Regulation of Hypoxia-Inducible Factor-1a by Reactive Oxygen Species : New Developments in an Old Debate
ABSTRACT
Hypoxia-Inducible Factor-1 (HIF-1) has been largely studied for its role in cell survival in hypoxic conditions. The regulation of HIF-1 is a complex process and involves a number of molecules and pathways. Among these mechanisms a direct regulatory role of reactive oxygen species (ROS) on HIF-1 alpha subunit has received a great deal of attention and the existing body of literature includes many contradictory findings. Other intermediates such as nitric oxide (NO), specific microRNAs (miR), and transcriptional and post-translational modification have also been implicated as players in ROS mediated HIF-1a regulation. The focus of this review is to present the past conflicting evidence along with more recent findings in order to relate various aspects of this complex process. Aside from the direct role of ROS on HIF-1a regulation under hypoxia and normoxia, we analyzed the effect of different sources and concentrations of NO and the interplay between superoxide (SO) and NO in this process. We also present findings on transcriptional and translational regulation of HIF-1a via ROS and the interplay with microRNAs in this process. This review further provides insight on ERK and PI3K/AKT signaling as a common mechanism relating several pathways of ROS mediated HIF-1a regulation. Ultimately further research and discovery regarding HIF-1 regulation by oxidative stress is warranted for better understanding of disease development and potential therapeutics for pathologies such as cancer, inflammatory diseases, and ischemia-reperfusion injury. J. Cell. Biochem. 116: 696–703, 2015. © 2014 Wiley Periodicals, Inc.
Abbreviations
-
- CoCl2
-
- cobalt (II) chloride
-
- DETA-NO
-
- 2,2′-(hydroxynitroso-hydrazono) bis-ethanimine
-
- DFO
-
- desferrioxamine
-
- DMOG
-
- dimethyloxalylglycine
-
- DMNQ
-
- 2,3-Dimethoxy-1,4-naphthoquinone
-
- ETC
-
- electron transport chain
-
- GSNO
-
- S-nitroglutathione
-
- H2O2
-
- hydrogen peroxide
-
- HIF-1a
-
- Hypoxia-Inducible Factor-1 alpha
-
- HRE
-
- hypoxia response element
-
- iNOS
-
- inducible nitric oxide synthase
-
- miR
-
- microRNA
-
- Mn-SOD
-
- Manganese Superoxide Dismutase
-
- NO
-
- nitric oxide
-
- ODDD
-
- oxygen dependent degradation domian
-
- OSCC
-
- oral squamous cell carcinoma
-
- PHD
-
- prolyl hydroxylases
-
- PDTC
-
- pyrrolidine dithiocarbamate
-
- RNAi
-
- RNA inhibitor
-
- ROS
-
- reactive oxygen species
-
- SDH
-
- succinate dehydrogenase
-
- SDHA
-
- succinate dehydrogenase complex, subunit A
-
- SDHD
-
- succinate dehydrogenase complex, subunit D
-
- shRNA
-
- short hairpin RNA
-
- siRNA
-
- RNA silencer
-
- SNAP
-
- S-nitro-N-acetylpenicillamine
-
- SO
-
- super oxide
-
- SOD
-
- super oxide dismutase
-
- TFAM
-
- mitochondrial transcription factor A
-
- TMPD
-
- 2,2,4-trimethyl-1,3-pentanediol
-
- VHL
-
- von Hippel-Lindau
Mammalian cells are able to adapt in a low oxygen level environment and Hypoxia-Inducible Factor-1 was discovered to be a master regulator of this process. HIF-1 is a transcription factor and a heterodimer consisting of an alpha subunit and a beta subunit. The beta subunit is constitutively expressed while the alpha subunit is inducible. In normoxia, two prolines on HIF-1a are hydroxylated by oxygen-dependent prolyl hydroxylases (PHD), tagging HIF-1 for ubiquination and destruction by the von Hippel-Lindau (VHL) complex. Under hypoxic conditions, this process is halted, allowing HIF-1a to accumulate and dimerize with the HIF-1b subunit to bind to hypoxia response elements (HREs) in the nucleus. A number of signaling molecules are transcriptionally induced by HIF-1 DNA binding which in turn increase cellular oxygenation and enhance metabolic adaptation to hypoxic states [Ratcliffe et al., 1998; Ivan et al., 2001; Jaakkola et al., 2001; Semenza, 2004].
After the discovery of HIF-1 protein, several studies have explored the mechanism of oxygen sensing and HIF-1a regulation under hypoxia. Originally, studies evaluated the importance of the mitochondrial respiration in hypoxic regulation of HIF-1a [Chandel et al., 1998, 2000; Brunelle et al., 2005; Bell et al., 2007]. Such studies also investigated the role of ROS in HIF-1 stabilization under hypoxia. A large body of evidence also evolved investigating ROS mediated PHD inhibition and HIF-1a stabilization under normoxia. These studies included nitric oxide, superoxide, and hydrogen peroxide (H2O2) as potential key players. More recent hypotheses suggest ROS mediated transcriptional and translational regulation of HIF-1a via the PI3K/AKT and ERK pathways and a potential role of microRNAs (miR) in this process. There continues to be conflicting evidence in many aspects of HIF-1a regulation by ROS, and several reviews have been written to address the existing discrepancies [Chun et al., 2002; Hagen, 2012]. However, more recent evidence adds more complexity to the picture and points to potential interplays between several regulatory pathways. The question of whether ROS regulates HIF-1a stabilization under hypoxia or normoxia, and the exact mechanism(s) of the types regulation reported has been a matter of debate with no clear consensus. However, consideration of the newly reported mechanism and careful evaluation of the interplay between some of the old and new findings seems to elucidate a potential common final step. In this review we briefly revisit the older hypotheses, discuss the newer findings, and evaluate the interplay between the old and the newer proposed pathways with the aim to specifically point out a unifying role of ERK and PI3K/AKT signaling in this process.
MITOCHONDRIAL ELECTRON TRANSPORT CHAIN AND ROS MODELS
Early studies evaluated the extent of mitochondrial involvement in HIF-1a stabilization. It has been shown that ρ°cells devoid of mitochondria are unable to activate HIF-1 in presence of 1.5% O2 levels [Chandel et al., 1998, 2000]. Short hairpin RNA (shRNA) targeting mitochondrial transcription factor A (TFAM), resulted in a reduction of HIF-1a stabilization [Bell et al., 2007]. TFAM is required to maintain mitochondrial DNA. Reduced HIF-1a levels in cells with TFAM shRNA reinforces the importance of the mitochondria in HIF-1a stabilization process [Bell et al., 2007].
It is known that the respiratory chain of the mitochondria responsible for transport of electrons requires oxygen for final transfer of electrons. During decreased O2 levels, electrons can accumulate in the respiratory compartments of the mitochondria and univalently reduce the existing O2 molecules to SO (O2-) radical [Lenaz, 2001]. It has been shown that the SO generated, from the mitochondria during hypoxic states, enhances HIF-1a levels. Chandel et. al. showed that hypoxic conditioning leads to ROS generation and stabilization of HIF-1a, while use of PDTC, which maintains reduced glutathione stores, abolished both ROS generation and HIF-1a stabilization [Chandel et al., 1998, 2000]. Further evidence suggests ROS produced particularly by mitochondrial complex III are responsible for stabilizing HIF-1a during hypoxia. Suppressing the Rieske iron-sulfur protein of complex III, in HEK293, and Hep3B cells, increased both ROS production and HIF-1a stabilization under hypoxia [Brunelle et al., 2005; Guzy et al., 2005]. It has also been shown that application of antimycin A which increases complex III ROS production, enhances hypoxic HIF-1a accumulation [Chandel et al., 2000]. It was found that cells deficient in cytochrome b subunit of complex III, which are respiratory incompetent, are still able to increase ROS levels and stabilize HIF-1a [Bell et al., 2007]. Also, cytosolic ROS inhibition by MitoQ, a mitochondrial-targeted antioxidant; was shown to reduce hypoxic HIF-1a stabilization in cells with wild type and cytochrome b deficient mitochondria [Bell et al., 2007]. Thus implying that cytochrome b part of complex III, is not responsible for ROS mediated HIF-1a stabilization. This group found the Qo site of complex III, to be the primary site of ROS generation during hypoxia. This was supported with the finding that hypoxic HIF-1a stabilization was inhibited by stigmatellin, a Qo site inhibitor and enhanced by antimycin A when used in cells with wild-type mitochondria [Bell et al., 2007].
A second model of mitochondrial oxygen sensing however, negates the involvement of ROS release from complex III in HIF-1a stabilization under hypoxia. This model indicates that reduced mitochondrial electron transport chain activity results in reduced consumption of O2 and an increase in cytosolic oxygen levels. The net result is more availability of oxygen to PHD, which then leads to HIF-1a degradation. In the case of mitochondrial ETC inhibition, similar increases in cytosolic O2 levels leads to HIF-1a degradation. In line with this hypothesis, loss of HIF-1a stabilization was observed with general mitochondrial ETC inhibitors as well as antimycin A, at concentrations that inhibit ETC, in hypoxic 143B and HEK 293 cells [Chua et al., 2010]. These experiments also showed HIF-1a stabilization after adding TMPD, an artificial electron donor to complex IV, in presence of complex III inhibition [Chua et al., 2010]. Providing electrons to complex IV and bypassing complex III, still stabilized HIF-1a during hypoxia [Chua et al., 2010]. This finding lead to the idea that stabilization of HIF-1a is not limited to complex III ROS production but is dependent on the mitochondrial ETC as a whole.
Further, there is evidence to suggest that ROS are not even, or at least directly, involved in HIF-1a stabilization under hypoxia. SOD (superoxide dismutase) 1 and SOD2 overexpression did not prevent HIF-1a from stabilizing during hypoxia, which indicates that mitochondrial superoxide was not directly required for HIF-1a stabilization under hypoxia [Guzy et al., 2005]. Similarly, the addition of an SOD mimetic, MnTBAP, or superoxide scavengers including N-acetylcysteine (NAC) and ascorbate, did not seem to affect the hypoxic stabilization of HIF-1a [Chua et al., 2010]. Aside from superoxide, earlier evidence had shown that exogenous H2O2 inhibited HIF-1a expression and DNA binding activity [Wang et al., 1995]. In a study by Haddad et. al, use of general ROS scavengers such as NAC and glutathione was shown to induce HIF-1a stabilization under hypoxic to hyperoxic O2 pressure change in a concentration-dependent manner [Haddad et al., 2000].
Mechanistic experiments also fail to show ROS species such as H2O2, directly inducing HIF-1a via PHD inhibition but rather through an asparaginyl hydroxlyase called factor inhibiting HIF (FIH) [Masson et al., 2012]. In an in vitro assay with cell lysates, it has further been shown that both low and high concentrations of H2O2 (50 uM and 200 uM) did not affect pVHL binding to a recombinant GST-HIF-1a protein. GST-HIF-1a is a recombinant fusion of GST and HIF-1a oxygen dependent degradation domain (ODDD) and it contains proline 564 that can be hydroxylated by PHD [Chua et al., 2010]. Thus it seems that H2O2 produced by SO released from mitochondria does not stabilize HIF-1a by direct PHD inhibition in a similar manner that has been shown during anoxia or when agents such as DMOG, DFO, or COCl2 are administered [Guzy et al., 2005]. Despite the conflicting evidence it appears that hypoxic HIF-1a stabilization, is an independent mechanism than ROS production from complex III. It is likely that HIF-1a stabilization under hypoxia depends more on mitochondrial ETC oxygen handling. Nevertheless, current evidence regarding the role of mitochondria and ROS fail to provide a clear mechanism for HIF-1a stabilization during hypoxia.
ROS REGULATION OF HIF-1 STABILIZATION UNDER NORMOXIA
Although, the role of mitochondrial SO or H2O2 on hypoxic HIF-1a stabilization remains controversial, normoxic HIF-1a stabilization by ROS seems to be more conclusive. Studies have shown that addition of exogenous H2O2 or glucose oxidase, which generates H2O2, is sufficient to stabilize HIF-1a protein under normoxia [Chandel et al., 2000; Brunelle et al., 2005; Guzy et al., 2005]. Bell et. al. noted that catalase, which breaks down H2O2 into water and O2, inhibited the stabilization that was observed with glucose oxidase [Bell et al., 2007]. Another group, found that both glucose oxidase and exogenous H2O2 stabilized HIF-1a and HIF-2a in Hep3B and HEK293 cells in normoxia [Guzy et al., 2005]. In a different approach, it was shown that HIF-1a levels increased when using siRNA against Manganese Superoxide Dismutase (Mn-SOD) [Kaewpila et al., 2008]. SOD1 inhibition via DCC also greatly increased expression of HIF-1a in normoxia, but not significantly in hypoxia [Chua et al., 2010]. Furthermore, use of DMNQ redox cycler was shown to increase normoxic HIF-1a levels, in a concentration dependent manner in epithelial lung carcinoma cell lines [Köhl et al., 2006]. These findings implicate SO and potentially H2O2 in normoxic HIF-1a stabilization. As far as mechanistic evidence, Chua et. al. have shown that in normoxia, SO, generated by xanthine/xanthine oxidase, partially inhibited pVHL from binding to HIF-1a. This was not seen with H2O2 [Chua et al., 2010]. In another study, siRNA of Mn-SOD was shown to inhibit pVHL-HIF binding, thus inhibiting PHD activity, under normoxia [Sasabe et al., 2010]. Studies involving pVHL-HIF binding assay provide strong evidence regarding oxidation state of the ODDD domain of HIF-1a protein and its stabilization in presence of SO. These observations are irrespective of hypoxic conditioning and indicate direct inhibiton of PHD activity in presence of SO. However, in terms of the exact mechanism of PHD inhibition by SO, the evidence is not conclusive. It has been suggested that O2- may oxidize the iron (Fe2+) co-factor of the PHD enzyme, thus inhibiting its activity [Brüne and Zhou, 2007].
NO EFFECT ON HIF-1a STABILIZATION AND ACTIVITY
NO has been shown to stabilize HIF-1a under both normoxia and hypoxia [Sandau et al., 2001; Mateo et al., 2003; Metzen et al., 2003]. Increased endogenous NO via iNOS induction leads to HIF-1a accumulation in both hypoxic and normoxic conditions [Mateo et al., 2003]. HIF-1a was shown to accumulate in iNOS overexpressing LLC-PK1 cells upon iNOS stimulation or when co-cultured with NO producing activated macrophages [Sandau et al., 2001]. However, when different sources and concentrations of NO are examined, the evidence becomes contradictory. Some findings show that 1 uM concentrations of endogenous NO, induced by iNOS, enhance HIF-1a accumulation and DNA binding activity in both normoxic and hypoxic conditions [Mateo et al., 2003]. Lower concentrations of endogenous NO (<400 nM), showed inhibition of HIF-1a stabilization under hypoxia 19. In the case of NO donors, normoxic HIF-1a accumulation was shown to be more rapid but less stable in presence of low concentrations (100 uM), whereas, at high concentrations (1 mM), the accumulation was slower but more stable [Sandau et al., 2001]. Kimura et. al. showed enhanced accumulation and DNA binding activity of HIF-1a with 500 uM of an NO-donor, S-nitro-N-acetylpenicillamine (SNAP) in A-172, and Hep3B cells under normoxia [Kimura et al., 2000]. In contrast, it was shown that under hypoxia, high concentrations of NO donors inhibit HIF-1a accumulation and DNA binding activity [Huang et al., 1999; Kimura et al., 2000]. Given the current evidence, it appears that moderate to high (500 uM to 1 mM) concentrations of NO donors induce HIF-1a accumulation and DNA binding activity under normoxia, but inhibit the same responses under hypoxia. With respect to endogenous NO, high concentrations (1 uM) seem to stabilize HIF-1a under both normoxia and hypoxia while lower concentrations (<400 nM) inhibit hypoxic stabilization of HIF-1a. These observations as mentioned above, are tested under variable oxygenation states, sources and concentrations of NO. Although, studies with exogenous NO application fail to mimic physiological NO effects, they do provide insight into the mechanism of regulation of HIF-1a by NO.
Several mechanisms have been proposed in regards to NO mediated HIF-1a stabilization. It has been proposed that S-nitrosation contributes to HIF-1a stabilization in a concentration dependent manner [Palmer et al., 2000; Metzen et al., 2003; Yasinska and Sumbayev, 2003]. GSNO, which donates nitrosonium, seems to induce HIF-1a accumulation; an effect which is reversible in the presence of antioxidants such as glutathione and ascorbate [Sumbayev et al., 2003]. Additionally, GSNO and similar agents were shown to induce S-nitrosation of all 15 of the free thiol groups of purified HIF-1a [Palmer et al., 2000; Sumbayev et al., 2003]. Aside from direct protein modification, another suggested mechanism of NO mediated HIF-1a accumulation is through PHD inhibition. GSNO was shown to inhibit the interaction of pVHL with HIF-1a ODDD in the presence of PHD1, PHD2, and PHD3 under normoxia [Metzen et al., 2003]. All of these observations were made in normoxic conditions. Overall, it appears that NO directly enhances HIF-1a stability and activity in normoxia either through post-translational modification of HIF-1a protein or by inhibiting PHD activity. The extent to which these mechanisms pertain to physiological levels of endogenous NO under hypoxia or normoxia is unclear from the available evidence.
THE INTERPLAY BETWEEN NO AND SO
Aside from direct effect of NO on HIF-1a, interactions between NO and SO also appear to mediate HIF-1a regulation. NO and SO interaction seems to be synergistic in certain conditions and antagonistic in others. On one hand, NO and SO react with each other to produce a number of reactive intermediates capable of nitrating, nitrosating, or oxidizing proteins. On the other hand, it has been shown that presence of SO leads to decreased endogenous NO mediated cellular signaling and post-translational modification of proteins [Thomas et al., 2006; Brüne and Zhou, 2007]. These variable effects result in differential regulation of HIF-1a stability in presence of NO and SO and has led to contradictions in the field.
Evidence suggests that NO mediated HIF-1a stabilization is independent of the mitochondrial ETC inhibition. In high concentrations of endogenous NO, hypoxic, and normoxic HIF-1a stabilization was unaffected in the presence of several mitochondrial respiratory chain inhibitors such as rotenone and myxothiazol [Mateo et al., 2003]. Whether ROS levels increased or decreased by these interventions, was not reported in this study [Mateo et al., 2003]. Meanwhile, the addition of H2O2 alongside NO has been shown to reduce HIF-1a stabilization [Thomas et al., 2006]. On the other hand, spermine-NONOate, a donator of NO radicals, caused S-nitrosation of purified HIF-1a protein in presence of SO under normoxia [Sumbayev et al., 2003]. Interaction of NO donor DETA-NO and the redox cycler DMNQ, which donates SO, was shown to have a dose dependent effect on HIF-1a accumulation in A-549 cells. Addition of DMNQ to cells treated with DETA-NO attenuated NO mediated HIF-1a accumulation at 1–40 uM concentrations. However, at greater than 40 uM concentrations of DMNQ, HIF-1a stabilization was recovered in cells treated with DETA-NO [Köhl et al., 2006]. The apparent contradiction in these observations could in part be due to source, concentrations, and the exact balance of NO and SO needed to stabilize HIF-1a. Interaction of low concentrations of endogenous NO and SO seem to inhibit HIF-1a stabilization. However, high concentrations of NO donors along with SO seem to play synergistic roles in stabilizing HIF-1a under normoxia. It has been proposed that SO may serve as a co-signal for NO mediated HIF-1a stabilization, however, the bioavailability of NO is affected by the amount of SO present, such that more NO is needed to achieve the same response in presence of SO [Brüne and Zhou, 2007b]. The interaction between NO and SO is especially noteworthy, as both agents are released endogenously under altered oxygenation states, and may pose differential regulatory roles on HIF-1a in a concentration dependent manner. In addition, the exact mechanism of HIF-1a regulation by either SO or NO, may not be conclusively determined without taking into account presence or absence of the other agent.
TRANSCRIPTIONAL AND TRANSLATIONAL REGULATION OF HIF-1a MEDIATED BY ROS
It has been shown that increased intracellular SO levels, secondary to knocking down Mn-SOD, enhances HIF-1a gene expression in oral squamous cell carcinoma (OSCC) cells under normoxia [Sasabe et al., 2010]. This effect seems to be through transcriptional and translational regulation of HIF-1a via ERK and PI3K/AKT. Specifically, SO-induced HIF-1a transcription and translation were inhibited in presence of PD98059 and LY294002, which inhibit ERK and PI3K, respectively [Sasabe et al., 2010]. Others have also reported an increase in transcription of HIF-1a under hypoxia by ROS through induction of PI3K/AKT and ERK phosphorylation [Koshikawa et al., 2009; Du et al., 2011]. In general, ROS seem to enhance the signaling activity of ERK and PI3K/AKT, which in return induce HIF-1a transcription and translation via a number of regulators such as p70S6K1, 4E-BP1, Rac1, HDAC, and mTOR [19,31–34]. Additional studies have suggested the involvement of PI3K and ERK in NO mediated HIF-1a accumulation [Sandau et al., 2000; Kasuno et al., 2004]. The increased levels of HIF-1a were due to increased HIF-1a protein synthesis via PI3K and ERK signaling and regulators such as p70S6K, 4E-BP1, and Elf-4E [Kasuno et al., 2004]. Interestingly, the interplay between SO and NO as indicated above, may play a role in HIF-1a transcription and translation via these common signaling intermediates. A recent report regarding thrombin induced angiogenesis, showed induction of NO and ROS as well as HIF-1a in response to thrombin, which was mediated by signaling intermediate Rac1. Furthermore, in this study the balance between ROS and NO regulated the HIF-1a mediated angiogenic response to thrombin in HMEC-1 cells [Petry et al., 2012].
Additional pathways that involve ROS mediated transcriptional and translational regulation of HIF-1a is through inflammatory mediators. It has been shown that GSH depletion or exogenous ROS, in specific H2O2, increase synthesis of inflammatory mediators such as TNF-α and IL-1β [Haddad, 2002a, 2002b]. TNF-α and IL-1β can in turn induce transcription and protein synthesis of HIF-1a under normoxia [Westra et al., 2007]. The suggested mechanisms for up-regulation of HIF-1a by inflammatory cytokines is MAPKp38 and via PI3K/AKT phosphorylation [Westra et al., 2007; De Lemos et al., 2013] (Fig. 1).
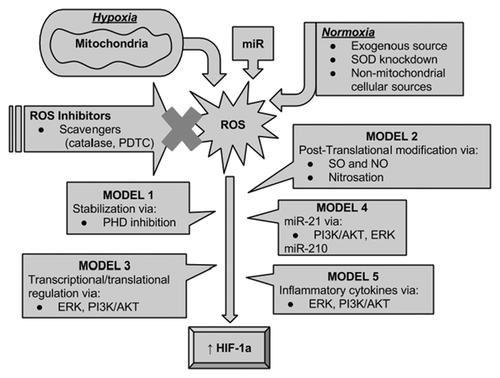
MICRORNA AND ROS MEDIATED REGULATION OF HIF-1a
miR-21
The links between miR-21 and HIF-1a have been the subject of investigation in cancer research [Loscalzo, 2010]. Liu et. al. demonstrated that inhibition of miR-21 caused a decrease in the activation of endogenous AKT and ERK as well as HIF-1a expression. Using inhibitors of AKT and ERK and knocking down HIF-1a, miR-21-induced angiogenesis was diminished to less than 40 percent [Liu et al., 2011]. Furthermore, inhibition of AKT and ERK abolished HIF-1a protein levels [Liu et al., 2011]. AKT and ERK regulation of HIF-1a has been reported in literature to be mediated by ROS [Du et al., 2011]. Interestingly, in a study by Zhang et. al. stable expression of miR-21 enhanced ROS levels under ischemia-reperfusion by reducing expression of SOD2 and SOD3 in NL20 cells lines [Zhang et al., 2012]. It has also been shown that miR-21 is induced by H2O2 in vascular smooth muscles [Lin et al., 2009]. It is therefore possible that the interplay between miR-21 and ROS may lead to activation of AKT and ERK pathways and contribute to miR-21 regulation of HIF-1a.
miR-210
MiR-210 has also been implicated in ROS mediated HIF-1a regulation. Overexpression of miR-210 in A549 cells was shown to induce HIF-1a levels 72 h after transfection and inhibition of miR-210 during hypoxic conditions reduced HIF-1a protein levels after 48–72 h [Puissegur et al., 2011]. Additional experiments showed, succinate dehydrogenase complex, subunit D (SDHD), a subunit of complex II of the mitochondrial ETC, to be a direct target of miR-210 [Puissegur et al., 2011]. Pre-miR-210 was shown to reduce luciferase reporter activity of SDHD in lung adenocarcinoma A549 cells [Puissegur et al., 2011]. These showed, mitochondrial dysfunction with decreased complex II subunit (SDHA) and succinate dehydrogenase (SDH) complex II activity [Puissegur et al., 2011]. These cells also showed increased luciferase reporter activity of hypoxia response element (HRE). The authors thus concluded that miR-210 induces HIF-1a via SDHD inhibition. Mutations in SDHD have shown to increase concentrations of superoxide in Chinese hamster fibroblasts [Owens et al., 2012]. It is possible that the interaction of miR-210 and SDHA potentially induce ROS release. Induction of ROS by miR-210 has been observed by Kim et. al. in adipose derived stem (ACS) cells [Kim et al., 2013]. Interestingly, in this study a reverse relationship between mitochondrial ROS and miR-210 was also seen in ACS cells, such that mitochondrial inhibition by rotenone and antimycin A induced miR-210 [Kim et al., 2013]. Taken together, these studies provide evidence for HIF-1a regulation by miR-210 through inhibition of mitochondrial complex II and possibly via ROS. Interestingly, other studies have shown up-regulation of miR-210 under hypoxia via HIF-1a or through p53 and AKT pathways [Huang et al., 2009; Mutharasan et al., 2011]. Along the same lines, induction of miR-210 by ROS was also shown to be AKT and ERK mediated [Kim et al., 2013]. These mechanism are similar to those suggested for ROS mediated HIF-1a induction. It is possible that ROS induces both HIF-1a and miR-210 via ERK and AKT pathways, HIF-1a then in turn induces mi-R-210, and miR-210 further induces HIF-1a. The involvement of ROS in miR-210 mediated HIF-1a induction is controversial and cell line specific. As mentioned above, in ACS cells miR-210 was shown to induce ROS [Kim et al., 2013]. However, Muthasaran et. al. showed that miR-210 adenoviral transfection in NRCM decreased antimycin A induced mitochondrial ROS production [Mutharasan et al., 2011]. It is therefore, unclear whether induction of HIF-1a by miR-210 or vice versa is ROS mediated (Fig. 2).
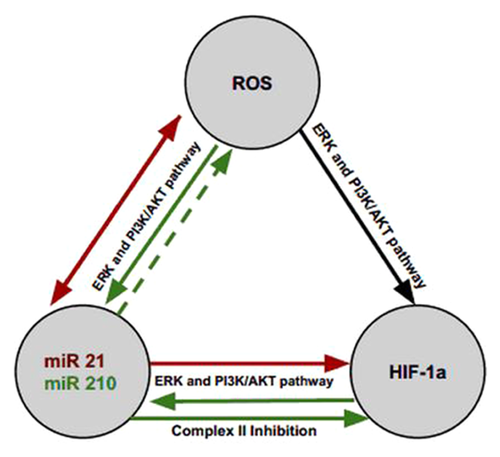
NEW DEVELOPMENTS IN AN OLD DEBATE
Earlier evidence regarding ROS regulation of HIF-1 primarily focused on HIF-1a stabilization via direct PHD inhibition and variable observations have been made with respect to the ROS species (SO vs. H2O2) as well as hypoxic versus normoxic states. Further studies regarding NO mediated post-translational modification of HIF-1a through nitrosation which also involves the interplay of SO and NO introduced additional regulatory pathways by ROS. However most such studies have been inconclusive due to variable sources and concentrations of SO and NO and the methods used such as chemical mitochondrial inhibition and use of exogenous NO donors, mostly because the tested conditions do not mimic physiological levels of either molecule. With growing evidence regarding ROS mediated transcriptional and translational regulation of HIF-1a, specifically through ERK and PI3K/AKT pathways, new and more conclusive insight develops into the regulatory process of HIF-1a under both hypoxic and normoxic conditions. HIF-1a transactivation by MAPK via p-300 co-activator protein, or its induction by Rac1; a PI3K and ERK signaling intermediate, are among such proposed regulatory pathways known to be induced by ROS [Sang et al., 2003; Du et al., 2011; Xue et al., 2011; Hielscher and Gerecht, 2014]. Signaling via ERK and PI3K/AKT are further shown to be involved in miRNA, ROS, and HIF-1a regulatory feedback loops (Fig. 2). This is especially noteworthy, since inhibitors of ERK and PI3K/AKT are shown to inhibit the angiogenic response of miRNAs through HIF-1a [Liu et al., 2011]. Taken together, it becomes apparent, that the old debate of whether mitochondrial ROS released under hypoxia regulate HIF-1a directly via PHD inhibition, gives way ROS mediated regulation of signaling molecules such as ERK and PI3K/AKT whose down stream effectors directly mediate HIF-1a protein stabilization, transactivation as well as transcription and translation, under both hypoxia and normoxia.
CONCLUSION
It has long been known that ROS and HIF-1 signaling are involved in many disease pathologies including cancer, inflammatory diseases, and ischemic injuries. ROS signaling via HIF-1a is a key process that is critical in cellular proliferation and angiogenesis. The growing body of evidence suggests that a number of intermediates play important roles in ROS mediated HIF-1a regulation even under normoxia. Ultimately, targeting specific players in these regulatory pathways, may serve as novel therapeutic approaches for cancer, ischemia and inflammatory diseases.




