The Paradoxical Effects of AMPK on Insulin Gene Expression and Glucose-Induced Insulin Secretion
ABSTRACT
The activation of AMP-activated protein kinase (AMPK) is known to repress the expression of the insulin gene and glucose-stimulated insulin secretion (GSIS). However, the mechanisms by which this occurs, as well as the effects of AMPK activation on glucolipotoxicity-induced β-cell dysfunction, have not been elucidated. To investigate the effects of 5-amino-4-imidazolecarboxamide ribonucleotide (AICAR) and peroxisome proliferator-activated receptorγ-coactivator-1α (PGC-1α) on β-cell-specific genes under glucolipotoxic conditions, we performed real-time PCR and measured insulin secretion by primary islets. To study these effects in vivo, we administered AICAR for 10 days (1 mg/g body weight) to 90% pancreatectomized hyperglycemic mice. The exposure of isolated rat and human islets to glucolipotoxic conditions and the overexpression of PGC-1α suppressed insulin and NEUROD1 mRNA expression. However, the expression of these genes was preserved by AICAR treatment and by PGC-1α inhibition. Exposure of isolated islets to glucolipotoxic conditions for 3 days decreased GSIS, which was also well maintained by AICAR treatment and by PGC-1α inhibition. The administration of AICAR to 90% pancreatectomized hyperglycemic mice improved glucose tolerance and insulin secretion. These results indicate that treatment of islets with an AMPK agonist under glucolipotoxic conditions protects against glucolipotoxicity-induced β-cell dysfunction. A better understanding of the functions of molecules such as PGC-1α and AMPK, which play key roles in intracellular fuel regulation, could herald a new era for the treatment of patients with type 2 diabetes mellitus by providing protection against glucolipotoxicity. J. Cell. Biochem. 117: 239–246, 2016. © 2015 Wiley Periodicals, Inc.
Abbreviations
-
- AMPK
-
- AMP-activated kinase
-
- AICAR
-
- 5 amino-4-imidazolecarboxamide riboside
-
- PGC-1α
-
- peroxisome proliferator activated receptorγ coactivator-1α
-
- NEUROD1
-
- neuronal differentiation 1
-
- PDX1
-
- pancreatic duodenal homeobox1
-
- Px
-
- partial pancreatectomy
Glucolipotoxicity plays an important role in the development and progression of type 2 diabetes. Once chronic hyperglycemia is established, this condition can affect pancreatic β-cell function and survival [Laybutt et al., 2002a]. However, recent evidence has suggested that elevated levels of circulating and intracellular lipids also play an important role in inducing β-cell dysfunction and decreased β-cell mass [Pick et al., 1998; Cnop et al., 2001; Prentki et al., 2002]. Therefore, the stabilization of metabolic changes induced by glucolipotoxicity in β-cells represents a potential new avenue for the treatment of patients with type 2 diabetes mellitus.
The activation of AMP-activated kinase (AMPK) has been pursued as a potential strategy for the treatment of type 2 diabetes, primarily due to the AMPK-mediated inhibition of gluconeogenesis in the liver during feeding [Shaw et al., 2005]. AMPK is a heterotrimeric enzyme consisting of α (catalytic) and β/γ (regulatory) polypeptides, and multiple isoforms exist for each. AMPKα1 and AMPKα2 proteins have been detected in the rodent insulinoma cell lines MIN6 and INS1 [Salt et al., 1998a; da Silva Xavier et al., 2000]. AMPKα1 is the major catalytic subunit found in both MIN6 cells and islets, and accounts for most AMPK catalytic activity [da Silva Xavier et al., 2000; Sun et al., 2010]. Although several publications have been directly devoted to this topic, the role of AMPK in glucose-stimulated insulin secretion (GSIS) still requires investigation because the results of previous studies have not been congruent, and some studies have reported that AMPK is both a positive and negative regulator of insulin secretion.
AMPK activity is clearly detectable under basal conditions in β-cell lines [Salt et al., 1998b; da Silva Xavier et al., 2003; Diraison et al., 2004a] and in isolated islets from rodents and humans [Salt et al., 1998a; da Silva Xavier et al., 2003]. Maintaining AMPK activity at elevated levels at low glucose concentrations suppresses GSIS from cell lines [Salt et al., 1998b] and isolated islets [Pick et al., 1998; da Silva Xavier et al., 2000; Leclerc et al., 2004]. Furthermore, culturing islets with the AMPK activator 5-amino-4-imidazolecarboxamide riboside (AICAR) leads to a marked accumulation of triglycerides in primary rat islets and to a profound decrease in glucose oxidation and GSIS [Diraison et al., 2004b]. Conversely, a dominant-negative form of AMPK stimulates insulin release at low glucose concentrations [da Silva Xavier et al., 2003; Diraison et al., 2004a]. Together, these results suggest that AMPK suppresses insulin gene expression and GSIS in normal β-cell lines and isolated islets.
Our group previously reported that the expression levels of the Neuronal differentiation 1 (NEUROD1) gene and the associated protein significantly decreased upon AICAR treatment, and that these decreases were associated with the suppression of NEUROD1 promoter and DNA-binding activity. We suggested that the expression of the NEUROD1 and insulin genes is regulated positively by glucose and negatively by AMPK [Kim et al., 2008]. Adenovirus-mediated overexpression of an activated form of AMPK markedly inhibits the improvement of glycemic control achieved by the transplantation of islets in streptozotocin-diabetic syngeneic mice [Richards et al., 2005]. Therefore, animal models of diabetes have clear discrepancies [Fiedler et al., 2001; Halseth et al., 2002; Pold et al., 2005]. Furthermore, there are no clear data on the role of AMPK activation in glucolipotoxicity-induced β-cell dysfunction in isolated islets and β-cell lines. In this study, we tested the effects of AMPK activation on glucolipotoxicity-induced β-cell dysfunction in vivo and in vitro.
MATERIALS AND METHODS
ISLET ISOLATION
Rat pancreatic islets were isolated from Sprague Dawley rats (200–230 g) by digesting the pancreatic duct with collagenase P (1 mg/mL PBS, Roche, Mannheim, Germany), as previously described [Sutton et al., 1986]. After digestion, the islets were separated with Histopaque-1077 (Sigma, St. Louis, MO). The islets were cultured in RPMI 1640 media (11.1 mM glucose) containing 10% FBS, penicillin (100 IU/mL), and streptomycin (100 g/mL). Human pancreas tissue was obtained from brain-dead organ donors (n = 2). For this, we obtained informed consent from the relatives of the organ donors and permission from the ethics committee of our institute. Human islets were isolated using liberase M/TF (2 mg/mL, Roche) digestion and purified using Ficoll gradient centrifugation [Linetsky et al., 1997].
GLUCOLIPOTOXIC CONDITIONS AND AICAR TREATMENT
Palmitate conjugated to fatty acid (FA)-free BSA (Sigma) was prepared as follows. First, 0.256 g palmitic acid (Sigma) was dissolved in 10 mL 0.1 N NaOH and boiled at 70°C, filtered using a 0.2 µm syringe filter, and stored at −20°C (100 mM FFA stock). Next, the palmitate stock solution (100 mM stock, 75 μL) was dissolved in 10% fatty-acid-free BSA (750 μL) in distilled water (675 μL) (5 mM palmitate/5% fatty-acid-free BSA). Finally, a palmitate solution (5 mM) was diluted to 1 mM concentration in growth media, and heated on a 60°C heat block for 5 min. Three experimental groups were formed: islets cultured in RPMI 1640 containing 10% FBS (control); islets cultured in RPMI 1640 (33.3 mM glucose) containing 10% FBS, 0.6 mM palmitic acid (glucoliptoxic conditions); and AICAR-treated islets cultured in glucolipotoxic conditions (glucoliptoxicity plus AICAR).
ADENOVIRUS INFECTION
PGC-1α RNA interference (RNAi) constructs were generated using the PGC-1α sequencing method of Koo et al. [2004]. For the adenovirus experiments, adenoviruses were generated in human embryonic kidney 293 cells. Prior to transduction, freshly isolated rat islets were cultured for 3 days in RPMI 1640 containing 10% FBS. Then the cells were divided into two groups: normal controls (11.1 mM glucose) and a glucolipotoxicity group (exposed to glucolipotoxic conditions of 33.3 mM glucose plus 0.6 mM palmitic acid). Ad-null or Ad-siPGC-1α were transduced at a 100 multiplicity of infection (MOI) for 2 h into islets in the glucolipotoxicity group. After infection with the adenovirus, the islets were cultured under glucolipotoxic conditions with or without AICAR (400 μM) for 3 days.
REAL-TIME QUANTITATIVE PCR (qRT-PCR)
Samples of mRNA (1 µg) were reverse-transcribed with 0.5 µg oligo-dT primers using the Superscript III system. Each cDNA product, obtained according to the manufacturer's protocol, was diluted to a concentration of 0.1 mg/mL in ultrapure water. Aliquots (0.1 µg) of cDNA were used as a template in 20 µL reaction mixtures including SYBR Mastermix, 10 pM concentrations of the primer pairs, and 0.4 mL ROX reference dye. The following primer sequences were used: for NEUROD1 (229bp): 5′-CTTGGCCAAGAACTATATCTGG-3′ (forward) and 5′-GGAGTAGGGATGCACCGGGAA-3′ (reverse); for INSULIN (187 bp): 5′-TGCCCAGGCTTTTGTCAAACAGCACCTT-3′ (forward) and 5′-CTCCAGTGCCAAGGTCTGAA-3′ (reverse); for PDX1 (225 bp): 5′-CTCGCTGGGAACGCTGGAACA-3′ (forward) and 5′-GCTTTGGTGGATTTCATCCACGG-3′ (reverse); for PGC-1α (173 bp): 5′-GGAGACGTGACCACTGACA-3′ (forward) and 5′-TGGTTTGCATGGTTCTG-3′ (reverse); for TBP (190 bp): 5′-ACCCTTCACCAATGACTCCTATG-3′ (forward) and 5′-ATGATGACTGCAGCAAATCGC-3′ (reverse). The specificity of the amplified product was determined via melting peak analysis. The magnitude of the fluorescence signal was determined using the MiniOpticonTM real-time system (Cat# CFB-3120, BioRad). The data were analyzed using Supports OpticonMonitorTM software (Biorad), which determines mRNA transcript levels using the threshold cycle (CT) method based on the CT measurements obtained during the reaction. The quantified values for each target gene were normalized to the housekeeping gene, TBP, and the relative quantity was normalized to the template obtained from rat islets.
GLUCOSE-STIMULATED INSULIN SECRETION (GSIS)
Prior to assessing the secretory function of freshly isolated rat islets, all cells were cultured for 3 days in RPMI 1640 media containing 10% FBS. Then they were divided into normal controls (11.1 mM glucose), a glucolipotoxicity group (exposed to glucolipotoxic conditions: 33.3 mM glucose plus 0.6 mM palmitic acid), and a glucolipotoxic + AICAR (400 μM) group for 1–3 days. A total of 100 islets (∼150 μm) were collected from each group, washed in KRB buffer (130 mM NaCl, 23.6 mM KCl, 1.5 mM CaCl2, 0.5 mM MgSO4, 0.5 mM KH2PO4, 2.0 mM NaHCO3, and 10 mM Hepes), and incubated in KRB buffer containing 5.5 mM glucose for 1 h. Then we collected the samples for assays and stored them at −70°C until measurement. Then the islets were washed two times with D-PBS and incubated in KRB buffer containing 25 mM glucose for 1 h. Finally, insulin concentrations were measured in the medium (5.5 or 25 mM) using a radioimmunoassay kit (Linco).
ANIMALS AND TREATMENTS
Male C57/BL6 mice weighing 19–20 g were anesthetized with ketamine and xylazine (5:1) before undergoing a partial pancreatectomy (Px). All of the tail portion and most of the head of the pancreas were removed by gentle abrasion with cotton applicators. The residual pancreatic tissue was within 1–2 mm of the common pancreatic duct to the first part of the duodenum [Bonner-Weir et al., 1983]. Based on the results of intraperitoneal glucose tolerance tests (IPGTTs) 3 weeks after the Px, the mice were allocated into two groups: saline-treated (0.9% NaCl) hyperglycemic Px mice (n = 6) and AICAR-treated (1 mg/g body weight) hyperglycemic Px mice (n = 7). The hyperglycemic Px mice were given a daily subcutaneous injection of AICAR (1 mg/g body weight) or saline for 10 days [Halseth et al., 2002; Song et al., 2002; Pruznak et al., 2008]. Non-fasting blood glucose was monitored every morning before AICAR or saline administration. Fasting insulin levels were measured using a mouse insulin RIA kit (Linco Research, Inc., St. Charles, MO).
INTRAPERITONEAL GLUCOSE TOLERANCE TEST (IPGTT)
Prior to grouping of pancreatectomized mice (n = 13), we conducted IP-GTTs. All mice were bled following overnight fasting to establish basal metabolite concentrations and were then injected intraperitoneally with a volume of a 20% glucose solution calculated to deliver a glucose dose of 2 g/kg body weight. Blood samples were collected at 30, 60, 90, and 120 min after injection. Blood glucose levels were measured immediately after sampling from the tail tip using glucoX (Arkray Factory, Inc., Japan), and the area under the glucose curve (AUCg) was calculated.
STATISTICAL ANALYSIS
The results are presented as the mean ± SE of at least three independent experiments. Analysis of variance was used to compare different groups. Statistical significance was determined using Student's t-tests and P < 0.05 was assumed to be significant.
RESULTS
GLUCOSE-STIMULATED INSULIN SECRETION AND INSULIN GENE EXPRESSION IN ISLETS EXPOSED TO GLUCOLIPOTOXIC CONDITIONS AND/OR TO CHRONIC AICAR TREATMENT
We found that the secretory function of islets under glucolipotoxicity was impaired compared to the control group (Fig. 1A). The depressed glucose-stimulated insulin secretion induced by glucolipotoxicity was prevented by simultaneous AICAR treatment during 3 days of culture (Fig. 1A). We confirmed the expression of insulin under the same conditions using qRT-PCR. Under glucolipotoxic conditions, insulin gene expression was suppressed in a time-dependent manner and was preserved by AICAR treatment (Fig. 1B).
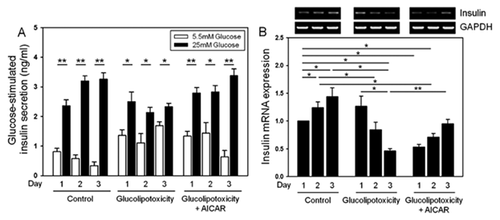
PRESERVATION OF NEUROD1 AND INSULIN GENE EXPRESSION LEVELS BY AICAR TREATMENT IN ISOLATED RAT AND HUMAN ISLETS
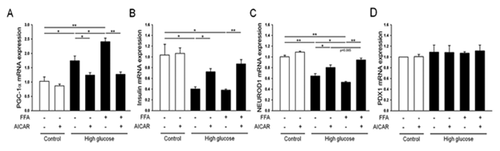
To investigate the effects of AICAR on β-cell-specific genes under glucolipotoxic conditions, we performed qRT-PCR on rat islets. Under normal conditions (control), AICAR caused no changes in β-cell-specific gene expression. The expression of PGC1α under glucotoxic or glucolipotoxic conditions was significantly increased (up to 1.7-fold) compared to controls (Fig. 2A). In addition, repression of the insulin and NEUROD1 genes was detected. This repression was prevented by AICAR treatment (Fig. 2B and C). All of these results were reproduced in human islets (Supplemental Fig. S1).
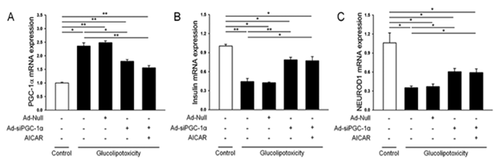
EFFECTS OF AICAR TREATMENT AND PGC-1α EXPRESSION ON INSULIN GENE EXPRESSION UNDER GLUCOLIPOTOXIC CONDITIONS
The expression of PGC1-α increased under glucolipotoxic conditions (up to 2.2-fold) compared to controls (Fig. 3A). In parallel with this increase, the expression of insulin and NEUROD1 mRNA was significantly decreased under glucolipotoxic conditions. The decrease in insulin expression was prevented when PGC1-α expression was suppressed by treatment with si-PGC1-α, with or without AICAR (Fig. 3B and C). These data indicate that PGC1-α plays a role in the gene expression of insulin and NEUROD1 in β-cells.
EFFECTS OF AICAR TREATMENT IN VIVO
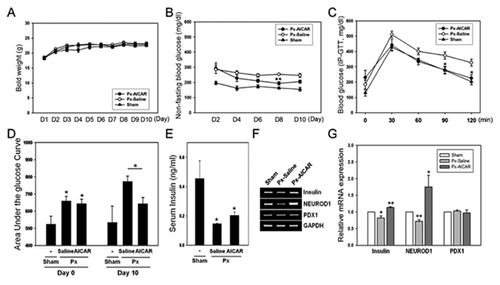
After Px, hyperglycemic mice were allocated into two groups, and were treated with saline or AICAR for 10 days. The body weights of the groups did not differ significantly during the study (Fig. 4A). The average morning, non-fasting blood glucose levels of the AICAR group were significantly lower than those of the saline-treated hyperglycemic group, 4 days after beginning AICAR treatment (Fig. 4B). The IPGTT results showed that glucose values at 90 and 120 min after a 2 g/kg glucose dose were significantly lower in the AICAR-treated group than in the saline-treated group (Fig. 4C). The mean AUCg was significantly higher in the saline-treated group than in the AICAR-treated group (Fig. 4D). Fasting insulin levels in serum were significantly higher in the AICAR-treated group than in the saline-treated group (Fig. 4E). About 7–10 islets were obtained from the remnant pancreases in each group, and RNA from these islets was isolated. The expression levels of NEUROD1 and insulin were repressed in the islets of the saline-treated hyperglycemic mice (Fig. 4F); however, AICAR treatment protected against the repression of NEUROD1 and insulin gene expression. PDX1 expression levels in the islets did not differ between the two groups (Fig. 4G).
DISCUSSION
This study demonstrated that AMPK can protect against glucolipotoxicity-induced β-cell dysfunction and preserve β-cell-specific gene expression levels. In previous studies, AMPK activation contributed to the inhibition of glucose metabolism and GSIS in pancreatic β-cells [Salt et al., 1998a; da Silva Xavier et al., 2000]. We observed similar results in this study, and performed additional experiments to determine the regulatory mechanisms of insulin gene expression by AMPK. First, we observed that the glucolipotoxicity-induced repression of p-AMPK level was prevented by AICAR treatment (Supplemental Fig. S2), and the stimulatory effects of glucose on NEUROD1 and insulin gene expression were blocked in the presence of AICAR [Kim et al., 2008]. The induction of NEUROD1 promoter activity by NEUROD1 and/or by the transcriptional coactivator p300 was inhibited dramatically by AICAR treatment, which then downregulated the transcription of the insulin gene. Thus, AMPK activation prevents the binding of activators to DNA and may modulate interactions between activators and the basal transcriptional machinery for insulin gene activation. We also showed that AMPK could protect against glucolipotoxicity-induced β-cell dysfunction and preserve β-cell-specific gene expression levels. Superficially, the findings of our previous studies that used acute AICAR treatment as well as glucolipotoxic conditions suggest that the role of this process in the protection of β-cell dysfunction is paradoxical. The role of AMPK in the control of malonyl-CoA metabolism in β-cells may provide a possible explanation that may reconcile these apparently contradictory data.
Chronically elevated levels of glucose (glucotoxicity) and lipids (lipotoxicity) synergistically influence β-cell energy metabolism, leading to modifications in the expression levels of key transcription factors [Prentki et al., 2002]. Under glucolipotoxic conditions, malonyl-CoA, which is a metabolic signaling molecule, regulates lipid partitioning through its inhibitory action on carnitine palmitoyltransferase-1 (CPT-1), which then catalyzes the rate-limiting step of mitochondrial fatty acid β-oxidation. As a result, free fatty acid (FFA)-derived long-chain acyl-CoA esters (FACoAs) accumulate in the cytoplasm and can cause impaired glucose-induced insulin secretion or promote β-cell apoptosis [Saha et al., 2000]. According to recent reports, the net effect of AMPK activation increases the oxidation of fatty acids and decreases their esterification and use in other non-β-oxidative pathways [Muoio et al., 1999]. All of these effects strongly suggest that AMPK activation decreases the accumulation of FACoAs and malonyl-CoA in β-cells under glucolipotoxic conditions, and protects against glucolipotoxicity-induced β-cell dysfunction. Several studies relevant to the effects of AICAR on potassium channels and glucose transport have demonstrated that AMPK activation via AICAR treatment increases GLUT-4 expression in muscle [Holmes et al., 1999; Ojuka et al., 1999; Pold et al., 2005). Lim et al. [2009] demonstrated that there is crosstalk between AMPK and ATP-sensitive K+ (KATP) channels in pancreatic β-cells, and described the regulation of KATP by energy status and AMPK.
In this study, glucose-stimulated insulin secretion increased under normal conditions; however, when islets were exposed to glucolipotoxic conditions, the fold increase in glucose-stimulated insulin secretion was reduced. Interestingly, this decrease was completely recovered by 3 days of treatment with AICAR (Fig. 1A). To test whether AICAR can mimic AMPK activation, a constitutively active AMPK (Ad-AMPKα1312[T172]) (CA-AMPK), and a dominant-negative AMPK (DN-AMPK), in which Asp157 was replaced with alanine, were used. These adenoviruses were provided by Dr. J. Ha at Kyung Hee University, Korea. After virus infection or AICAR treatment, RT-PCR was performed to evaluate gene expression. Glucolipotoxicity-induced PGC-1α was reduced successfully by Ad-CA-AMPK and AICAR treatment, and the insulin gene expression levels, which had been suppressed by Ad-DN-AMPK and glucolipotoxicity, were normalized (Supplemental Fig. S3). These results suggest that the activation of AMPK protects against β-cell dysfunction by restoring glucose-stimulated insulin secretion. An unknown regulator or mediator causes this paradoxical phenomenon following glucolipotoxicity. We hypothesized that one mediator of this phenomenon is PGC-1α. PGC-1α is a highly regulated transcriptional coactivator of nuclear receptors and other transcription factors that have been linked to the control of energy metabolism in multiple cell types, particularly in liver and muscle cells [Puigserver et al., 1998; Yoon et al., 2001]. PGC-1α has also been implicated as a major regulator of mitochondrial biogenesis. In addition, chronic AICAR treatment can cause remarkable changes in skeletal muscle, and increases glycogen stores, GLUT-4, and the activity of hexokinase and mitochondrial oxidative enzymes [Holmes et al., 1999; Ojuka et al., 2000]. Several studies relevant to glucoliptoxic conditions have recently been performed. Somesh et al. [2013] recently demonstrated that mitochondrial activity in pancreatic islets was dysregulated in chronic glucolipotoxic conditions. Heilbronn et al. [2007] recently reported that insulin-resistant subjects have reduced markers of mitochondrial metabolism. PGC-1α levels in islets are elevated in diabetic animals with defective insulin secretion [Yoon et al., 2003; Kim et al., 2009], and this occurs along with an altered pattern of metabolic gene expression that resembles type 2 diabetes [Russell, 2005]. These results suggest that PGC-1α plays an important role in the pathogenesis of β-cell dysfunction in type 2 diabetes. However, there is no clear evidence of the role of PGC-1α in glucolipotoxic-induced β-cell dysfunction and the relationship between AMPK and PGC-1α in β-cells. In this study, PGC-1α overexpression was induced by an adenoviral vector under glucolipotoxic conditions. This overexpression caused repressed insulin and NEUROD1 gene expression. However, the overexpression of PGC-1α was not suppressed by AICAR treatment (Fig. 3). This suggests that PGC-1α may mediate AMPK downstream signaling, and that it is closely related to insulin and NEUROD1 gene expression.
Several previous studies have shown that AICAR administration in insulin-resistant animal models, such as transgenic KKAy-CETP mice [Fiedler et al., 2001], db/db mice, and ob/ob mice [Halseth et al., 2002], improves glucose tolerance. However, in those experiments, glucose tolerance was improved not through β-cell function but primarily by enhancing peripheral insulin sensitivity. However, Laybutt et al. [2002b] reported that hyperglycemia in a 90% partial pancreatectomy rat model was associated with induced ACC and FAS gene expression in islets and reduced PPAR-α mRNA expression. Therefore, we hypothesized that the activation of AMPK in 90% partially pancreatectomized diabetic mice might improve glucose tolerance and maintain β-cell-specific gene expression levels, including that of insulin, via the same mechanism as glucolipotoxic conditions in vitro. We administered AICAR to hyperglycemic partially pancreatectomized mice and observed improvements in glucose tolerance, GSIS, and insulin and NEUROD1 gene expression levels.
In conclusion, our findings indicate that hyperglycemia- and hyperlipidemia-induced glucolipotoxicity increased PGC-1α gene expression and repressed NEUROD1 and insulin gene expression. The activation of AMPK inhibited glucolipotoxic-induced events and downregulated PGC-1α expression. Finally, NEUROD1 and insulin gene expression were rescued by the suppression of PGC-1α. Therefore, we suggest that AMPK may function as a key molecule that provides protection against glucolipotoxicity-induced β-cell dysfunction through the attenuation of PGC-1α overexpression, and may represent an attractive pharmacological candidate for enhancing β-cell function in type 2 diabetes. The specific mechanism of AMPK downstream signaling requires further study.
ACKNOWLEDGMENTS
We thank Dr. Joohun Ha (Kyung Hee University) for adenovirus for AMPK activation and In-Kyu Lee (Kyungpook National University) for expression vector for the insulin promoter gene. We appreciate the expert technical assistance provided by Marie Rhee. This study was supported by a Korean Healthcare Technology R&D Project grant (HI14C3417) and the Ministry of Health, Welfare & Family Affairs, and by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (20110009075).