Heat Shock Protein 90 Regulates Subcellular Localization of Smads in Mv1Lu Cells
ABSTRACT
Heat shock protein 90 (HSP90) regulates the stability of various proteins and plays an essential role in cellular homeostasis. Many client proteins of HSP90 are involved in cell growth, survival, and migration; processes that are generally accepted as participants in tumorigenesis. HSP90 is also up-regulated in certain tumors. Indeed, the inhibition of HSP90 is known to be effective in cancer treatment. Recently, studies showed that HSP90 regulates transforming growth factor β1 (TGF-β1)-induced transcription by increasing the stability of the TGF-β receptor. TGF-β signaling also has been implicated in cancer, suggesting the possibility that TGF-β1 and HSP90 function cooperatively during the cancer cell progression. Here in this paper, we investigated the role of HSP90 in TGF-β1-stimulated Mv1Lu cells. Treatment of Mv1Lu cells with the HSP90 inhibitor, 17-allylamino-demethoxy-geldanamycin (17AAG), or transfection with truncated HSP90 (ΔHSP90) significantly reduced TGF-β1-induced cell migration. Pretreatment with 17AAG or transfection with ΔHSP90 also reduced the levels of phosphorylated Smad2 and Smad3. In addition, the HSP90 inhibition interfered the nuclear localization of Smads induced by constitutively active Smad2 (S2EE) or Smad3 (S3EE). We also found that the HSP90 inhibition decreased the protein level of importin-β1 which is known to regulate R-Smad nuclear translocation. These data clearly demonstrate a novel function of HSP90; HSP90 modulates TGF-β signaling by regulating Smads localization. Overall, our data could provide a detailed mechanism linking HSP90 and TGF-β signaling. The extension of our understanding of HSP90 would offer a better strategy for treating cancer. J. Cell. Biochem. 117: 230–238, 2016. © 2015 Wiley Periodicals, Inc.
The 90-kDa heat-shock protein, HSP90, is a molecular chaperone for various client proteins and plays an essential role in cellular homeostasis [Kamal et al., 2004; Taipale et al., 2010]. HSP90 activity is enhanced by heat shock or oxidative stress [Kaplan and Li, 2012]. Many client proteins of HSP90 are involved in cell growth, survival, and migration; processes that are generally accepted as participants in tumorigenesis [Kamal et al., 2004]. Indeed, inhibitors of HSP90 are known to be effective in cancer treatment [Holzbeierlein et al., 2010; Kabakov et al., 2010; Garcia-Carbonero et al., 2013]. For example, 17-allylamino-demethoxy-geldanamycin (17AAG), an inhibitor of HSP90, is currently undergoing clinical trials for the treatment of acute myelogenous leukemia [Ramanathan et al., 2005; Newman et al., 2012]. These inhibitors competitively bind to the ATP binding site of HSP90 and inhibit the ATPase activity of HSP90 [Chiosis et al., 2003]. Inhibition of HSP90 results in destabilization of client proteins, which are ultimately degraded via the ubiquitin-dependent proteasome pathway.
Transforming growth factor (TGF)-β1 is also involved in cell growth, survival, and migration [Heldin et al., 2009]. TGF-β1 acts through binding to the TGF-β receptors, serine–threonine receptor kinases that, when activated by TGF-β1 binding, phosphorylate the receptor-regulated Smads (R-Smad), Smad2 and Smad3. Phosphorylated R-Smads interact with Smad4 and together translocate to the nucleus, where they participate in the transcription of various target genes. Rapid and precise transport of Smads to the nucleus is important for the signal transduction, and the mechanism of Smad transport between cytoplasm and nucleus is beginning to be elucidated. A basic bipartite NLS sequence is essential for the Smad nuclear translocation. Among transport machinery, importin-β1 seems to be crucial for the R-Smad nuclear transport; R-Smad phosphorylation promotes both its interaction with importin-β1 and its nuclear import [Xiao et al., 2000; Kurisaki et al., 2001].
Recent research has shown that HSP90 is capable of regulating the TGF-β signaling pathway by increasing the stability of the TGF-β receptor [Wrighton et al., 2008; Yun et al., 2010]. Treatment with 17AAG has been shown to decrease Smad2/3 phosphorylation and subsequent transcription of target genes. They also reported that 17AAG takes significantly longer than SB431542 (type I TGF-β receptor inhibitor) to block TGF-β signaling, suggesting that the two compounds act via different mechanisms. Since aberrant TGF-β signaling has been implicated in cancer, a possible connection between TGF-β signaling and HSP90 during tumor cell progression could be inferred. Dysregulation of TGF-β signaling and up-regulation of HSP90 activity are associated with tumor cell progression [Kamal et al., 2004; ten Dijke and Hill, 2004], and both TGF-β1 and HSP90 have been reported to regulate cell migration [Jakowlew, 2006; Taiyab and Rao Ch, 2011]. These observations suggest the possibility that these two molecules share a common effector.
In this paper, we examined whether HSP90 plays a critical role in TGF-β1-stimulated Mv1Lu, mink lung epithelial carcinoma cell line. In doing so, we used both 17AAG and a truncated HSP90 construct (ΔHSP90) to inhibit HSP90 activity. Our data suggest that HSP90 can affect TGF-β signaling through the regulation of nuclear transport machinery. This also provides importin-β1 as a new client protein of HSP90, which could regulate TGF-beta signaling. These results suggest a detailed mechanism linking HSP90 and TGF-β signaling, extend our understanding of the role of HSP90 during tumorigenesis, and offer a potential new strategy for treating cancer.
MATERIALS AND METHODS
DNA CONSTRUCTS, REAGENTS, AND ANTIBODIES
N-terminally Flag-tagged Smad2EE (S2EE) and Smad3EE (S3EE) constructs were generated by replacing the serine residues at 465/467 and 423/425, respectively, in the C-terminus with glutamic acid [An et al., 2013]. Hemagglutinin (HA)-tagged, truncated HSP90 (ΔHSP90) containing a C-terminal amino acid (EEVD) deletion was kindly provided by Dr. S.W. Bae (Korean Institute of Radiological and Medical Sciences, Seoul, Korea) [Scheufler et al., 2000]. HA-tagged ubiquitin construct is a gift from Dr. D. Bohmann (European Molecular Biology Laboratory, Heidelberg, Germany) [Treier et al., 1994]. TGF-β1 was purchased from R&D Systems (Minneapolis, MN). 17AAG, MG132 and importazole (Sigma–Aldrich, St. Louis, MO) were dissolved in dimethyl sulfoxide (DMSO) and used at the indicated concentrations (1, 5, or 30 µM).
Antibodies against phospho-Smad2, Smad2, Smad3, and MEK-1 were purchased from Cell Signaling Technologies (Beverly, MA). Antibodies against phospho-Smad3 and importin-β1 were purchased from Abcam (Cambridge, UK). Antibodies against Smad4, Lamin A/C, β-actin, and HA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody against Flag was obtained from Sigma–Aldrich.
CELL CULTURE AND TRANSIENT TRANSFECTION
The mink lung epithelial carcinoma cell line, Mv1Lu, was cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin in a humidified incubator containing 5% CO2. For transient transfection experiments, Mv1Lu cells were transfected with the indicated plasmids using FugeneHD (Roche, Basel, Switzerland) according to the manufacturer's protocol.
CELL MIGRATION ASSAY
For analyses of cell migration, Mv1Lu cells were transfected with the appropriate plasmids, as indicated in the text. The next day, cells were pretreated with 17AAG (5 µM) or DMSO (mock treatment). After 6 h, cells were trypsinized and distributed into Transwells with 8.0-µm pore membranes (Corning Costar, NY) at 1 × 105 cells/well. Three hours later, serum-free DMEM containing 17AAG (5 µM) and/or TGF-β1 (0.5 ng/ml) was added. After 16-h incubation, the cells on the upper surface of the membrane were removed with a cotton swab, and the migrated cells (lower surface) were analyzed with a Hemacolor Rapid staining set (Merck KGaA, Darmstadt, Germany). The migrated cells were determined from four randomly selected areas per sample.
WESTERN BLOT ANALYSIS
Mv1Lu cell extracts were prepared using a lysis solution (Cell Signaling Technology). Protein concentration in lysates was determined using a BCA Protein Assay kit (Pierce, Rockford, IL) according to the manufacturer's protocol. Equal amounts of protein were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) on 7–10% gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk in phosphate-buffered saline (PBS) containing 0.2% Tween (PBST) for 1 h, and then incubated with primary antibodies overnight at 4°C. After three washes with PBST for 1 h, the blots were probed with horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature (RT). Protein bands were detected with enhanced chemiluminescence (ECL) reagents (Amersham Pharmacia Biotechnology, Buckinghamshire, UK) and X-ray film (AGFA, Mortsel, Belgium).
IMMUNOFLUORESCENCE MICROSCOPY
For immunofluorescence analyses, Mv1Lu cells were grown on 13-mm glass coverslips. After the treatment as indicated in the main text, the cells were fixed in formaldehyde (3.7% in PBS) at RT for 20 min, permeabilized with 0.2% Triton X-100, and blocked with normal horse serum (20% in PBS) for 1 h. The cells were washed three times with PBS, and incubated overnight with anti-Smad4 antibody (Santa Cruz Biotechnology). After additional three washes with PBS, the cells were incubated with appropriate fluorescence-conjugated secondary antibody (Vector Labs, Peterborough, England) for 1 h at RT. The attached cells were mounted in 4′,6-diamidino-2-phenylindole (DAPI)-containing Vectashield mounting medium (Vector Labs) and photographed using laser-scanning microscopy (LSM710; Carl Zeiss, Munich, Germany).
SUBCELLULAR FRACTIONATION
Mv1Lu cells were collected in PBS and then suspended in hypotonic buffer (20 mM Tris–HCl pH 7.5, 10 mM NaCl, 3 mM MgCl2) containing protease inhibitors. After 15 min, 1/20th volume of 10% NP-40 was added and cells were centrifuged at 900g for 10 min. The supernatants (cytosolic fraction) were saved and the pellets were resuspended in nuclear extraction buffer (100 mM Tris–HCl pH 7.5, 100 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10% glycerol, 1 mM EGTA, 0.1% SDS, 0.5% deoxycholate). After incubating for 30 min, extracted nuclei were centrifuged at 18,000g for 30 min and the supernatants (nuclear fraction) were saved. The protein concentrations in cytosolic and nuclear fractions were determined using a BCA Protein Assay kit (Pierce) according to the manufacturer's protocol.
IMMUNOPRECIPITATION
Mv1Lu cells were transfected with HA-tagged ubiquitin and incubated for 48 h. The cells were treated with MG132 (5 μM) for 2 h, then treated with 17AAG (5 µM) for 6 h, and total cell lysates were prepared. The samples were immunoprecipitated overnight with importin-β1 antibody (1:200) and protein G–sepharose beads (GE Healthcare Bioscience, Uppsala, Sweden). After extensive washing with lysis buffer, the immunocomplexes were dissociated by boiling in SDS sample buffer and subjected to SDS–PAGE, followed by immunoblotting with the indicated antibodies.
STATISTICAL ANALYSIS
All data were analyzed using Microsoft Office Excel (Microsoft Corp.) and are presented as means ± standard deviations (SDs). P-values (Student's t-test) less than 0.05 are considered significant.
RESULTS
INHIBITION OF HSP90 REDUCES TGF-β1-INDUCED MIGRATION OF Mv1Lu CELLS
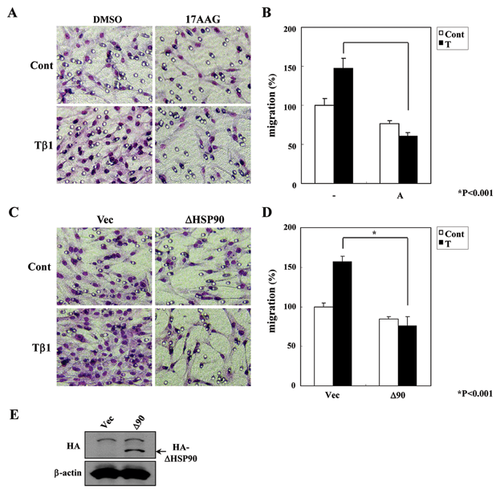
TGF-β1 stimulation induces cell migration in various cell types [Wakefield and Roberts, 2002; Jakowlew, 2006]. We also observed that Mv1Lu cell migration is induced by TGF-β1 treatment, and this TGF-β1-induced migration was inhibited by SB431542 (type I TGF-β receptor inhibitor) treatment (Supplementary Figure S1). To investigate the role of HSP90 in TGF-β1-stimulated Mv1Lu cells, we performed migration assays using the HSP90 inhibitor, 17AAG, which binds the N-terminal ATP-binding domain and inactivates HSP90 protein [Chiosis et al., 2003]. Stimulation of Mv1Lu cells with TGF-β1 induced a 1.5-fold increase in cell migration compared to control cells (Fig. 1A and B). Pretreatment with 17AAG significantly inhibited this TGF-β1-induced cell migration. In order to confirm that the effect of 17AAG is mediated through HSP90, a truncated HSP90 (ΔHSP90), which contains a deletion of four C-terminal amino acids, was used. Mv1Lu cell was transfected with ΔHSP90 and cell migration was examined. Consistent with the results of 17AAG experiments, transfection with ΔHSP90 inhibited TGF-β1-induced cell migration (Fig. 1C and D). Western blotting confirmed the expression of transfected HA-ΔHSP90 (Fig. 1E). Taken together, these data suggest that HSP90 inhibition reduces TGF-β1-induced cell migration.
INHIBITION OF HSP90 REDUCES TGF-β SIGNALING ACTIVITY IN Mv1Lu CELLS
Wrighton et al. [2008] reported that TGF-β1-induced Smads activation is blocked by inhibition of HSP90. The authors of those studies argue that HSP90 acts as a modulator of Smurf2-mediated TGF-β receptor ubiquitination and thereby regulates TGF-β receptor stability. They further suggest that HSP90 affects Smads phosphorylation and downstream transcriptional processes. Consistent with this, we found that the levels of phospho-Smad2 and phospho-Smad3 were decreased by 17AAG treatment or ΔHSP90 transfection (Fig. 2A).
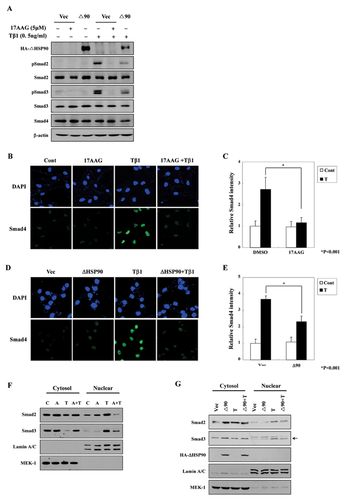
Activation of TGF-β signaling induces nuclear translocation of Smad4 along with Smad2/3, and the degree of nuclear Smads correlates with the activation of TGF-β signaling. Therefore, we investigated the nuclear translocation of Smad4 using immunofluorescence assays. As predicted, Smad4 nuclear translocation was induced by TGF-β1 treatment and was blocked by 17AAG pretreatment (Fig. 2B and C) and ΔHSP90 transfection (Fig. 2D and E). Under these experimental conditions, total Smad4 expression was grossly unchanged by HSP90 inhibition (Fig. 2A).
Next, to measure the subcellular localization of R-Smads, we fractionated Mv1Lu cells into cytosolic and nuclear fractions and performed Western blot analyses. TGF-β1 treatment induced the nuclear localization of Smad2 and Smad3 proteins within 1 h (Fig. 2F and G). However, this TGF-β1-induced nuclear localization was inhibited with 17AAG pretreatment or ΔHSP90 transfection.
Taken together, these data suggest that HSP90 inhibition decreases TGF-β1-stimulated cell migration by attenuating the nuclear localization of Smads.
INHIBITION OF HSP90 REDUCES THE ACTIVE R-SMADS-INDUCED MIGRATION OF Mv1Lu CELLS
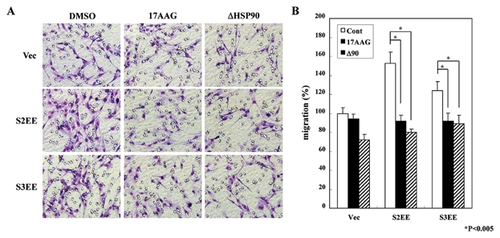
It has been reported that the effects of HSP90 inhibition could be mediated by a decrease in the phosphorylation of R-Smads caused by destabilization of the TGF-β receptor [Wrighton et al., 2008; Yun et al., 2010], a comparable effect we also observed (see Fig. 2A). To clarify the mechanism of HSP90 inhibition in the TGF-β signaling pathway, we used constitutively active R-Smads and examined their relationship to HSP90 inhibition. Mv1Lu cells were transfected with active forms of Smad2 (S2EE) or Smad3 (S3EE), and migration assays were performed with or without 17AAG treatment or ΔHSP90 co-transfection (Fig. 3A and B). Western blot analyses confirmed the correct expressions of ΔHSP90, S2EE, and S3EE (Supplementary Figure S2). As predicted, migration was enhanced in cells transiently transfected with S2EE or S3EE compared with controls (Fig. 3A and B). Surprisingly, the HSP90 inhibition blocked the active Smad-induced cell migration, suggesting a possiblity that HSP90 inhibition could block TGF-β signaling pathway downstream of R-Smads phosphorylation.
INHIBITION OF HSP90 ATTENUATES NUCLEAR LOCALIZATION OF SMADS IN Mv1Lu CELLS
Having demonstrated that HSP90 inhibition blocks constitutively active Smads (Fig. 3), we hypothesized that HSP90 might regulate the localization of Smads. To test this hypothesis, we performed immunofluorescence analyses of Smad4 after transfection of the constitutively active R-Smads (Fig. 4A–C) and depicted in a graph (Fig. 4D). As shown in Figure 4D, transfection of active R-Smad (S2EE or S3EE) increased the nuclear localization of Smad4 more than fourfold. This increase in nuclear Smad4 was eliminated by treatment with 17AAG or transfection of ΔHSP90.
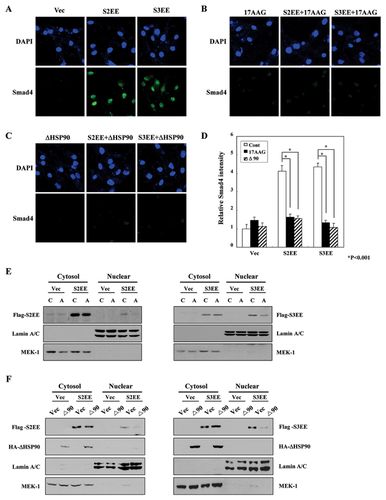
Next, we investigated the subcellular localization of constitutively active R-Smads by fractionating Mv1Lu cells into cytosol and nuclear fractions and performing Western blot analyses. Activated R-Smads were detected in the nuclear fraction in the absence of TGF-β1 treatment (Fig. 4E and F). However, 17AAG treatment or ΔHSP90 transfection reduced the amount of nuclear localized R-Smads. Thus, these data suggest that HSP90 might play a role in the translocation of activated Smads to the nucleus.
INHIBITION OF HSP90 DECREASED THE LEVEL OF IMPORTIN-β1
We found that the inhibition of HSP90 attenuated the nuclear localization of Smads. In an effort to investigate the mechanism contributing to this effect, we speculated that the machinery for nuclear translocation is affected by HSP90 inhibition. Researchers reported that importin-β1 interacts with R-Smad [Xiao et al., 2000; Kurisaki et al., 2001], therefore, we tested whether the effect of HSP90 inhibition on Smads localization is mediated via importin-β1. Interestingly, 17AAG treatment or ΔHSP90 transfection decreased the protein level of importin-β1 (Fig.5A and B). This decrease was not affected by TGF-β1. Next, we investigated whether the decrease in importin-β1 level is mediated by protein ubiquitination. The cells were transfected with HA-tagged ubiquitin and treated with MG132. After immunoprecipitated with importin-β1 antibody, the amount of ubiquitinated importin-β1 was checked (Fig. 5C). Consistent with the level of importin-β1 upon 17AAG treatment, the ubiquitination of importin-β1 was increased in the presence of 17AAG.
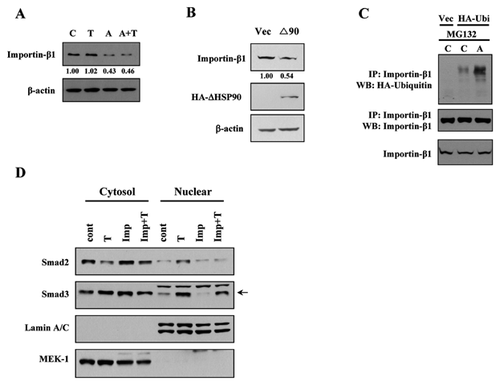
In addition, to verify the involvement of importin-β1 during Smad nuclear translocation, we performed nuclear fractionation in the presence of importin-β inhibitor, importazole. Stimulation of Mv1Lu cells with TGF-β1 induced the nuclear localization of Smad2 and Smad3 proteins (Fig. 5D). However, this TGF-β1-induced nuclear localization was inhibited with importazole pretreatment. These data show a correlation between the activity of importin-β and R-Smad nuclear translocation.
Taken together, we could conclude that the inhibition of HSP90 induces proteasomal degradation of importin-β1 and subsequently blocks the nuclear localization of Smads and TGF-β signaling pathway.
DISCUSSION
Deficient TGF-β signaling and increased HSP90 in response to stressful conditions, including heat shock or oxidative stress, are frequent phenomena in human diseases [Kaplan and Li, 2012; ten Dijke and Hill, 2004]. Recent studies have demonstrated that HSP90 may be involved in TGF-β signaling [Wrighton et al., 2008; Yun et al., 2010]. The authors of those studies argue that HSP90 acts as a modulator of Smurf2-mediated TGF-β receptor ubiquitination and thereby regulates TGF-β receptor stability. They further suggest that HSP90 affects Smads phosphorylation and downstream transcriptional processes.
In the present study, we focused on the effects of HSP90 inhibition in TGF-β1-stimulated Mv1Lu cells. To inhibit HSP90 function, we used two different methods: 17-allylamino-demethoxy-geldanamycin (17AAG), a small specific inhibitor of HSP90 that disrupts the ATP binding site [Chiosis et al., 2003], and a ΔHSP90 DNA construct containing a four amino-acid deletion at the C-terminus that interferes with the interaction between HSP90 and its target proteins.
Consistent with previous studies [Wrighton et al., 2008], we found that TGF-β1 treatment increased cell migration in Mv1Lu cells, and further showed that this increase was abolished by treatment with 17AAG or transfection of ΔHSP90 (Fig. 1). In addition, we demonstrated that HSP90 inhibition with 17AAG or ΔHSP90 significantly suppressed TGF-β1-induced Smad2/3 phosphorylation and nuclear translocation (Fig. 2).
A reasonable interpretation of these results is that HSP90 inhibition decreases cell migration by inducing destabilization of the TGF-β receptor and reducing Smad2/3 phosphorylation. However, we could not rule out the possibility that HSP90 inhibition acts at multiple points in the TGF-β signaling pathway. To clarify the mechanism, we used DNA constructs for constitutively active Smad2 (S2EE) and Smad3 (S3EE) [An et al., 2013]. Exogenous expression of S2EE or S3EE mimicked TGF-β treatment, inducing cell migration (Fig. 3) and enhancing the nuclear localization of Smads (Fig. 4). Surprisingly, inhibition of HSP90 blunted the enhanced migration and increased nuclear localization of Smads, induced by transfection of constitutively active Smads (Figs. 3 and 4). These data suggest that, in addition to regulating TGF-β receptor stability, HSP90 inhibition controls the nuclear translocation of Smads.
In an effort to investigate the mechanism contributing to this effect, we focused on the nuclear transport machinery. Among these complex process, both R-Smad [Kurisaki et al., 2001] and HSP90 [Echeverria et al., 2009] are known to interact with importin-β1. Importin-β1 is known to function in nuclear protein import, either in cooperation with an adapter protein, like an importin-α subunit, or by serving itself as NLS receptor. The importin/substrate complex interacts with a nuclear pore complex (NPC) through nucleoporin FxFG repeats. Subsequently, the complex is translocated through the pore by Ran-dependent mechanism. In case of Smads, Importin-β1 functions as NLS receptor [Kurisaki et al., 2001; Xiao et al., 2000]. Also, Luo et al. [2006] showed that HSP90 are among the identified Smad-interacting proteins in Mv1Lu cells. Therefore, we tested the possibility that HSP90 inhibition affects R-Smad nuclear translocation via importin-β. Indeed, we found that the treatment of 17AAG enhanced the ubiquitination of importin-β1 and subsequently decreased the protein level of importin-β1 (Fig. 5A and B). We conjecture that the enhanced ubiquitination of importin-β1 may indicate that importin-β1 is a substrate of HSP90. During the nuclear transport, which is energy-requiring Ran-dependent process, importin-β1 may be more prone to denaturation, and therefore, susceptible to 17AAG treatment. Without proper function of HSP90, this partially denatured importin-β1 could be tagged with ubiquitin for proteasomal degradation. Importin-β1 is also responsible for nuclear transport of other proteins, many of which are relevant to cancer [Chook and Suel, 2011]. Increased levels of importin-β1 are found in transformed cells lines [Kuusisto et al., 2012]. These increased importin-β1 levels could contribute the increased rates of nuclear import in transformed cells, therefore, the degradation of importin-β1 could interfere the nuclear localization of its cargo proteins. We speculate that the anticancer effect of HSP90 inhibitors may be, in part, due to the low level of importin-β1 protein.
In summary, we propose that HSP90 inhibition could act through at least two mechanisms: (i) controlling R-Smads phosphorylation, consistent with previous reports [Wrighton et al., 2008; Yun et al., 2010]; and (ii) decreasing the level of importin-β1, and therefore, inhibiting the nuclear translocation of Smads. We presented evidence for a novel function of HSP90 in which HSP90 controls the nuclear transport machinery and nuclear translocation of Smads in the context of TGF-β signaling. Although the inhibition of HSP90 shows potent anti-cancer activity and is in clinical trial, the understanding of the inhibition of HSP90 is obviously incomplete. We hope that our data could provide new experimental support and better understanding of the function of HSP90.
ACKNOWLEDGMENTS
This work was supported by a grant (NRF-2012M2A2A7012377) from Nuclear Research and Development Program through the Korea Science and Engineering Foundation (KOSEF) funded by the Ministry of Science, ICT and Future Planning (MSIP).