBSB), followed by incubation for 90 min at RT with goat anti-fibronectin antibody C-20 at a dilution of 1:100 in PBSB; cells were then washed with PBSF, and incubated for 1 h with HRP-conjugated rabbit anti-goat IgG secondary antibody (Jackson) at a dilution of 1:2500 in PBSB. After rinsing the cells with PBSF twice, 2,2-Azino-bis(3-ethylbenzathiazoline-6-sulfonic acid) (ABTS, Sigma)-ELISA staining was performed: cells were incubated with ABTS solution (0.1 M citrate-PO4, pH 4 and 3% hydrogen peroxide). Bound Fn was assessed from the absorbance determined with a microplate reader at 405 nm, with a reference wavelength of 490 nm.
Cell adhesion to extracellular matrix (ECM) components
On day 0, L6(ΔNCEA), L6(ΔNCEA + NCAM) and Caco-2(ΔNCEA) transfectant cells were seeded at a density of 104 cells per cm2. On day 2, subconfluent cultures were subjected to anti-CEA antibody cross-linking (primary anti-CEA mAb J22 followed by donkey anti-mouse secondary Ab), detached from Petri dishes with PBS-citrate (Screaton et al., 2000), and added to 96-well tissue culture plates pre-coated with fibronectin (Fn), vitronectin (Vn), laminin (Ln), collagen I (C I) or collagen IV (C IV) (CytoMatrix adhesion kit, CHEMICON, Temecula, CA) for 1 h at 37°C. Staining of adherent cells to the ECM components was assessed according to the manufacturer's protocol. Briefly, cells attached to the wells were stained for 5 min with 0.2% crystal violet in 10% ethanol. Wells were then gently washed three times with PBS. Solubilization buffer (a 50/50 mixture of 0.1 M NaH2PO4, pH 4.5 and 50% ethanol) was added for 5–10 min. The optical density, which increases with increased cell binding, was determined at a wavelength of 540 nm using a microplate reader.
Western blot analysis
Total cell lysates or sucrose gradient fractions were subjected to SDS–PAGE and transferred electrophoretically to 0.45 µm PDVF membranes (Millipore, Mississauga, ON). Western blots were evaluated using antibodies specific to α5 and α2 integrins, CEA, ILK, Akt, and MAPK before and after anti-CEA antibody-mediated cross-linking of ΔNCEA. Following extensive washing, blots were developed with HRP-conjugated antibodies and visualized using ECL reagent according to the manufacturer's instructions (Amersham Life Sciences, Piscataway, NJ).
Statistical analysis
Statistical comparison of means between groups was performed using the two-tailed unpaired Student t test. P < 0.05 was considered significant.
RESULTS
Antibody-mediated ΔNCEA clustering increases integrin α5 activity
Previous results (Ordonez et al., 2007) implicated α5β1 integrin perturbation in the inhibitory effects of CEA on cell differentiation and anoikis. An increase in cell adhesion to Fn and enhanced Fn matrix assembly was observed in a variety of cell lines upon CEA/CEACAM6 over-expression (Ordonez et al., 2007). We therefore measured the kinetics of the change in the intensity of α5β1-fibronectin interaction after antibody-mediated cross-linking of ΔNCEA in L6(ΔNCEA) cells, by assessing the binding of purified soluble Fn to cells as well as the cell adhesion to immobilized Fn. Cross-linking of ΔNCEA showed a relatively rapid increase (within 5 min) of Fn binding to the cells, reaching maximum levels after 15 min (Fig. 1A). The latter increase was not observed using the control mAb, D13, in which the epitope is missing in ΔNCEA; therefore the increase in Fn binding with time was not due to slow saturation of cellular binding sites by the added Fn.
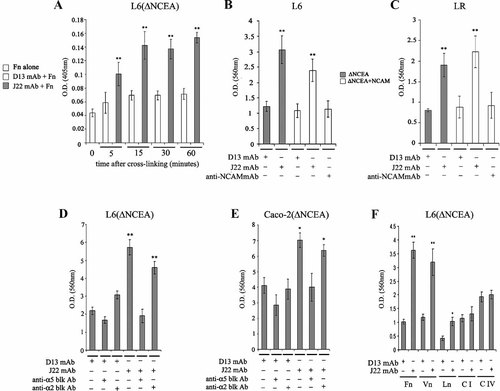
Activation of α5β1 integrin as a consequence of CEA clustering. Subconfluent cultures of L6(ΔNCEA; A, B, D, F), L6(ΔN + NCAM; B), LR-73(ΔN; C), LR-73(ΔN + NCAM; C) and Caco-2(ΔNCEA; E) transfectants were cross-linked with mouse anti-CEA mAb antibody (J22) without (A) or with (B, C, D, E, F) secondary anti-mouse IgG Ab, as described in M&M, and then subjected to binding of soluble Fn for various times (A) or to cell adhesion to immobilized Fn (B, C, D, E, F), Vn, Ln, C I and C IV (F). Control cells were treated with an isotype-matched mouse IgG anti-CEA mAb (D13) that binds to an epitope in the N domain of CEA that is deleted in ΔNCEA. The time course of soluble Fn binding to cross-linked L6(ΔNCEA) cells shows a rapid increase, with the maximum level of binding being reached 15 min after cross-linking (A). ΔNCEA cross-linking increases cell adhesion of L6(ΔNCEA; B, D), LR(ΔNCEA; C) and Caco-2(ΔNCEA; E) cells to immobilized Fn. In contrast to ΔNCEA cross-linking, NCAM cross-linking in double transfectant cell lines L6(ΔNCEA + NCAM; B) and LR(ΔNCEA + NCAM; C) did not show any increase in cell binding to immobilized Fn. Pre-treatment with blocking anti integrin α5 but not α2 mAbs reduced the increase in cell adhesion to Fn almost to control levels upon ΔNCEA cross-linking (D, E). Mean values, ±SD from a representative experiment out of at least three independent experiments are shown (*P < 0.003, **P < 0.002).
A similar increase in binding of L6(ΔNCEA) cells to immobilized Fn within 15 min of cross-linking was observed, whereas no such increase was found utilizing the control anti-CEA mAb D13 directed against an epitope missing in ΔNCEA plus secondary anti-mouse IgG antibody (Fig. 1B). This effect was also observed for ΔNCEA transfectants of the CHO fibroblast LR cell line (Fig. 1C) and the human colonocyte Caco-2 cell line (Fig. 1E), indicating the generality of the effect. In order to demonstrate the specificity of the ΔNCEA cross-linking effect, we used LR and L6 transfectant cell lines expressing both ΔNCEA and the GPI-anchored variant of the immunoglobulin superfamily member adhesion molecule, NCAM, which normally has no effect on cell adhesion to Fn (Ordonez et al., 2007). In contrast to the increase of cell adhesion to Fn observed upon ΔNCEA cross-linking in L6(ΔNCEA) and L6(ΔNCEA + NCAM) cells (Fig. 1B), NCAM cross-linking did not show any increase in cell adhesion to Fn. Similar results were obtained for LR-73(ΔNCEA + NCAM) cells (Fig. 1C). Thus the increase in binding to Fn was not due to non-specific reorganization of cell surface elements due to ΔNCEA cross-linking.
To demonstrate the involvement of the integrin α5 in this effect, both rat myoblast L6(ΔNCEA; Fig. 1D) and human colonocyte Caco-2(ΔNCEA; Fig. 1E) transfectants were pretreated with blocking anti-α5 or control anti-α2 mAbs, then assessed for binding to immobilized Fn. As shown in Figure 1D,E, incubation with integrin α5, but not α2 mAb, completely prevented the increase in cell adhesion to Fn after ΔNCEA cross-linking.
It has been previously described that ligand occupancy of integrin receptors can alter the function of other integrins present on the same cell surface (Diaz-Gonzalez et al., 1996; Blystone et al., 1999; Ly et al., 2003). Therefore, effects of CEA clustering on cell binding to other ECM components were investigated. Whereas no effect of antibody-mediated clustering of ΔNCEA in L6(ΔNCEA; Fig. 1F) and LR(ΔNCEA; not shown) transfectants on cell binding to immobilized collagens I and IV (major integrin receptor: α2β1) was observed, there was an increase in cell binding to Vn (αvβ3) and Ln (α6β4) (Fig. 1F). The effect on binding to Vn could be completely reversed by anti-α5 mAb treatment (not shown), which is presumably due to cross-talk between α5 and αv integrins. We have chosen to focus on the α5β1 integrin because of the complete reversal of the CEA-mediated Fn binding increase (Fig. 1D,E) and of the reversal of inhibition of anoikis by anti-α5 mAb (Ordonez et al., 2007).
ΔNCEA and integrin α5, but not integrin α2, co-cluster in membrane microdomains
Previous studies have demonstrated the functional role of cholesterol and sphingolipid-rich membrane microdomains as platforms for cellular signaling (Simons and Ikonen, 1997; Brown and London, 2000). Recent studies showed that association of integrins with these membrane rafts positively regulates integrin activity (Hogg et al., 2002; Leitinger and Hogg, 2002; Baron et al., 2003; Decker and ffrench-Constant, 2004; del Pozo et al., 2004). In order to evaluate whether changes in membrane microdomain localization of integrin α5 could correlate with the observed ΔNCEA cross-linking-mediated integrin α5 activation, isopycnic sucrose gradient centrifugation of cold Brij 98 L6(ΔNCEA) cell extracts was performed. Western blot analysis of ΔNCEA in the sucrose gradient fractions showed, as expected, ΔNCEA localization in low density sucrose fractions containing membrane microdomains, as confirmed by the presence of the ganglioside GM1, a marker of raft-type membrane microdomains (Fig. 2A). Integrin α5, but not α2, was also found in the same low density fractions (Fig. 2A). Thus both ΔNCEA and α5 integrin co-existed in membrane microdomains prior to antibody-mediated clustering. Western blot analysis of ΔNCEA and α5 integrin in the sucrose gradient fractions (Fig. 2A) as well as α5 cell surface expression levels by FACS analysis (Fig. 2B) did not show differences in cell surface levels after cross-linking. From these results, we suggest that ΔNCEA and α5 integrin could exist in the same membrane microdomain prior to activation, which co-cluster upon ΔNCEA cross-linking, leading to activation of integrin α5.
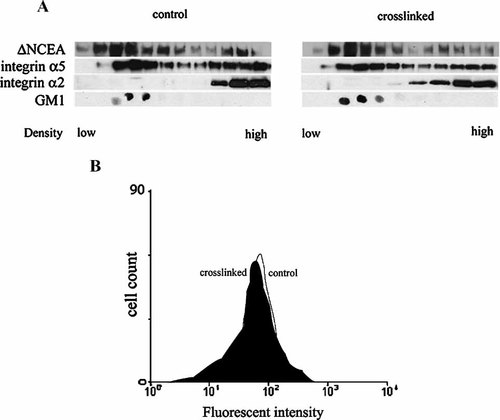
ΔNCEA and α5β1 integrin are both initially localized in membrane microdomains. L6(ΔNCEA) transfectant cells were lysed with cold 1% Brij-98 and subjected to isopycnic centrifugation in a sucrose density step gradient before and after 10 min of antibody-mediated CEA cross-linking. Twelve fractions (350 µl each) were collected from the top of the gradient. Gradient fractions were subjected to SDS–PAGE and Western blot analysis for α5 integrin and CEA; for GM1, dot blot detection was performed (A). Cytofluorometric analyses performed as previously described (Taheri et al., 2000) showed that, after ΔNCEA cross-linking, cell surface levels of integrin α5, remained the same (B). Three independent experiments were performed with similar results.
In support of this possibility, confocal microscopy experiments were performed. L6(ΔNCEA) transfectants were treated with either hamster anti-α5 (Fig. 3A,B) or hamster anti-α2 (Fig. 3C,D) mAbs together with mouse anti-ΔNCEA mAb at 37°C for 20 min; primary antibodies were then clustered at 37°C for another 20 min with FITC-conjugated goat anti-mouse Ab (green dye) to visualize ΔNCEA and rhodamine-conjugated rabbit anti-hamster Ab (red dye) to visualize the integrins; cells were fixed at RT at the end of the Ab labeling (Fig. 3B,D). The results show that, after cross-linking ΔNCEA, integrin α5 followed ΔNCEA into the same patches (Fig. 3B). Integrin α2, on the other hand, showed little co-localization with ΔNCEA and did not acquire the punctate staining pattern of ΔNCEA after cross-linking (Fig. 3D). When the experiment was performed with cells fixed either before any Ab treatment (Fig. 3A,C) or after only the primary Ab treatment (data not shown), in order to reveal pre-existing co-localization, integrin α5 showed some co-localization with ΔNCEA in very fine “particles,” whereas integrin α2 was more diffuse and showed much less co-localization. These results support the suggestion that co-clustering of integrin α5, but not of integrin α2, occurs as a result ΔNCEA cross-linking, probably because of their co-habitation of the same membrane microdomain.
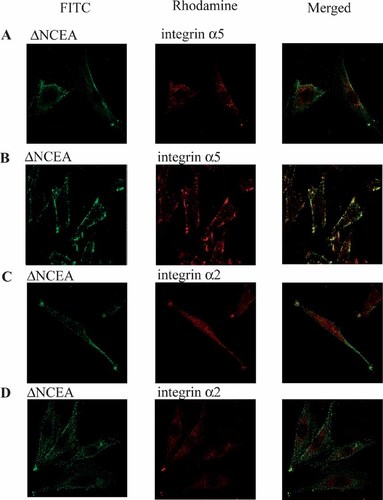
ΔNCEA cross-linking induces co-clustering of integrin α5 but not integrin α2 on L6 myoblast surfaces. L6(ΔNCEA) cells were labeled with primary anti-CEA (J22) mAb and integrin α5(HMα5-1; A, B) or α2(Hα1/29; C, D) mAbs for 20 min at 37°C. Primary antibodies were next clustered with FITC-conjugated goat-anti-mouse antibodies (green dye; ΔNCEA, left column) and with rhodamine-conjugated rabbit anti-hamster antibodies (red dye; integrins, middle column) for 20 min at 37°C and then fixed with 4% PFA at room temperature (B, D). Control cells where fixed prior to antibody labeling (A, C). Right column shows little colocalization of ΔNCEA with either α5 (A) or α2 (C) integrins before patching. Co-localization was observed by yellow patches for integrin α5 (B) but not for α2 (D) after patching. These results were repeated in three independent experiments.
Involvement of ILK in α5β1 integrin activation upon ΔNCEA cross-linking
The identity of cytoplasmic signaling elements that are rapidly activated in response to ΔNCEA clustering was then investigated. Since activation of ILK was previously shown to mimic closely the molecular and cellular consequences of up-regulation of CEA (see Introduction), ILK was the first such element to come under scrutiny. ILK was found to move into low density sucrose fractions within 5 min of antibody-mediated cross-linking of ΔNCEA in L6(ΔNCEA) transfectant cells (Fig. 4A). Reduction in ILK kinase activity by disassociation from membrane microdomains, that is, the opposite of the shift seen here, has been shown to be correlated positively with differentiation in other systems (Huang et al., 2000; Vespa et al., 2003).
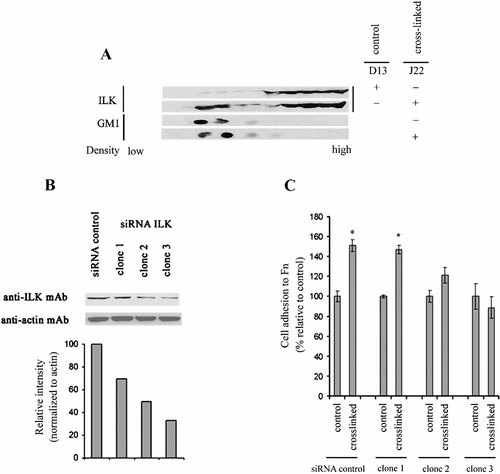
ILK activation is implicated in the increase of cell adhesion to immobilized fibronectin upon ΔNCEA cross-linking. Cross-linked L6(ΔNCEA) transfectants were lysed with cold 1% Brij 98 and subjected to isopycnic sucrose gradient centrifugation. SDS–PAGE and Western blot analyses were performed to detect the presence of ILK and GM1 in membrane microdomains fractions (A). In (B), three clones generated by stable ILK siRNA transfections in LR(ΔNCEA) cells were shown to “knockdown” about 30%, 50%, and 67% of the endogenous expression of ILK (ILK expression was normalized to β-actin expression levels). ILK siRNA-transfected LR-73(ΔNCEA) cells were removed from tissue culture plates by a non-enzymatic method (PBS-citrate) after ΔNCEA cross-linking. These transfectants showed reduced cell adhesion to Fn after ΔNCEA cross-linking directly related to their degree of reduction in ILK levels (C). The results are the mean of ±SD of a representative experiment out of three independent experiments (*P < 0.003).
The endogenous level of ILK was then reduced by stable expression of specific siRNA. Since ILK siRNA was poorly expressed in L6(ΔNCEA) cells, siRNA experiments were performed in LR(ΔNCEA) transfectants. Three LR-73(ΔNCEA) siRNA transfectant clones were isolated with reduced expression of ILK by 30%, 50%, and 67% of control levels, as shown by Western blotting (Fig. 4B). Relative to the siRNA control, ILK siRNA clones prevented the increased cell adhesion to immobilized Fn after antibody-mediated ΔNCEA cross-linking, which was directly related to the degree of reduction in ILK levels, with clone 3 at 67% reduction showing no increase in cell-Fn adhesion (Fig. 4C). These results indicate that ILK activation may be involved in the regulation of inside-out integrin activation upon CEA clustering.
Involvement of the PI3-K/Akt signaling pathway
The PI3-K/Akt cascade is a signaling pathway frequently altered in human cancers (Franke et al., 2003). Activation of PI3-K and Akt is implicated in the regulation of cell differentiation (Sayama et al., 2002; Peng et al., 2003), proliferation (Sayama et al., 2002) and apoptosis. Since ILK activation has been described to be downstream of PI3-K activation (Delcommenne et al., 1998), and Akt phosphorylation occurs in a PI3-K/ILK dependent fashion (Delcommenne et al., 1998; Persad et al., 2001; Troussard et al., 2003), we determined whether CEA clustering induced both Akt activation, by Ser 473 phosphorylation, and a rapid shift to membrane microdomain association. The dependence of ILK and Akt activation on PI3-K activity was also investigated using the specific pharmacological PI3-K inhibitor LY294002. Figure 5A shows that Akt rapidly shifts to membrane microdomains after ΔNCEA cross-linking in L6(ΔNCEA) cells and that this localization correlates with its phosphorylation at Ser473. The addition of LY294002 before ΔNCEA cross-linking blocked the shift of Akt (Fig. 5A) and ILK (Fig. 5B) into low density sucrose fractions, as well as increased Akt phosphorylation in total cell lysates (Fig. 5C). To examine whether these changes were linked with the status of integrin α5, cells were pre-treated with an anti-α5 integrin antibody. Inhibition of integrin α5 using antibodies strongly reduced Akt phosphorylation (Fig. 5D). No change in Akt phosphorylation was observed, however, upon reduction of ILK expression in the siRNA clones of Figure 4 (data not shown). Finally, we determined whether PI3-K inhibition by LY294002 prevented the increase of cell binding to Fn induced by ΔNCEA cross-linking. Figure 6 shows that, in L6 and Caco-2(ΔNCEA) cells, pre-treatment with LY294002 but not the MAPK phosphorylation-specific inhibitor, PD098059, reduced this increase and completely in the case of L6(ΔNCEA).
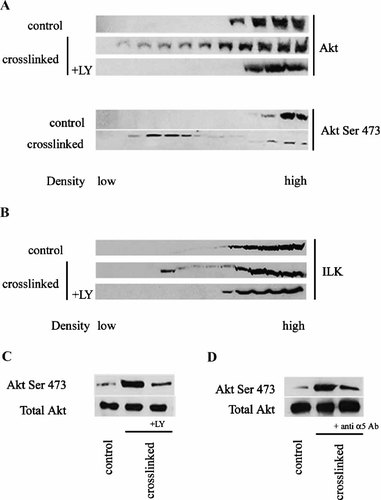
Integrin α5-mediated activation of AKT and ILK localization in membrane microdomains upon ΔNCEA clustering is prevented by PI3-K inhibition in L6 (ΔNCEA) cells. PI3-K inhibition prevented recruitment of Akt (A) and ILK (B) to membrane microdomains. Total expression levels of Akt and Akt phosphorylation on Ser 473 (A) or ILK (B) were evaluated after ΔNCEA cross-linking by western blots of isopycnic sucrose gradient fractions. Decreased Akt phosphorylation was observed after pre-treatment of 50 µM LY294002 (C) or anti-α5 integrin mAb (D) for 30 min at 37°C. This experiment was repeated three times with identical results.
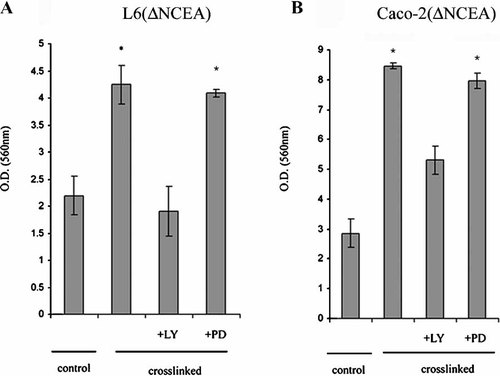
The CEA-mediated increase in cell adhesion to fibronectin was abrogated by inhibition of PI3-K but not by MAPK inhibition. L6 (ΔNCEA) (A) and Caco-2 (ΔNCEA) (B) cultures were incubated with inhibitors LY294002 at 50 µM and PD098059 at 30 µM for 30 min at 37°C before ΔNCEA cross-linking. Values shown are means ± SD for a representative experiment out of three independent experiments (*P < 0.002).
In summary, ILK and the PI3-K/Akt pathway appear to be involved bi-directionally in the activation of integrin α5 initiated by antibody-mediated ΔNCEA clustering, in that anti-α5 mAb treatment blocks the activation of Akt, and down regulation of ILK or PI3-K block the activation of α5.
The MAPK pathway is activated by ΔNCEA cross-linking/integrin α5 activation independent of PI3-K
Integrin regulation of cell differentiation and anoikis has also implicated the MAPK signaling cascade (Sastry et al., 1999; Howe et al., 2002). Interactions between the PI3-K and the Raf/MEK/Erk signaling pathways have been reported in multiple cell systems (Moelling et al., 2002; Levinthal and DeFranco, 2004), as either antagonistic (Rommel et al., 1999; Guan et al., 2000) or agonistic (King et al., 1997; Berra et al., 1998). In addition, the block of terminal myogenic differentiation by ILK was shown to be due to p44/p42 MAP kinase inactivation (Huang et al., 2000). We therefore decided to look for effects on MAPK activation as a result of CEA clustering. Although Figure 6 showed that pre-treatment with PD098059, but not the PI3-K specific inhibitor, LY294002, had no effect on the increase of cell binding to Fn after ΔNCEA cross-linking, we found that MAPK/Erk1/2 shifted to membrane microdomain localization within 10 min of cross-linking ΔNCEA in L6(ΔNCEA) cells (Fig. 7A). This observation correlated with MAPK/Erk1/2 activation, since total cell extracts showed a marked increase in MAPK p44/42 phosphorylation after cross-linking ΔNCEA in L6(ΔNCEA) cells, without a measurable increase in total MAPK levels in the same cell extracts (Fig. 7B). In addition, MAPK activation required integrin α5 activation, since L6(ΔNCEA) cells pretreated with an anti-α5 mAb before ΔNCEA cross-linking showed little if any increase in MAPK p44/42 phosphorylation (Fig. 7B), presumably due to the blockage of α5 integrin Fn binding activity (Fig. 1), thus implying a direct involvement of α5 integrin activation in this pathway. The latter demonstrated that unlike the PI3-K signal, MAPK appears to be uni-directional, since the increase in cell adhesion to Fn upon ΔNCEA cross-linking was not prevented by MAPK inhibition, but its downstream activation was abolished by pre-treatment with blocking anti-α5 mAb.
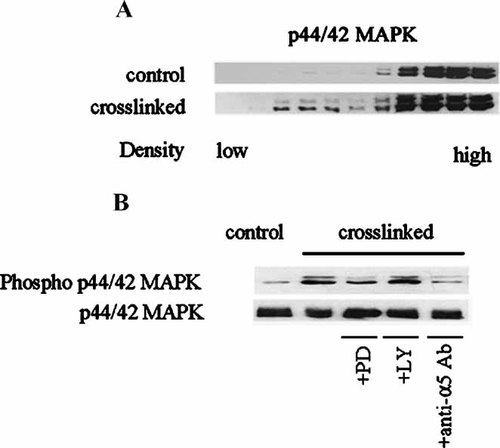
Inhibition of PI3-K by LY294002 has no effect on MAPK phosphorylation mediated by CEA cross-linking. L6(ΔNCEA) cells were cross-linked and subjected to isopycnic fractionation (A) or total cell extracts were obtained from cross-linked/pretreated L6(ΔNCEA) cells with PI3-K or MAPK inhibitors. MAPK was found to shift to membrane raft fractions after CEA cross-linking (A). Inhibition of PI3-K with LY294002 had no effect on the phosphorylation levels of MAPK (Ser 44 and Thr 42). The specific inhibitor of MAPK, was used as a positive control for the inhibition of MAPK phosphorylation. Cells pre-treated with anti-α5 integrin antibody at 37°C and then cross-linked for 10 min showed inhibition of MAPK phosphorylation (B). The effects of MAPK localization and activation were reproducible at least in three independent experiments.
Finally, in order to explore possible cross-talk between the PI3-K and the MAPK signaling cascade, we evaluated the effect of LY29002 on the activation of MAPK in L6(ΔNCEA) transfectants. PI3-K inhibition did not prevent the increase in phosphorylation of MAPK/Erk1/2 after ΔNCEA cross-linking (Fig. 7B), suggesting that MAPK signaling is a collateral pathway activated by CEA clustering independent of PI3-K signaling.
DISCUSSION
CEA structural and functional analysis implicated the importance of self-binding extracellular domains attached to the CEA-specific GPI anchor in blocking myoblast differentiation (Screaton et al., 2000). It was also shown that antibody-mediated cross-linking of a self-binding-incompetent CEA mutant, ΔNCEA, which we suggest mimics the CEA clustering due to cell surface concentration increases seen in human cancer, was sufficient to re-instate the CEA differentiation block (Taheri et al., 2003). This result afforded a method of synchronizing the molecular events initiated by the clustering of CEA and allowed the study of any early transient events not requiring changes in gene expression that could be involved in changing cell state. We show here that a relatively rapid (<5 min) activation of integrin α5β1 and accompanying changes in a group of signaling elements, are all set in motion by antibody-mediated cross-linking of ΔNCEA. Rapid re-localization of these elements to membrane microdomains was observed, which implies the existence of complexes of elements that can rapidly and efficiently undergo interdependent changes in activation state. Co-localization studies by confocal microscopy demonstrated that antibody-mediated clustering of ΔNCEA resulted in co-localization of integrin α5 (Fig. 3), consistent with the view that these proteins inhabit the same membrane microdomains, thereby recruiting and increasing the local concentration of attached cytoplasmic signaling elements above a threshold required for activation.
We demonstrated that two signaling cascades, previously shown to determine cellular attributes which mimic those observed with CEA expression, were activated upon ΔNCEA clustering: the PI3-K/ILK/Akt and MAPK cascades. We showed significant differences in how these two molecular pathways are integrated into the scheme of outside-in and inside-out integrin signaling resulting in the CEA-mediated build-up of integrin α5 activity. Altogether these observations establish a potential molecular mechanism involved in the tumorigenic effects of CEA.
The issue of integrin specificity deserves discussion. It is well known that integrins exhibit functional “cross talk” (Diaz-Gonzalez et al., 1996; Blystone et al., 1999; Ly et al., 2003), in that any effect on a given integrin is usually accompanied by functional changes on others, making it impossible to isolate investigation on any particular integrin when dealing with studies on whole cells. Indeed, our original observation that CEA over-expression perturbed cell-ECM interactions was followed by the observation of increases in cell binding to both Fn and Vn and, less reproducibly in different cell types, to Ln, implicating both integrins α5β1 and αvβ3 and possibly α6β4, respectively. Here, we showed rapid increases in cell binding to the same three ECM components after cross-linking ΔNCEA in L6(ΔNCEA) transfectants (Fig. 1). Our decision to focus on α5β1-Fn interactions was predicated on previous results involving cells (see Introduction) and the observation that the CEA-mediated increase in binding to Vn (data not shown) and even to whole unfractionated ECM (Ordonez et al., unpublished results) could be completely reversed by the administration of anti-α5 mAb. Thus α5β1 seems to represent a dominant controlling integrin in response to CEA clustering.
Since no change in the cell surface levels of α5β1 after ΔNCEA cross-linking was observed, and both of these molecules showed prior localization in membrane microdomains (Fig. 2), it is possible that an initial increase in integrin affinity for Fn, resulting from a α5β1 valency increase (Weitzman et al., 1997; Bazzoni and Hemler, 1998; van Kooyk and Figdor, 2000), came first, perhaps from the generation of larger membrane rafts (Harris and Siu, 2002). No direct binding (that withstands cold detergent treatment) between CEA and α5β1 could be demonstrated in multiple cell systems (data not shown), indicating perhaps that the CEA and α5β1 association that results in the putative α5β1 valency increase is simply due to their occupation of the same membrane microdomains. At present, however, it is impossible to identify whether the initial event in this process is external, due to the above-mentioned valency increase, or internal, such as PI3-K activation arising from integrin priming. Note that increased Fn binding was seen both as cell binding to immobilized Fn and binding of soluble Fn to immobilized cells, which distinguishes our situation from those showing only the former (Bazzoni and Hemler, 1998). Our present model includes the formation of a “cocoon” of polymerized Fn around the cells, due to the more tenacious binding of Fn produced by the cells and the consequent failure to deposit it in the ECM (Ordonez et al., 2007), which could explain the observed loss of cell polarity, disruption of tissue architecture (Ilantzis et al., 2002) as well as the inhibition of cell differentiation (Eidelman et al., 1993; Screaton et al., 1997; Taheri et al., 2003) and anoikis (Ordonez et al., 2000).
The signaling events initiated by the clustering of ΔNCEA include the activation of PI3-K. The PI3-K family of enzymes are localized in the cytosol of unstimulated cells, but in response to lipid phosphorylation, accumulate at the plasma membrane due to their ability to associate with newly formed phosphoinositides (Vanhaesebroeck and Alessi, 2000). At the membrane these proteins become activated and initiate various local responses, including the activation of several downstream targets such as Src family kinases (Fincham et al., 2000), the protein serine-threonine kinase Akt (protein kinase B, PKB) (Vanhaesebroeck and Alessi, 2000), phosphoinositide-dependent kinase 1 (PDK1) (McManus et al., 2004) and ILK (Delcommenne et al., 1998). In our study we showed that ILK and Akt (Fig. 5) were rapidly relocated to membrane rafts after ΔNCEA cross-linking. These results implicate ILK and Akt as part of the CEA-associated signaling network.
The phenotypic changes previously observed in cells over-expressing ILK (Wu et al., 1998; Attwell et al., 2000; Huang et al., 2000) led to the hypothesis that ILK could be a component of the CEA-induced signal transduction pathway since, as for these previous observations, the forced expression of CEA in both mouse C2C12 and L6 myoblasts inhibits myogenic differentiation (Screaton et al., 1997), inhibits anoikis in many cell types including human colonocytes and L6 myoblasts (Ordonez et al., 2000) and increases fibronectin matrix assembly (Ordonez et al., 2007). Consistent with previous results (Delcommenne et al., 1998; Troussard et al., 2003), we identified ILK and Akt as downstream targets of PI3-K, since inhibition of PI3-K prevented ILK localization to membrane microdomains (Fig. 5B) and Akt phosphorylation (Fig. 5A,C). Although down-regulation of ILK expression with siRNA reduced the normal increase in cell adhesion to fibronectin caused by ΔNCEA cross-linking (Fig. 4B), reflecting a requirement for ILK in the regulation of CEA-mediated inside-out integrin α5 activation, we did not see a significant reduction of Akt phosphorylation in the siRNA ILK clones (data not shown). This could be due to residual expression levels of ILK observed in these clones, but is also consistent with results obtained by other groups showing that ILK is not directly responsible for Akt phosphorylation (Lynch et al., 1999; Zervas et al., 2001; Hill et al., 2002; Grashoff et al., 2003). Further studies will be required to clarify this question. Our results thus support a model in which membrane microdomain localization of signaling elements, resulting from membrane raft aggregation induced by CEA clustering, may allow interaction of integrins with relocated and activated effector molecules such as ILK, leading to increased integrin-Fn binding activity and the activation of downstream signaling cascades, including the Akt/PKB pathway.
Another signaling pathway that has been linked to integrin signaling is the ERK/MAPK cascade. Various findings support a role for ERK in the regulation of the adhesion/cytoskeletal network, cell differentiation and apoptosis (Sastry et al., 1999; Howe et al., 2002). Our experiments demonstrated the activation and relocation of MAPK to membrane microdomains as a result of CEA clustering (Fig. 7A); this activation was dependent on integrin activation, as pretreatment with α5 integrin antibodies inhibited MAPK phosphorylation (Fig. 7B). However, inhibition of PI3-K did not prevent MAPK activation (Fig. 7B) and furthermore, a specific inhibitor of MAPK did not prevent the increase of cell adhesion to Fn in response to ΔNCEA cross-linking as did PI3-K inhibition (Fig. 6). Thus it appears that MAPK activation represents a uni-directional collateral signal regulated by outside-in integrin signaling.
As noted in the introduction, the interaction of integrins with intracellular signaling elements can be bi-directional, resulting in a positive feedback loop that drives a rapid and robust activation of the integrin. ILK and PI3-K could be thought of as multi-directional elements in that integrin clustering/activation can result in their re-localization and activation which, in turn, could result in further integrin activation. Our results implicate the PI3-K signaling cascade as an important bi-directional regulator of the activation state of the α5β1 integrin receptor. The MAPK cascade, on the other hand, appears not to be involved in this positively feedback activation of the integrin and is a uni-directional consequence of α5β1 activation. In conclusion, our results suggest a model for CEA signaling in which clustering of CEA molecules causes membrane microdomain aggregation and subsequent activation of the α5 integrin receptor. We show data supporting the view that the CEA GPI anchor plays a key role in CEA function by determining a specific subset of membrane rafts and their associated signaling molecules that all rapidly relocate and become activated upon CEA clustering. We find that signaling complexes such as the PI3-K and MAPK pathways are specifically activated. We propose that the MAPK and PI3-K/Akt signaling pathways are modulators of a CEA-mediated integrin activation mechanism that may contribute to aberrant cell–ECM interactions characteristic of the CEA phenotype, leading to inhibition of cell differentiation and anoikis, and a distortion of tissue architecture, thus promoting malignant progression.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research and the National Cancer Institute of Canada, with funds from the Canadian Cancer Society. P.C.-L. was supported by the National Science Council, (CONACYT), Mexico. We thank Luisa De Marte and J. Laliberte for assistance with the confocal microscopy experiments.




