miRNA27a Is a Biomarker for Predicting Chemosensitivity and Prognosis in Metastatic or Recurrent Gastric Cancer
Abstract
We previously identified five miRNAs (miR-1, miR-20a, miR-27a, miR-34a, and miR-423-5p) that are up-regulated in gastric cancer. The goal of this study was to investigate the value of these miRNAs as potential biomarkers for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer patients who received first-line chemotherapy. A total of 82 patients with metastatic or recurrent GC receiving first-line chemotherapy were included in our study. The expression levels of the five miRNAs were evaluated using hydrolysis probe-based stem-loop quantitative reverse transcription polymerase chain reaction (qRT-PCR) in individual samples before first-line chemotherapy. Patients receiving first-line chemotherapy with fluoropyrimidine combined with oxaliplatin or paclitaxel were chosen for the chemosensitivity analysis. The relationships between expression of the five-miRNAs and clinicopathological parameters, response to chemotherapy and prognosis were analyzed statistically. Patients with higher miRNA1 expression levels tended to have a higher rate of liver metastasis, and higher miRNA34a expression levels occurred more frequently in males (P = 0.022). The expression of the remaining three miRNAs showed no obvious relationship to any of the clinicopathological features. The partial response rates of the patients with high miRNA1 expression and low miRNA1 expression were 11.1% and 23.1%, respectively (P = 0.048). Similar results were observed for miRNA27a (the partial response rate was 7.7% vs. 25.9%, P = 0.018). Patients with up-regulated miRNA27a expression had a significantly worse overall survival (OS) than patients with lower miRNA27a expression (P = 0.024). In patients with MRGC, miRNA27a is a potential biomarker for predicting resistance to fluoropyrimidine-based chemotherapy and a novel prognostic marker for gastric cancer. J. Cell. Biochem. 115: 549–556, 2014. © 2013 Wiley Periodicals, Inc.
Gastric cancer (GC) is the second most common cause of cancer death worldwide [Jemal et al., 2010]. Curative surgical resection is the most effective treatment modality for gastric cancer, but only 30–60% of patients receive this treatment [Wanebo et al., 1993], and a recurrence rate of 40–60% after curative surgery is observed [Yoo et al., 2000; D'Angelica et al., 2004]. Most patients die of recurrent or metastatic disease, leading to a poor prognosis for gastric cancer. Therefore, the role of palliative chemotherapy in the treatment of gastric cancer is especially important. The commonly used first-line combination chemotherapy regimens consist of a fluoropyrimidine plus a platinum agent with or without docetaxel [Van Cutsem et al., 2006; Kim et al., 2008; Choi et al., 2012; Jiang et al., 2013]. However, the response to chemotherapy differs widely among GC patients, and patients who show a poor response to first-line chemotherapy always have a poor prognosis. The usefulness of traditional serum tumor biomarkers such as carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), carbohydrate antigen 242 (CA242) and carbohydrate antigen 724 (CA724) for screening, diagnosis, and follow-up monitoring of gastric cancer has been recognized for decades. However, the ability of these biomarkers to predict the response to chemotherapy and treatment outcome for GC patients who receive first-line chemotherapy is limited. Palliative chemotherapy is an essential therapeutic approach for the metastatic or recurrent GC (MRGC). The identification of novel biomarkers that are predictive of treatment outcome and survival should allow a better identification of different patient subpopulations and provide clinicians with improved individualized treatment options.
MicroRNAs (miRNAs) are a class of small, non-coding RNAs (20–22 nucleotides in length) that mediate gene expression through complementary binding to the 3′ untranslated regions (UTRs) of target genes and the subsequent regulation of messenger RNA translation and degradation [Bartel, 2004]. It has been reported that approximately 50% of miRNAs are located in cancer-associated genomic regions or at fragile sites [Calin et al., 2004]. The misexpression of miRNAs correlates with poor overall survival in cancer patients, and increasing evidence indicates that miRNAs are associated with sensitivity or resistance to chemotherapeutic drugs in various cancers [Ji et al., 2008; Kovalchuk et al., 2008; Yang et al., 2008]. Several miRNAs, such as miRNA let-7i [Liu et al., 2012], miR-181b, and miR-21 [Jiang et al., 2011], have been reported to correlate with the outcome of GC. However, most studies have focused on the use of miRNAs for early cancer diagnosis using tumor tissues. Serum and plasma miRNAs are more stable and relatively easier to access than tissue samples. Human serum miRNAs have been extracted successfully in previous studies, and different miRNA expression patterns can help identify various types of cancer [Chen et al., 2008; Mitchell et al., 2008; Ng et al., 2009]. Recently, our group has identified a five-miRNA (miR-1, miR-20a, miR-27a, miR-34a, and miR-423-5p) signature for GC diagnosis using genome-wide serum miRNA expression profiling. We found that the expression levels of these five serum miRNAs were significantly up-regulated in GC and correlated with tumor stage. Therefore, we have demonstrated that this profile can serve as a novel diagnostic biomarker for early GC detection [Liu et al., 2011]. In the present study, we examined whether the levels of these five miRNAs in serum samples taken prior to first-line chemotherapy could be a useful biomarker for predicting the response to chemotherapy and outcome in patients with metastatic or recurrent GC (MRGC).
MATERIALS AND METHODS
PATIENTS AND SERUM SAMPLES
A total of 82 blood samples were collected from patients who were diagnosed with metastatic or recurrent GC before first-line chemotherapy at Department of Gastrointestinal Medical Oncology, Tianjin Medical University Cancer Institute and Hospital between December 2011 and July 2012 for this study. Patients with other malignancies and those who had no measurable lesions were excluded. To evaluate tumor responses to first-line chemotherapy, the lesions were measured after every two courses of chemotherapy using CT or MRI imaging. The Response Evaluation Criteria for Solid Tumors (RECIST 1.1) was used to classify the tumor responses [Watanabe et al., 2009]. Progression-free survival (PFS) was defined as the time elapsed between chemotherapy initiation and tumor progression or death from any cause. Overall survival (OS) was defined as the time from the first day of chemotherapy to death from any cause. This study was performed with the approval of the Hospital Ethics Committee.
SERUM PREPARATION, RNA ISOLATION, AND qRT-PCR ASSAY
All samples were processed within 1 hour and stored at 4°C. Serum separation was accomplished by centrifugation at 3,000g for 10 min, followed by a 15-min high-speed centrifugation at 12,000g to completely remove cell debris. The supernatant serum was collected and stored at −80°C until analysis.
The RNA isolation and qRT-PCR assay were performed as described in previous studies [Chen et al., 2005, 2005, 2008; Liu et al., 2011]. Briefly, we extracted total RNA from 100 µl serum by phenol/chloroform purification and centrifugation in isopropyl alcohol.
For qRT-PCR assays, 2 µl of total RNA was reverse-transcribed to cDNA using AMV reverse transcriptase (TaKaRa, Dalian, China) and a stem-loop RT primer (Applied Biosystems, Foster City, CA). Hydrolysis probes (Applied Biosystems) were used for the qRT-PCR analysis. This system is highly specific for the target miRNA. Real-time PCR was performed using a TaqMan PCR kit on the Applied Biosystems 7500 Sequence Detection System (Applied Biosystems). All reactions were performed in triplicate.
The expression levels of the five miRNAs were normalized to the internal miR-16 control. The absolute expression values were calculated according to standard curves generated using synthetic miRNA oligonucleotides (miR-16) at known concentrations. The reliability and reproducibility of all these methodologies have been confirmed in our previous studies.
STATISTICAL ANALYSIS
The χ2 test was performed to investigate the relationships between the expression levels of the five miRNAs and clinicopathological features. Fisher's exact test was used if necessary. Cumulative survival rates were calculated using the Kaplan–Meier method. Univariate and multivariate Cox regression analyses were used to examine whether the expression of the five miRNAs effectively predicted patient survival. All statistical analyses were performed using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL). P values <0.05 were considered statistically significant.
RESULTS
THE CLINICOPATHOLOGICAL SIGNIFICANCE OF THE EXPRESSION OF FIVE-miRNAs IN MRGC
Our previous study [Liu et al., 2011] demonstrated that the expression levels of the five miRNAs were significantly higher in the GC cases compared to the control cases in both the training set and validation set. In this study, we investigated the correlations between the five miRNAs and the clinicopathological parameters for the MRGC patients. The results are presented in Tables I and II. The 82 MRGC cases were classified into two groups according to the median expression of the five miRNAs. The five miRNAs showed no relationship to any of the clinicopathological features investigated including age, performance status, tumor location, tumor differentiation, peritoneal dissemination, number of metastatic organs and previous gastrectomy history. However, it was noted that patients with higher miRNA1 expression levels tended to have a greater liver metastasis burden than the patients with lower expression levels (46.3% vs. 26.8%, P = 0.067). Furthermore, higher miRNA34a expression levels were more likely to occur in male patients (P = 0.022). The specific molecular mechanisms underlying these observations should be studied further.
| Characteristics | miRNA1 | miRNA20a | miRNA27a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | Low | High | P | |
| Age (years) | |||||||||
| ≤60 | 29 | 22 | 0.111 | 25 | 26 | 0.820 | 25 | 26 | 0.820 |
| >60 | 12 | 19 | 16 | 15 | 16 | 15 | |||
| Gender | |||||||||
| Male | 22 | 30 | 0.067 | 23 | 29 | 0.169 | 25 | 27 | 0.647 |
| Female | 19 | 11 | 18 | 12 | 16 | 14 | |||
| KPS | |||||||||
| ≥80 | 34 | 32 | 0.577 | 32 | 34 | 0.577 | 35 | 31 | 0.265 |
| <80 | 7 | 9 | 9 | 7 | 6 | 10 | |||
| Location | |||||||||
| Proximal stomach | 7 | 9 | 0.952 | 7 | 9 | 0.831 | 9 | 7 | 0.540 |
| Body stomach | 8 | 7 | 9 | 6 | 5 | 10 | |||
| Distal stomach | 22 | 21 | 21 | 22 | 23 | 20 | |||
| Entire stomach | 4 | 4 | 4 | 4 | 4 | 4 | |||
| Tumor differentiation | |||||||||
| Moderately differentiated | 8 | 10 | 0.780 | 9 | 9 | 0.937 | 9 | 9 | 0.937 |
| Moderately–poorly differentiated | 4 | 5 | 5 | 4 | 4 | 5 | |||
| Poorly differentiated | 29 | 26 | 27 | 28 | 28 | 27 | |||
| Liver metastasis | |||||||||
| Present | 11 | 19 | 0.067 | 12 | 18 | 0.169 | 15 | 15 | 1.000 |
| Absent | 30 | 22 | 29 | 23 | 26 | 26 | |||
| Peritoneal dissemination | |||||||||
| Present | 19 | 18 | 0.824 | 19 | 18 | 0.824 | 17 | 20 | 0.506 |
| Absent | 22 | 23 | 22 | 23 | 24 | 21 | |||
| Number of metastatic organs | |||||||||
| One | 15 | 19 | 0.370 | 16 | 18 | 0.654 | 14 | 20 | 0.179 |
| Two or more | 26 | 22 | 25 | 23 | 27 | 21 | |||
| Gastrectomy history | |||||||||
| Present | 17 | 14 | 0.494 | 15 | 16 | 0.820 | 19 | 12 | 0.111 |
| Absent | 24 | 27 | 26 | 25 | 22 | 29 | |||
| CEA (mg/dl) | |||||||||
| ≤5.0 | 20 | 18 | 0.658 | 22 | 16 | 0.184 | 20 | 18 | 0.658 |
| >5.0 | 21 | 23 | 19 | 25 | 21 | 23 | |||
| CA19-9 (mg/dl) | |||||||||
| ≤37 | 21 | 24 | 0.506 | 24 | 21 | 0.506 | 22 | 23 | 0.824 |
| >37 | 20 | 17 | 17 | 20 | 19 | 18 | |||
| NLR | |||||||||
| ≤2.5 | 22 | 25 | 0.503 | 23 | 24 | 0.823 | 25 | 22 | 0.503 |
| >2.5 | 19 | 16 | 18 | 17 | 16 | 19 | |||
- NLR, neutrophil lymphocyte ratio; KPS, karnofsky performance status.
| Characteristics | miRNA34a | miRNA423-5p | ||||
|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | |
| Age (years) | ||||||
| ≤60 | 26 | 25 | 0.820 | 26 | 25 | 0.820 |
| >60 | 15 | 16 | 15 | 16 | ||
| Gender | ||||||
| Male | 21 | 31 | 0.022 | 23 | 29 | 0.169 |
| Female | 20 | 10 | 18 | 12 | ||
| KPS | ||||||
| ≥80 | 33 | 33 | 1.000 | 34 | 32 | 0.577 |
| <80 | 8 | 8 | 7 | 9 | ||
| Location | ||||||
| Proximal stomach | 7 | 9 | 0.838 | 8 | 8 | 0.639 |
| Body stomach | 8 | 7 | 9 | 6 | ||
| Distal stomach | 21 | 22 | 19 | 24 | ||
| Entire stomach | 5 | 3 | 5 | 3 | ||
| Tumor differentiation | ||||||
| Moderately differentiated | 7 | 11 | 0.557 | 9 | 9 | 0.937 |
| Moderately–poorly differentiated | 5 | 4 | 4 | 5 | ||
| Poorly differentiated | 29 | 26 | 28 | 27 | ||
| Liver metastasis | ||||||
| Present | 12 | 18 | 0.169 | 14 | 16 | 0.647 |
| Absent | 29 | 23 | 27 | 25 | ||
| Peritoneal dissemination | ||||||
| Present | 19 | 18 | 0.824 | 16 | 21 | 0.267 |
| Absent | 22 | 23 | 25 | 20 | ||
| Number of metastatic organs | ||||||
| One | 14 | 20 | 0.179 | 15 | 19 | 0.370 |
| Two or more | 27 | 21 | 26 | 22 | ||
| Gastrectomy history | ||||||
| Present | 15 | 16 | 0.820 | 15 | 16 | 0.820 |
| Absent | 26 | 25 | 26 | 25 | ||
| CEA (mg/dl) | ||||||
| ≤5.0 | 21 | 17 | 0.376 | 21 | 17 | 0.376 |
| >5.0 | 20 | 24 | 20 | 24 | ||
| CA19-9 (mg/dl) | ||||||
| ≤37 | 22 | 23 | 0.824 | 22 | 23 | 0.824 |
| >37 | 19 | 18 | 19 | 18 | ||
| NLR | ||||||
| ≤2.5 | 25 | 22 | 0.503 | 25 | 22 | 0.503 |
| >2.5 | 16 | 19 | 16 | 19 | ||
miRNA1 AND miRNA27a ARE PREDICTIVE FOR CHEMOSENSITIVITY TO FLUOROPYRIMIDINE + OXALIPLATIN/PACLITAXEL TREATMENT
We investigated the relationships between the levels of the five miRNAs in pretreatment serum samples and patient response to first-line chemotherapy. Among the 82 MRGC patients, 53 patients received a fluoropyrimidine + oxaliplatin or fluoropyrimidine + paclitaxel regimen and the other 29 patients were treated with different regimens. The overall objective response rate (PR) and disease control rate of patients receiving first-line treatment were 12.2% and 46.3%, respectively. For the 53 patients who received fluoropyrimidine-based chemotherapy the objective response rate and disease control rate were 17.0% and 56.6%, respectively. As fluoropyrimidine-based chemotherapy remains a major treatment option for advanced gastric cancer patients, we selected the 53 patients who received fluoropyrimidine + oxaliplatin or fluoropyrimidine + paclitaxel regimens as the target population to evaluate the ability of the five miRNAs to predict the response to chemotherapy. Of the 53 patients, 35 patients received fluoropyrimidine + oxaliplatin treatment, and 18 patients received a fluoropyrimidine + paclitaxel regimen. There was no significant difference between the two groups in response to chemotherapy (Table III). Among the 27 patients with high miRNA1 expression, only 3 (11.1%) patients showed partial response to fluoropyrimidine-based treatment, whereas the remaining 26 patients showed stable or progressive disease. In contrast, among the 26 patients with low expression, 6 (23.1%) had a partial response to treatment (P = 0.048). Similar results were observed for miRNA27a (partial response rates of 7.7% vs. 25.9%, P = 0.018, Table IV). We did not find any predictive ability for the other three miRNAs (data not shown). As for the 29 patients who did not receive fluoropyrimidine-based chemotherapy, the objective response rate (PR) and disease control rate was 3.4% and 55.2% respectively. However, a significant difference between the five-miRNAs expression and the response to chemotherapy was not found for this population.
| Fluoropyrimidine + oxaliplatin | Fluoropyrimidine + paclitaxel | P | |
|---|---|---|---|
| PR | 6 | 3 | 0.760 |
| SD | 15 | 6 | |
| PD | 14 | 9 |
- PR, partial response; SD, stable disease; PD, progressive disease.
| miRNA1 expression | P | miRNA27a expression | P | |||
|---|---|---|---|---|---|---|
| Low | High | Low | High | |||
| PR | 6 | 3 | 0.048 | 7 | 2 | 0.018 |
| SD | 6 | 15 | 6 | 15 | ||
| PD | 14 | 9 | 14 | 9 | ||
OUTCOME OF THE PATIENTS RECEIVING FLUOROPYRIMIDINE-BASED FIRST-LINE CHEMOTHERAPY
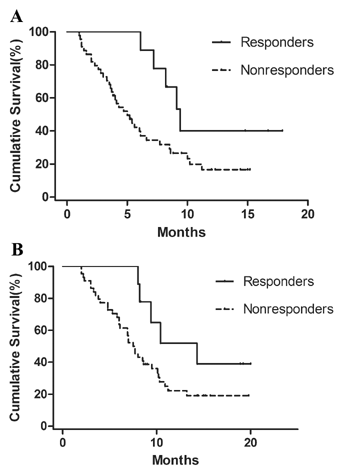
The median PFS and OS values for the patients who received fluoropyrimidine-based chemotherapy were 5.6 and 8.2 months, respectively. Among the 53 patients, 9 showed a partial response (PR) to chemotherapy, whereas the remaining 44 showed stable or progressive disease. The PFS was significantly longer in responders to fluoropyrimidine + oxaliplatin/paclitaxel treatment than the non-responders (9.4 vs. 5.0 months, P = 0.044, Fig. 1A). A survival advantage was found in the responders compared with the non-responders (14.3 vs. 7.5 months) for OS. However, no significant difference was found (P = 0.071, Fig. 1B).
miRNA27a EXPRESSION IS PROGNOSTIC FOR PATIENTS WITH MRGC RECEIVING FIRST-LINE CHEMOTHERAPY
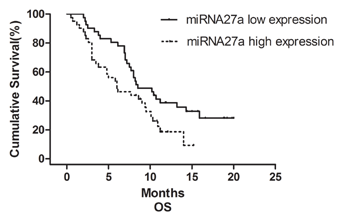
To determine whether any of the five miRNAs has potential as prognostic biomarkers for MRGC, we performed a patient survival analysis using the Kaplan–Meier method. Patients with higher miRNA27a expression had a significantly worse OS than those with lower miRNA27a expression (6.0 vs. 8.5 months, P = 0.024, Fig. 2). In addition to the low miRNA27a expression group, male patients (P = 0.026), KPS ≥ 80 (P = 0.003), those with previous gastrectomy (P = 0.008), those receiving second-line chemotherapy (P = 0.031), and those with a neutrophil lymphocyte ratio (NLR) ≤ 2.5 (P = 0.003) exhibited a better prognosis than those in the alternate groups (Table V). Furthermore, multivariate analysis revealed that miRNA27a expression was an independent predictive factor for OS in these patients (P = 0.043, Table VI).
| Characteristics | N | Median survival (months; 95% CI) | Log-rank χ2 value | P |
|---|---|---|---|---|
| Age (years) | ||||
| ≤60 | 51 | 7.2 (5.7–8.7) | 0.508 | 0.476 |
| >60 | 31 | 10.1 (8.1–12.1) | ||
| Gender | ||||
| Male | 52 | 9.5 (7.7–11.3) | 4.936 | 0.026 |
| Female | 30 | 6.0 (3.7–8.3) | ||
| KPS | ||||
| ≥80 | 66 | 9.0 (6.7–11.3) | 8.971 | 0.003 |
| <80 | 16 | 5.3 (1.2–9.4) | ||
| Location | ||||
| Proximal stomach | 16 | 8.2 (5.5–10.9) | 2.619 | 0.454 |
| Body stomach | 15 | 10.1 (5.3–14.9) | ||
| Distal stomach | 43 | 8.0 (5.6–10.4) | ||
| Entire stomach | 8 | 6.1 (3.5–8.7) | ||
| Tumor differentiation | ||||
| Moderately differentiated | 18 | 10.1 (6.3–13.9) | 1.475 | 0.478 |
| Moderately–poorly differentiated | 9 | 9.5 (5.8–13.2) | ||
| Poorly differentiated | 55 | 7.5 (5.5–9.5) | ||
| Liver metastasis | ||||
| Present | 30 | 10.4 (5.9–14.9) | 3.223 | 0.073 |
| Absent | 52 | 7.7 (6.2–9.2) | ||
| Peritoneal dissemination | ||||
| Present | 37 | 7.7 (5.4–10.0) | 0.119 | 0.730 |
| Absent | 45 | 8.5 (6.3–10.7) | ||
| Number of metastatic organs | ||||
| One | 34 | 8.2 (5.8–10.6) | 0.001 | 0.972 |
| Two or more | 48 | 8.0 (5.6–10.4) | ||
| Gastrectomy history | ||||
| Present | 31 | 10.9 (6.1–15.7) | 6.988 | 0.008 |
| Absent | 51 | 6.9 (5.2–8.6) | ||
| Second-line chemotherapy | ||||
| Present | 20 | 10.9 (7.3–14.5) | 4.678 | 0.031 |
| Absent | 62 | 7.2 (5.4–9.0) | ||
| CEA (mg/dl) | ||||
| ≤5.0 | 38 | 8.6 (5.7–11.5) | 1.286 | 0.257 |
| >5.0 | 44 | 7.2 (4.8–9.6) | ||
| CA19-9 (mg/dl) | ||||
| ≤37 | 45 | 8.3 (6.2–10.4) | 0.630 | 0.427 |
| >37 | 37 | 8.0 (5.9–10.1) | ||
| NLR | ||||
| ≤2.5 | 47 | 10.1 (7.2–13.0) | 8.967 | 0.003 |
| >2.5 | 35 | 6.1 (4.8–7.4) | ||
| miRNA1 | ||||
| High expression | 41 | 8.0 (3.6–12.4) | 0.000 | 0.984 |
| Low expression | 41 | 8.2 (6.9–9.5) | ||
| miRNA20a | ||||
| High expression | 41 | 7.7 (3.5–11.9) | 0.010 | 0.920 |
| Low expression | 41 | 8.3 (7.2–9.4) | ||
| miRNA27a | ||||
| High expression | 41 | 6.0 (2.6–9.4) | 5.071 | 0.024 |
| Low expression | 41 | 8.5 (5.5–11.5) | ||
| miRNA34a | ||||
| High expression | 41 | 7.7 (3.6–11.8) | 0.011 | 0.916 |
| Low expression | 41 | 8.3 (7.2–9.4) | ||
| miRNA423-5p | ||||
| High expression | 41 | 6.9 (3.8–10.0) | 0.398 | 0.528 |
| Low expression | 41 | 8.6 (7.1–10.1) | ||
| Variable | χ2 | P-Value | Hazard ratio (95% CI) |
|---|---|---|---|
| Gender | 3.041 | 0.081 | 1.618 (0.942–2.780) |
| KPS | 6.207 | 0.013 | 2.175 (1.180–4.009) |
| Gastrectomy history | 3.780 | 0.052 | 1.792 (0.995–3.227) |
| Second-line chemotherapy | 3.876 | 0.049 | 0.519 (0.270–0.997) |
| NLR | 4.903 | 0.027 | 1.844 (1.073–3.170) |
| miRNA27a | 4.098 | 0.043 | 1.751 (1.018–3.014) |
DISCUSSION
The dysregulation of miRNA expression in GC has been widely reported. As oncogenes or tumor suppressor genes, miRNAs play important roles in GC. Chan et al. [2008] found that miR-21 was overexpressed in 92% of GC patients, and patients with higher expression levels of miR-20b or miR-150 in undifferentiated GC had poorer overall survival [Katada et al., 2009]. Our previous studies identified a five-miRNA signature for GC diagnosis using genome-wide serum miRNA expression profiling. We have demonstrated that this profile can serve as a novel diagnostic biomarker for early GC detection. In this study, we further selected the stage IV GC patients as our target population and investigated the correlations between the miRNAs expression and patient treatment outcome. To our knowledge, there have been few studies investigating the relationship between serum miRNAs and the outcome of stage IV MRGC patients.
Previous studies have reported that tumor response to first-line chemotherapy is correlated with patient survival in advanced GC [Ichikawa and Sasaki, 2006; Kodera et al., 2011]. Consistent with these studies, we showed that the PFS was significantly longer for responders to fluoropyrimidine + oxaliplatin/paclitaxel treatment than for non-responders (9.4 vs. 5.0 months, P = 0.044, Fig. 1A). Although we could not draw a similar conclusion for the OS because of our small sample size, an obvious increase in survival time was observed in the responders compared with the non-responders (14.3 vs. 7.5 months, P = 0.071, Fig. 1B). Thus, the outcome in patients with stage IV MRGC is markedly dependent on the response to first-line chemotherapy. However, chemosensitivity differs among individuals, and primary or acquired drug resistance may become a critical factor in treatment failure. In this context, it is very important to identify biomarkers that can be used to predict the response to first-line chemotherapy. Therefore, we investigated the expression of five miRNAs in serum samples obtained prior to first-line chemotherapy. We demonstrated that miRNA1 and miRNA27a were useful biomarkers for predicting the response to fluoropyrimidine-based treatment and those patients with low levels of miRNA1 and miRNA27a expression demonstrated better chemosensitivity.
In the subsequent univariate and multivariate analysis of the whole population, we demonstrated that the upregulation of miRNA27a expression was independently correlated with poor OS. However, miRNA1 was not found to be an independent prognostic factor. The importance of miRNA27a in tumors has been strongly supported by previous studies. For instance [Li et al., 2013] found that the miR-23a/24-2/27a cluster, which is regulated by c-myc, promotes mammary carcinoma cell invasion and hepatic metastasis by targeting sprouty2. With regard to GC, researchers have shown that miR-27a is up-regulated in human gastric adenocarcinoma. By targeting the 3′ untranslated region (3′UTR) of prohibitin, the mRNA and protein levels of prohibitin are down-regulated [Liu et al., 2009]. These results strongly support our findings. The up-regulated miRNA27a reduces the expression of prohibitin. So in this way, it may promote gastric cancer cell growth and lead to a dismal prognosis for gastric cancer patients. Another reason for the worse survival may due to resistance to chemotherapy. Patients with up-regulated miRNA27a showed a poor response to first-line chemotherapy. Resistance to chemotherapy resulted in the rapid progression in a very short period of time and patients with systemic metastases died of multiple organ failure in the end. It is possible to conclude that miRNA27a has the potential to serve as a biomarker for predicting the response to chemotherapy and subsequent prognosis in patients with MRGC. Meanwhile, miRNA1 may function as an indicator of chemosensitivity but not as a prognostic indicator. However, our study may have some limitations, including its small sample size and retrospective design. A large cohort, multi-center clinical trial is needed in the future to validate our findings.
In our study, a relatively lower objective response rate was found for patients with elevated levels of these two miRNAs (23.1% and 25.9%, respectively) possibly because signet-ring cell and mucous cell adenocarcinoma accounted for a large proportion (28.3%) of patients. This distribution is not consistent with the overall epidemiology of GC. Therefore, a more balanced study should be conducted in the future.
Previous studies have indicated that activation of the anti-apoptotic pathway and the overexpression of multidrug transporter proteins are involved in chemotherapy resistance. This raises the question of how miRNA1 and miRNA27a confer resistance to chemotherapy in GC. The higher miRNA1 expression was correlated with the worse response rate to the first-line chemotherapy, which may due to the increased liver metastasis. However, the statistical difference was not found (P = 0.067). miRNA1 mediates muscle atrophy by regulating the levels of HSP70, and it is therefore considered to be a muscle-specific miRNA [Kukreti et al., 2013]. There were seldom studies concentrating on the correlations between miRNA1 expression and tumorigenesis. So the molecular mechanisms on how the up-regulated miRNA1 expression mediates tumorigenesis and whether it can confer resistance to chemotherapy by sensitizing liver metastasis need to be studied further by in vitro and in vivo experiments. miRNA-27a is an oncogene that contributes to drug resistance in various types of cancer, such as ovarian cancer [Li et al., 2010] and esophageal squamous cell carcinoma [Zhang et al., 2010]. The expression of miR-27a was up-regulated in multidrug resistant (MDR) cancer cell lines A2780DX5 and KB-V1. The treatment of A2780DX5 cells with miR-27a antagomirs decreased the expression of P-glycoprotein and MDR1 mRNAs [Zhu et al., 2008]. Zhao et al. [2011] found that the down-regulation of miRNA27a could significantly decrease the expression of P-glycoprotein and the transcriptional activity of cyclinD1 and up-regulate the expression of p21 to inhibit the proliferation and drug resistance of gastric cancer cells.
Additionally, we demonstrated that an elevated NLR was independently correlated with poor overall survival in MRGC patients using univariate and multivariate analyses. Only limited information on the clinical and prognostic significance of NLR in patients with advanced gastric cancer has been reported [Yamanaka et al., 2007; Jung et al., 2011]. The imbalance of NLR in the peripheral blood of cancer patients may affect tumor development by counteracting the antitumor immune response [Petrie et al., 1985]. This result may suggest that a systemic inflammatory response is associated with the progression of gastric cancer in addition to the dysregulation of microRNAs found in the present study.
In summary, these data suggest that miRNA27a is a novel indicator of chemosensitivity and prognosis in patients with metastatic and recurrent gastric cancer who receive first-line chemotherapy. miRNA27a may be a potential therapeutic target for gastric cancer patients and improve their prognosis.