A Proteomics-Driven Assay Defines Specific Plasma Protein Signatures in Different Stages of Ménière's Disease
ABSTRACT
Ménière's disease (MD) is a common disorder of the inner ear whose hallmarks are vertigo, tinnitus, aural fullness, and progressive hearing loss. The degree of severity of the disease is quite heterogeneous, and so is its pathogenesis. A multifactorial inheritance of intrinsic and extrinsic factors has been described, but there is not a common agreement on the molecular basis of MD. In a recent article, we have demonstrated that patients suffering from MD share a common plasma proteomic signature, characterized by the presence of several up- and down-regulated proteins. In this study, we have further extended our analysis and show that the differential expression of plasma proteins can identify specific subsets of MD-affected individuals, depending on their stage. Our findings confirm our plasma proteomics-driven approach as a powerful tool for early diagnosis of MD and uncover a potentially starring role for some proteins in the development and fate of this frustrating disease, whose pathogenesis still remains unclear. J. Cell. Biochem. 115: 1097–1100, 2014. © 2013 Wiley Periodicals, Inc.
Abbreviation
-
- MD
-
- Ménière disease
First described in Ménière [1861] MD is clinically characterized by episodic vertigo, fluctuating hearing loss, aural fullness and tinnitus. MD is disabling, significantly affecting quality of life, and often fallouts in clinical depression.
The epidemiology of the disorder is still not clearly defined. Prevalence rates of MD have varied widely, ranging from 3.5 per 100,000 to 513 per 100,000 [Alexander and Harris, 2010], with more recent data accounting for 190 per 100,000 [Harris and Alexander, 2010]. Such a broad range is likely due to methodological differences, changes over time in diagnostic criteria, difficulty in differential diagnosis from related conditions. MD is more likely to occur in women and the prevalence increases with aging [Alexander and Harris, 2010].
Despite many years of research, the exact causes, pathophysiology and treatments of MD still remain elusive.
Histologically, post-mortem examination reveals endolymphatic hydrops [Hallpike and Cairns, 1938; Yamakawa, 1938].
The American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) Hearing and Equilibrium Subcommittee has published guidelines for the classification and reporting of MD. These guidelines, which specify character, frequency, and duration of vertigo attacks necessary to achieve a diagnosis of MD, have been updated periodically, with the last update in 1995 [Committee on Hearing and Equilibrium, 1995]. The diagnostic scale for MD is presently based on clinical criteria. As suggested in AAO-HNS guidelines, only definite and certain categories of patients are to be considered as affected by MD.
Regardless of this classification, the question as to whether endolymphatic hydrops is the initial trigger of the disease or whether hydrops is an epiphenomenon deriving from biochemical alterations, still remains unresolved. In addition, the diagnosis of MD is still based on clinical criteria, and no test is available to conclusively identify the disease.
In the present study, we extended the analysis of our previous work [Chiarella and Saccomanno, 2012], aimed at evaluating the proteomic pattern of plasma proteins from patients suffering from MD. Moreover, we have attempted to correlate specific protein profiles with the stage of the disease.
MATERIALS AND METHODS
PARTICIPANTS
The study was performed at the Department of Experimental and Clinical Medicine, Audiology and Phoniatrics Unit and Laboratory of Proteomics and Mass Spectrometry (Proteomics@UMG), Magna Graecia University of Catanzaro (Italy). The research protocol has been cleared by our Institution Ethics Review Board for human studies and patients as well as healthy donors have signed an informed consent.
Fifteen patients (7 men, 8 women, mean age 53.4 years) affected by definite, unilateral, MD and 12 healthy anonymous donors (pair-matched by sex and age) were enrolled. The recruitment was performed according to the following AAO-HNS criteria for definite MD: two or more definitive spontaneous episodes of vertigo 20 min or longer, audiometrically documented hearing loss on at least one occasion, tinnitus or aural fullness in the treated ear, other causes excluded. Staging was based on the four-tone average (arithmetic mean rounded to the nearest whole number) of the pure-tone thresholds at 0.5, 1.2, and 3 kHz of the worst audiogram during the interval 6 months before treatment. Stage 1: <25 dB; stage 2: 26–40 dB; stage 3: 41–70 dB; stage 4: >70 dB [Monsell et al., 1995]. All MD patients had a follow up of more than twelve months (Table I).
| Stage | Patients | Sex (F/M) |
|---|---|---|
| 1 | 2 | 1/1 |
| 2 | 1 | 1/0 |
| 3 | 11 | 6/5 |
| 4 | 1 | 0/1 |
To confirm affected side and to exclude other pathologies, all subjects underwent complete otoneurological assessment including caloric and kinetic tests, positional maneuvers, electrophysiological tests (auditory brainstem responses, vestibular evoked myogenic potentials (VEMPs) and ocular VEMPs) and imaging studies [gadolinium enhanced Cerebral Magnetic Resonance and Cerebral MR venography as in Chiarella et al., 2012] according to our protocol for assessment of vestibular pathology.
SAMPLE PREPARATION
Ten milliliter of whole blood were drawn by venipuncture and collected in K2EDTA tubes (Becton Dickinson and Company, Franklin Lakes, NJ) according to Human Proteome Organization (HUPO) plasma proteome guidelines [Rai et al., 2005]. Each sample was immediately centrifuged (1,300g, 10 min) and 20 µl aliquots were stored into 0.5 ml Eppendorf tubes at −80°C until use.
Depletion of highly abundant protein from whole plasma was performed as previously described [Chiarella and Saccomanno, 2012]. Briefly, each sample was subjected to the Proteome-Lab™ IgY-12 Proteome Partitioning Kit (Beckman Coulter, Crea, CA) according to the manufacturer's specifications in order to remove the 12 most abundant proteins (albumin, total IgGs, α1-antitrypsin, IgA, IgM, transferrin, haptoglobin, α1-acid glycoprotein, α2-macroglobulin, HDL-binding apolipoproteins A-1 and A-2, fibrinogen).
GEL ELECTROPHORESIS
Separation of the proteins by 1D sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) was performed according to Laemmli [1970] using a Bio-Rad apparatus (Bio-Rad, Hercules, CA). A 12.5% linear resolving gel and a 2.5% stacking gel were used to separate the proteins. Concentration of the low-abundance proteins-enriched plasma was determined by the bicinchoninic acid (BCA) protein assay reagent (Thermo Fisher Scientific, Rockford, IL). The samples were diluted 1:1 vol/vol with loading buffer, heated to 95°C for 3–4 min, and 25 to 50 µg protein were loaded on the gel. SDS–PAGE was run at constant voltage (120 V) until the bromophenol blue front reached the end of the gel.
When required, gels were stained overnight with Coomassie Brilliant Blue R-20 solution (1 mg dye, 250 ml methanol, 35 ml acetic acid in a final volume of 500 ml) and destained in 100 ml of distilled water containing 20% glacial acetic acid and 10% methanol.
WESTERN BLOTTING
Plasma proteins obtained from each MD-patient and healthy control were separated as described and subsequently electro-transferred from the gel onto Immobilon-P PVDF (0.45 µm) membrane (Merk Millipore, Billerica, MA) using a Bio-Rad apparatus (Bio-Rad, Hercules, CA). The blots were blocked in 5% non-fat milk solution in Tris buffered saline (TBS) prior to incubation with specific antibodies.
The following primary antibodies were used to demonstrate the differential expression of plasma proteins in MD versus healthy individuals: (a) anti-complement factor H (sheep polyclonal), (b) anti-complement factor B (rabbit monoclonal), (c) anti-fibrinogen (α-chain) (rabbit polyclonal), (d) anti-fibrinogen-(γ-chain) (rabbit polyclonal), (e) anti-vitamin D-binding protein (rabbit polyclonal), (f) anti-β-actin (rabbit polyclonal), (g) anti-pigment epithelium-derived factor (rabbit polyclonal), (h) anti-apolipoprotein A-1 (rabbit polyclonal), (j) anti β-2-glycoprotein (rabbit polyclonal). All antibodies were purchased from Abcam (Cambridge, UK). Excess antibody was removed by washing the blot three times with TBS and the membrane was subsequently probed with secondary antibody. Antibody binding was visualized by using the ECL chemiluminescence method (Amersham-GE Healthcare, Piscataway, NJ).
DENSITOMETRIC ANALYSIS
Densitometric analysis of the differentially expressed protein bands was performed by using the free-software Image Processing and Analysis in Java—Image J (http://rsbweb.nih.gov/ij/).
RESULTS
WESTERN BLOTTING ANALYSIS
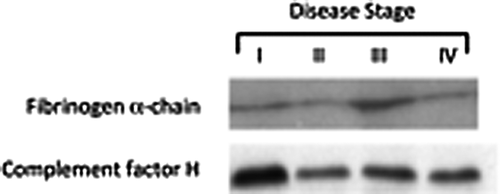
Three technical replicates of Western blots from each sample were performed in order to reach a statistical significance. Figure 1 shows the results of the densitometric analysis. Interestingly, the trend of expression of the plasma proteins probed in our experimental setting fully confirmed the findings reported in our previous work [Chiarella and Saccomanno, 2012]. In detail, complement factor-B and C, fibrinogen α-chain, β-actin, pigment epithelium-derived factor and fibrinogen γ-chain were found to be upregulated in MD-affected patients with respect to the healthy donors, while vitamin D-binding protein, apolipoprotein A-1 and β-2-glycoprotein were under-expressed in patients versus controls plasma.

PLASMA PROTEINS EXPRESSION AND CLINICAL STAGE OF MD
- All stage III patients show a significant and homogeneous increase in the expression of fibrinogen α- and γ-chain.
- β-2-Glycoprotein is modestly under-expressed in the stage IV patient compared to the overall MD-population.
- Stage I individuals have a consistently higher expression of complement factor H and B proteins with respect to the other patients.
DISCUSSION AND CONCLUSIONS
Ménière's disease is inner-ear disease characterized by the clinical association of recurrent rotatory vertigo, fluctuating sensorineural hearing loss, aural fullness and tinnitus. Even though genetic, epigenetic and environmental factors have been considered to play a significant role in its pathogenesis, the molecular mechanisms underlying the development of MD are still unclear [Pirodda and Brandolini, 2010; Salt and Plontke, 2010].
In this study we have performed a targeted Western blotting analysis of plasma proteins from patients with different stages of MD, with the aim: (i) to confirm our recent work which suggests a correlation between the presence of the disease and a “proteomic signature,” and (ii) to further extend this analysis, in the attempt to link the disease status to a specific protein pattern.
To this end, we have enrolled 15 patients with different degree of definite MD (2 in stage I, 1 in stage II, 11 in stage III and 1 in stage IV). Following a depletion of the 12 most abundant plasma proteins, in order to enrich the low abundancy-fraction, each sample was probed with nine distinct antibodies, reacting with the proteins that were found to be differentially expressed in our previous work.
More specifically, the following proteins were detected and their amount determined by a densitometric assay: complement factor H, complement factor B, fibrinogen (α-chain), fibrinogen (γ-chain), vitamin D-binding protein, β-actin, pigment epithelium-derived factor, apolipoprotein A-1, β-2-glycoprotein.
As already stated in the Results Section, the analysis performed on this specific set of patients not only confirms the findings reported earlier, but also adds new interesting insights in to the pathophysiology of MD, by linking peculiar protein profiles with a specific stage of the disease. In particular, the fibrinogen α- and γ-chain proteins are significantly and homogeneously over-expressed in stage III individuals, while a not analogously consistent and uniform upregulation could be detected in the rest of MD patients. On the other hand, both patients with a stage I disease show a higher amount of complement factor H and B in their plasma when compared to the other samples, suggesting that expression of these inflammation-related proteins might be negatively regulated during disease progression. A third interesting finding coming out from the densitometric analysis of protein patterns is the slight decrease of the β-2-glycoprotein in stage IV patient. This protein, known to bind to heparin and other negatively charged molecules, avoiding the activation of the intrinsic blood coagulation cascade through an interaction with phospholipids on the surface of damaged cells, is significantly downregulated in the plasma of MD patients form stage I-to-III. Again, it appears that a transcriptional/post-translational modulation of the β-2-glycoprotein is occurring in the advanced form of the disease.
Overall, the findings reported in the present study confirm and strengthen our previous findings and pinpoint a potential role of a set of plasma proteins an effective tool for a biomarker-oriented diagnosis and staging of MD. Moreover, even though our work was not primarily aimed at providing molecular insights into the pathogenesis of this common disorder of the inner ear, we can postulate that, at least few of the proteins here identified, might play a role in the development and/or the clinical evolution of Ménière's disease.




