Inhibition of the canonical Wnt pathway by high glucose can be reversed by parathyroid hormone-related protein in osteoblastic cells†
The authors have declared that no competing interests exist.
Abstract
Recent in vivo findings suggest that the bone sparing effect of parathyroid hormone-related protein (PTHrP) in diabetic mice might occur at least in part through targeting a suppressed Wnt/β-catenin pathway in osteoblasts. We here aimed to examine the inhibitory action of a high glucose environment on specific components of the canonical Wnt pathway, and the putative compensatory effects of PTHrP, in osteoblastic cell cultures. Mouse osteoblastic MC3T3-E1 cells and primary cultures of fetal mouse calvaria were exposed to normal (5.5 mM) or high (25 mM) D-glucose (HG), with or without PTHrP (1–36) or PTHrP (107–139) for different times. In some experiments, MC3T3-E1 cells were incubated with the Wnt pathway activators Wnt3a and LiCl, or were transfected with plasmids encoding either a mutated β-catenin that cannot be targeted for degradation or a human PTHrP (−36/+139) cDNA, or the corresponding empty plasmid, in the presence or absence of HG. The gene expression of Wnt3a and low density receptor-like proteins (LRP)-5 and 6, as well as β-catenin protein stabilization and β-catenin-dependent transcription activity were evaluated. Oxidative stress status under HG condition was also assessed. The present data demonstrate that HG can target different components of the canonical Wnt pathway, while β-catenin degradation appears to be a key event leading to inhibition of Wnt/β-catenin signaling in mouse osteoblastic cells. Both PTHrP peptides tested were able to counteract this deleterious action of HG. These in vitro findings also provide new clues to understand the underlying mechanisms whereby PTHrP can increase bone formation. J. Cell. Biochem. 114: 1908–1916, 2013. © 2013 Wiley Periodicals, Inc.
Osteoporosis is an important complication increasing the risk of fracture in diabetic patients [Blakytny et al., 2012]. In these patients, mainly in those with type 1 diabetes mellitus (T1D), a decreased bone formation is a hallmark determining bone loss, but the underlying mechanisms are poorly understood. A reduction in insulin and insulin-like growth factor 1, accumulation of advanced glycation end products, as well as changes in the synthesis of, and/or response to, calciotropic hormones [namely, parathyroid hormone (PTH) and vitamin D] might all be contributing factors [Wongdee and Charoenphandhu, 2011].
The canonical Wnt/β-catenin pathway is now known to be an important modulator of osteoblast function and bone formation [Glass and Karsenty, 2007; Deschaseaux et al., 2009]. This pathway is triggered by binding of Wnt glycoprotein family members (such as Wnt-1 and 3) to a co-receptor complex including Frizzled (Fzd), a member of the family of G protein-coupled receptors, and low density receptor-like proteins (LRP)-5 and 6 [Mao et al., 2001a, b]. This is followed by phosphorylation (inactivation) of glycogen synthase kinase (GSK)3β, leading to β-catenin translocation into the nucleus where it forms a complex with a T-cell factor (TCF) to induce the transcription of osteoblastic genes [e.g., Runx2 and osteoprotegerin (OPG)] that are Wnt targets [Gaur et al., 2005; Sato et al., 2009].
Several lines of evidence indicate that the canonical Wnt signaling can be directly targeted by PTH in osteoblasts. This hormone, within 24 h, up-regulates LRP-6 and Fzd1 gene expression while decreasing that of the Wnt pathway inhibitor Dickkopf 1 (DKK1) in the rat metaphysis, and in rat osteoblast-like UMR 106 cells, related to an increased β-catenin [Kulkarni et al., 2005]. Activation of canonical Wnt pathway is also in part responsible for the stimulatory effect of PTH on fracture repair in mice [Kakar et al., 2007]. Also of interest, PTH and the N-terminal fragment of PTH-related protein (PTHrP), a PTH counterpart as physiological modulator of bone remodeling at local level [Datta and Abou-Samra, 2009], modulate several components of the canonical Wnt pathway associated with various osteogenic actions in rodents [Bellido et al., 2005; Li et al., 2007; Guo et al., 2010; de Castro et al., 2012]. Interestingly, β-catenin/TCF-dependent transcription activation by Wnt3a was synergistically increased in human osteoblastic SaOS2 cells upon exposure to PTH [Suzuki et al., 2008]. In fact, the bipartite transcription factor β-catenin/TCF, the major effector of the canonical Wnt pathway, can be induced in a Wnt-independent manner by PTH. Thus, this hormone acting through Smad3 can enhance β-catenin levels as part of its anti-apoptotic action in osteoblastic MC3T3-E1 cells [Tobimatsu et al., 2006]. Furthermore, PTH has been reported to interact with its type 1 receptor (PTH1R) in osteoblasts to elicit the formation of a ternary complex containing this receptor, its ligand and LRP6 which promotes β-catenin stabilization [Wan et al., 2008]. The PTH1R has recently been shown to directly interact with β-catenin itself, affecting this receptor signaling [Yano et al., 2013], and also with disheveled, which then recruits axin and thus disrupts the destruction complex of β-catenin, leading to its transcriptional activation [Romero et al., 2010]. Collectively, these studies strongly support the implication of Wnt/β-catenin signaling in the bone anabolic action of PTH family of peptides.
Recent findings indicate a suppression of the Wnt pathway in the long bones of streptozotozin (STZ)-induced T1D mice [Portal-Núñez et al., 2010; Hie et al., 2011]. It was also shown that PTHrP downregulation appears to be an important mechanism contributing to bone loss in this model [Lozano et al., 2009, 2011]. Furthermore, intermittent administration of two different PTHrP peptides exerted bone anabolic effects related to an increased β-catenin immunostaining in the diabetic mouse tibia [Lozano et al., 2009, 2011; Portal-Núñez et al., 2010]. These findings suggest that PTHrP interacts with the Wnt/β-catenin pathway to re-establish the decreased bone formation in diabetic osteopenia.
In the present study, we aimed to explore the putative mechanisms whereby a high glucose environment would inhibit the canonical Wnt pathway using osteoblastic cell cultures. We also examined the ability of PTHrP—through its N-terminal and C-terminal domains—to interact with specific components of this pathway in these cells.
MATERIALS AND METHODS
Cell Cultures
Mouse osteoblastic MC3T3-E1-subclone four cells (CRL-2593; American Type Culture Collection, Mannassas, VA) were grown in α-minimum essential medium (MEM) with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin, and 2 mM glutamine in 5% CO2 at 37°C. Cells (10–15 × 103 cells/cm2) were incubated with normal (5.5 mM) or high (25 mM) D-glucose (HG), in the presence or absence of the conditioned medium from Wnt3a-overexpressing L-cells (CM-Wnt3a), at fourfold dilution [Nuche-Berenguer et al., 2010], 25 mM LiCl, or the PTHrP peptides tested, at 100 nM, in standard medium for 1 day or medium supplemented with 50 µg/mL ascorbic acid and 10 mM β-glycerophosphate (osteogenic medium) for 5 days. Cell culture medium and the agonists were replaced every other day to avoid any unwanted pH alterations which might affect the results.
Primary cultures of mouse osteoblastic cells (mOBs), kindly provided by Dr. V. Ruíz (Instituto de Investigaciones Biológicas-Alberto Sols, Madrid), were prepared by sequential collagenase and dispase digestion of calvariae from E18.5 mouse embryos [Pacheco et al., 2012]. These cells were maintained in the aforementioned osteogenic medium for 2 weeks before exposure to HG with or without different agonists for 1 day.
Some MC3T3-E1 cell cultures were stably transfected with pcDNA3.1 plasmid containing a human PTHrP (−36/+139) cDNA and an epitope tag at the C-terminus corresponding to human influenza hemaglutinin (HA; for detection by Western blot; kindly provided by Dr. A. García-Ocaña; Department of Medicine, Division of Endocrinology and Metabolism, University of Pittsburgh, Pittsburgh, PA) or empty plasmid. Transfections were carried out in serum-free medium with 1 µg of each plasmid and 6 µl of X-tremeGENE 9 DNA Transfection Reagent (Roche Diagnostics, Indianapolis, IN). Stably transfected clones were selected by treatment with 500 µg/ml geneticin (G418, Life Technologies, Paisley, UK). Human PTHrP overexpression was confirmed by Western analysis. To prevent clonal selection artifacts, two different PTHrP-overexpressing clones were used for experiments as described below.
Western Blot Analysis
Cell total protein was extracted with 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS, supplemented with protease inhibitor cocktail P8340 (Sigma–Aldrich, St. Louis, MO), and phosphatase inhibitor cocktail Set II (Calbiochem, La Jolla, CA). Cell protein content was determined by a bicinchoninic acid-based standard method (Thermo Fisher Scientific, Rockford, IL), using bovine serum albumin (BSA) as standard. Protein extracts (5–20 µg) were separated on 8–12% polyacrylamide–SDS gels under reducing conditions. After electrophoresis, samples were transferred onto nitrocellulose membranes, followed by blocking with 2.5% defatted milk in 50 mM Tris–HCl, pH 7.5, 150 mM NaCl with 0.05% Tween-20. We used the following primary antibodies: a rabbit polyclonal anti-β-catenin antibody (Abcam, Cambridge, UK), a rabbit polyclonal anti-Ser9-phospho-GSK3β (p-GSK3β) antibody, and mouse monoclonal antibodies against GSK3β or HA (Cell Signaling, Danvers, MA). After overnight incubation at 4°C with these antibodies, corresponding horseradish peroxidase-conjugated IgG was added for 1 h, at room temperature. As loading control, anti-α-tubulin antibody was used. Protein bands were developed with Supersignal West Dura (Thermo Fisher Scientific) and analyzed by densitometric scanning.
Gene Expression Analysis
Total RNA was extracted with Trizol (Invitrogen, Groningen, the Netherlands). RNA retrotranscription was carried out with 0.5–1 µg of total RNA performed with the cDNA High capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Real-time-PCR was done with unlabeled mouse specific primers for the following genes: 5′-GCACCACCGTCAGCAACAG-3′ (sense) and 5′-GGGTGGCTTTGTCCAGAACA-3′ (antisense; Wnt3a); 5′-CAACGTGGACGTGTTTTATTCTTC-3′ (sense) and 5′-CAGCGACTGGTGCTGTAGTCA-3′ (antisense; LRP-5); and 5′-AGATCCATCAAGTGGGTTCATGTA-3′(sense) and 5′-GAAGCGACTTGAGCCATCCA-3′ (antisense; LRP-6), and SYBR Premix Ex-Taq green (Takara, Otsu, Japan), or an Assay-by-Design™ system using TaqMan MGB probes (Applied Biosystems) for OPG, Runx2, lymphoid enhancer-binding factor-1 (Lef-1), Cyclin D1 (Ccnd1), connexin-43, catalase, and “growth arrest and DNA damage 45” (GADD45) and Premix Ex-Taq (Takara) in an ABI PRISM 7500 system (Applied Biosystems). Melting curves were used to verify single PCR product amplification. Results were expressed in mRNA copy numbers, calculated for each sample using the cycle threshold (Ct) value, and normalized against 18S rRNA as described [Lozano et al., 2009, 2011].
Topflash Luciferase Assay
MC3T3-E1 cells were grown with normal glucose or HG for up to 5 days. Thereafter, these cells were transfected with a mixture of 1 µg of TOPFLASH TCF reporter plasmid, containing six copies of the TCF/LEF DNA binding site upstream of a thymidine kinase (TK) minimal promoter and a luciferase reporter gene (Millipore, Billerica, MA), and 5 ng of a renilla coding plasmid (pRL-TK; Promega, Madison, WI; transfection control) using X-tremeGENE 9 DNA Transfection Reagent, as described above. In some experiments, 1 µg of pCI-neo β-catenin S33Y plasmid (Addgene, Cambridge, MA), which codes for a mutated β-catenin protein (S33Y) that cannot be targeted for degradation, was co-transfected in MC3T3-E1 cells. After 24 h of transfection, cells were further exposed to HG, in the presence or absence of CM-Wnt3a, at 25% (v/v), or 25 mM LiCl, with or without the p38 inhibitor, SB203580 (Calbiochem), at 10 µM, for 24 h. Two different PTHrP-overexpressing MC3T3-E1 cell clones were transfected with the TOPFLASH TCF reporter plasmid in the aforementioned conditions, and then were exposed to normal glucose or HG for 24 h. Some TOPFLASH-transfected MC3T3-E1 cell cultures were exposed to each PTHrP peptide tested, at 100 nM, with or without the protein kinase (PK) A inhibitor, adenosine 3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-cAMPS; Santa Cruz Biotechnology, Santa Cruz, CA), at 25 µM, or the PKC inhibitor, calphostin C (Calbiochem), at 200 nM, for 24 h. In all cases thereafter, cells were homogenized with lysis buffer and luciferase/renilla activity was quantified with a Dual Luciferase kit, following the manufacturer's instructions (Promega) using a luminometer FB/12 Sirius (Berthold technologies, Bad Wildbad, Germany). Renilla activity values were used to normalize the luciferase values [Nuche-Berenguer et al., 2010].
Forkhead Box (FOXO) Luciferase Assay
Subconfluent MC3T3-E1 cells were transfected with 1 µg of FoxO-luciferase reporter plasmid (gently provided by M. Almeida, Ph.D., University of Arkansas, Little Rock, AR) [Almeida et al., 2007] and 10 ng of pRL-TK plasmid mixture, as described above. After 24 h of transfection, cells were pre-incubated with CM-Wnt3a for 1 h, followed by addition of HG or normal glucose medium for 24 h. Then, luciferase/renilla activity was quantified as described above.
Reactive Oxygen Species (ROS) Measurement
Subconfluent MC3T3-E1 cells were treated or not with HG for 24 h in standard culture medium. Then, cells were washed with phosphate-buffered saline and loaded with 2,7-dichlorofluorescein diacetate (2,7-DCFH-DA), at 5 µM, for 10 min. HG-induced increase of fluorescence was quantified by flow citometry using a FACscalibur (BSbioscience, San José, CA), following a previously described protocol [Peiró et al., 2001].
Immunocytochemical Localization of β-Catenin
Subconfluent native or PTHrP-overexpressing MC3T3-E1 cells grown on coverslips were exposed to HG, in the presence or absence of CM-Wnt3a, at 25% (v/v), for 24 h. Cells were then fixed with Merckofix (Merck, Whitehouse Station, NJ), and permeabilized with 0.1% Triton-X-100 for 10 min. After blocking with 5% BSA for 30 min, cells were incubated with polyclonal anti-β-catenin antibody (Abcam) followed by a fluorescein isothiocyanate-conjugated secondary antibody (Sigma–Aldrich) for 1 h. Absence of primary antibody was used as negative control. Cells were also stained with the DNA fluorescent dye propidium iodide. Samples were mounted in Mowiol 4-88 (Calbiochem) and examined using a Leica DM-IRB confocal microscope.
Statistical Analysis
Results are expressed as mean ± SEM throughout the text. Differences between experimental and control conditions were analyzed by using two-tail t-test or Mann–Whitney test, when appropriate. P < 0.05 was considered significant. Statistical analysis was performed using statistical software (GraphPad InStatTM V2.04a+).
RESULTS
HG Reduces β-Catenin Accumulation in Osteoblastic Cells
Consistent with our recent in vivo results [Portal-Núñez et al., 2010], we found here that exposure of osteoblastic MC3T3-E1 cells to HG for up to 5 days in osteogenic medium elicits a decrease in β-catenin protein levels, associated with decreased p-GSK3β protein levels in these cells (Fig. 1A, top). This inhibitory effect of HG on β-catenin protein stabilization was confirmed in primary fetal mouse osteoblastic cells (mOBs; Fig. 1A, top). Therefore, the underlying mechanisms of this negative interaction of HG with the Wnt/β-catenin pathway in osteoblastic cells were subsequently explored. We initially focused on some key upstream elements in this pathway which might be affected by HG in osteoblastic cells. Gene expression of Wnt3a, a major Wnt protein activating this canonical pathway [Ling et al., 2010], was found to be downregulated by HG in MC3T3-E1 cells (Fig. 1B). In addition, LRP-5 and 6 co-receptors, which have proven to be essential for Wnt signaling [Mao et al., 2001a, b], were also downregulated by HG in these cells (Fig. 1B). However, using immunoblotting and/or immunocytochemistry, we observed that HG-medium was also able to prevent β-catenin accumulation induced by exogenously added Wnt3a in MC3T3-E1 cells and mOBs (Fig. 1A, bottom, and C).
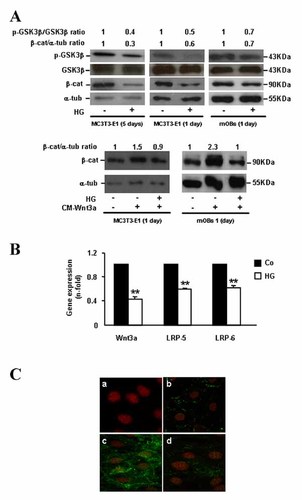
High glucose (HG) affects several components of the canonical Wnt pathway related to β-catenin destabilization in osteoblastic cells. A: MC3T3-E1 cells and mOBs were exposed to normal glucose (control; Co) or HG, without or with CM-Wnt3a, for 1–5 days. β-catenin and p-GSK3β protein expression was assessed by Western blot. Representative autoradiograms are shown. Relative intensities of β-catenin and p-GSK3β signals, which were normalized with α-tubulin and total GSK3β protein levels, respectively, corresponded to at least three independent measurements, and are indicated at the top (fold-induction, means). B: Changes in expression of Wnt-related genes (assessed by real-time-PCR) elicited by exposure to HG for up to 5 days in MC3T3-E1 cells. Values are mean ± SEM of three experiments in duplicate. **P < 0.01 versus Co. C: MC3T3-E1 cells were incubated with normal glucose (panels b and c) or HG (d) for up to 5 days, in the presence (panels c and d) or absence (panel b) of CM-Wnt3a for the last 24 h. Negative control without primary antibody was also shown (panel a). Double immunofluorescence staining was performed to visualize the nucleus (with propidium iodide; red) and β-catenin using a fluorescein isothiocyanate-labeled IgG (green). Images represent results in three independent experiments. β-cat, β-catenin; α-tub, α-tubulin.
HG Inhibits Wnt Signaling by Targeting β-Catenin Degradation in Osteoblastic Cells
Consistent with the aforementioned results by examining β-catenin protein levels, Wnt3a induction of Wnt/β-catenin signaling—assessed by TOPFLASH-luciferase transcriptional activity—was shown to be inhibited by HG in MC3T3-E1 cells (Fig. 2A). Of interest, this inhibitory effect of HG was prevented by a p38 mitogen-activated protein kinase (MAPK) activation inhibitor, SB203580, at 10 µM (Fig. 2A). Putative downstream mechanisms affecting the Wnt/β-catenin pathway which might be targeted by HG in osteoblastic cells were next evaluated. LiCl is known to favour β-catenin stabilization by inhibiting GSK3β [Stambolic et al., 1996]. Exposure of MC3T3-E1 cells to HG-medium was found to reduce LiCl-stimulated TCF-dependent transcription, as shown by analysis of TOPFLASH reporter activity (Fig. 2A), and by analyzing the expression of well characterized Wnt/β-catenin responsive genes [van der Heyden et al., 1998; Filali et al., 2002; Gaur et al., 2005; Dong et al., 2006], namely OPG, Runx2, Lef-1, and connexin-43 (Fig. 2B). These data indicate that promoting β-catenin stabilization by inhibiting GSK3β is not sufficient for preventing the negative effects of HG in these osteoblastic cells. Furthermore, the effect of HG on TCF-dependent transcription was examined in MC3T3-E1 cells co-transfected with a plasmid encoding a S33Y-mutated β-catenin protein that cannot be targeted for degradation. We observed that HG—either in the presence or absence of a p38 MAPK inhibitor—was inefficient to affect TOPFLASH-luciferase reporter activity induced by the β-catenin mutant (Fig. 2A). These aggregated data strongly suggest that β-catenin degradation is a key event targeted by HG to hamper activation of the Wnt pathway.
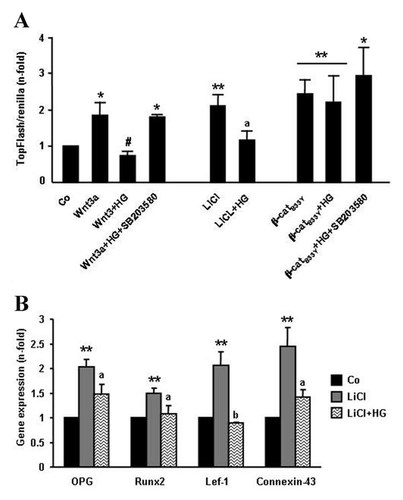
High glucose (HG) induces β-catenin degradation in osteoblastic cells. MC3T3-E1 cells were grown with normal glucose (control; Co) or HG for up to 5 days, and subsequently, cells were transfected with a TOPFLASH TCR reporter plasmid, co-transfected or not with pCI-neo β-catenin S33Y plasmid (β-catS33Y), as described in Materials and Methods Section. Twenty-four hours thereafter, cells were again exposed to HG, in the presence or absence of CM-Wnt3a or 25 mM LiCl, with or without SB203580, at 10 µM, for 24 h. A: TOPFLASH-luciferase reporter activity was determined as described in the text. B: Following total cell RNA isolation, gene expression of TCF-dependent transcriptional activity was determined by real-time-PCR. Values are mean ± SEM of three experiments in duplicate. *P < 0.05; **0.01 versus Co; #P < 0.05 versus Wnt3a value; aP < 0.05; b0.01 versus LiCl value.
Oxidative Stress May Contribute to Inhibition of Wnt Signaling by HG in Osteoblastic Cells
Oxidative stress has been suggested to play an important role in diabetic osteopenia by affecting osteoblast function [Mody et al., 2001; Hamada et al., 2007]. In fact, we here found that ROS production as well as FoxO transcriptional activity, evaluated by FoxO luciferase assay, and FoxO-modulated genes, namely catalase and GADD45, which represent a defense mechanism against oxidative stress [Manolagas, 2010], were all significantly up-regulated by exposure of MC3T3-E1 cells to HG for at least 24 h (Fig. 3A–C). Interestingly, the negative effect of HG on Wnt3a-stimulated TCF-dependent transcription was mirrored by its induction of FoxO transcriptional activity in these cells (Figs. 2A and 3B). In fact, it has been recognized that members of the FoxO protein family can sequester β-catenin thus preventing its interaction with TCF [Almeida et al., 2007]. In this way, oxidative stress might contribute to the observed inhibition of β-catenin activity by HG in mouse osteoblastic cells.
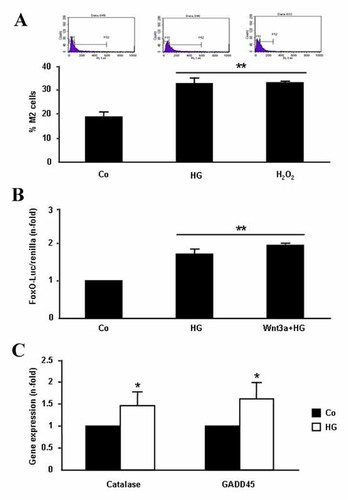
Effect of high glucose (HG) on oxidative stress in MC3T3-E1 cells. These cells were exposed or not (control; Co) to HG for 24 h (A,B) or 5 days (C). A: ROS production was detected in 2,7-DCFH-DA-loaded cells by flow cytometry (representative profiles are shown at the top). Stimulation with 100 µM H2O2 for 10 min was used as positive control. B: FoxO transcriptional activity was determined by using a FoxO-luciferase reported plasmid, as described in Materials and Methods Section. C: Expression of oxidative stress-related genes was analyzed by real-time-PCR, as described in the text. Values are mean ± SEM of at least three experiments in duplicate. *P < 0.05; **0.01 versus Co.
PTHrP Counteracts the Deleterious Effects of HG on the Wnt/β-Catenin Pathway in Osteoblastic Cells
We found here that PTHrP-overexpressing MC3T3-E1 cells (Fig. 4A) exhibited a dramatic increase of β-catenin accumulation (assessed by immunofluorescence; Fig. 4B) as well as of TCF-dependent transcription (Fig. 4C), and both effects were unaffected by exposure to HG. Recently, it was found that in vivo intermittent administration of the N-terminal PTHrP (1–36) fragment or the C-terminal PTHrP (107–139) peptide normalized the diminished β-catenin immunoreactivity in osteoblastic cells in the T1D mouse tibia [Portal-Núñez et al., 2010]. In agreement with this previous in vivo findings, we observed here that exogenous addition of each of these PTHrP peptides was able to prevent the HG-induced decrease of β-catenin accumulation and/or activity in native MC3T3-E1 cells and mOBs (Fig. 5A and B). As expected, based on the previously reported stimulation of the Wnt pathway by PTH in other osteoblastic cell preparations [Kulkarni et al., 2005] PTH (1–34) (Sigma-Aldrich), at 100 nM, was also efficient in this regard in MC3T3-E1 cells exposed to HG. On the other hand, L-glucose (Sigma-Aldrich), at 25 mM, failed to affect the expression of various Wnt pathway target genes in these cells, discarding an osmotic effect of HG as responsible for the observed changes in β-catenin activity (Fig. 5B). Moreover, this positive effect of both PTHrP peptides on the expression of Lef-1, a well characterized β-catenin target gene, was dose-dependent in MC3T3-E1 cells exposed to HG (Fig. 5C).
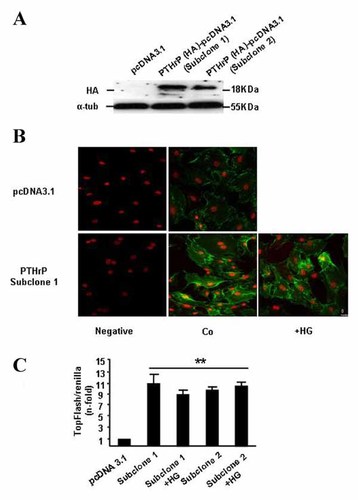
High glucose (HG) exposure does not prevent Wnt pathway activation by PTHrP overexpression in MC3T3-E1 cells. These cells, stably transfected with either pcDNA3.1 plasmid containing a human PTHrP (−36/+139) construct and HA as tag, or empty plasmid, were transfected or not with the TOPFLASH reporter plasmid before exposure to normal glucose (control, Co) or HG for 24 h. A: For experiments, two PTHrP-overexpressing clones were selected, as shown by a representative autoradiogram corresponding to Western analysis. B: Double immunofluorescence staining was carried out to visualize the nucleus (red) and β-catenin (green), as described in the legend to Figure 1C. Negative control without primary antibody was also shown. Images represent results from two independent experiments (C) TOPFLASH-luciferase reporter activity was determined as described in the text. Values are means ± SEM of at least three independent measurements in triplicate. **P < 0.01 versus pcDNA3.1 value.
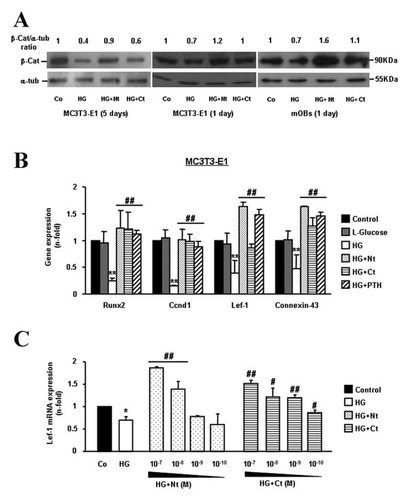
Exogenous PTHrP peptides counteract the inhibitory effect of high glucose (HG) on the Wnt/β-catenin pathway in osteoblastic cells. Native MC3T3-E1 cells and mOBs were exposed to normal glucose (control; Co) or HG, in the presence or absence of PTHrP (1–36; Nt), PTHrP (107–139; Ct), or PTH (1–34; a positive control), each at 100 nM, for up to 5 days. A: β-Catenin protein expression was assessed by Western blot. Representative autoradiograms are shown. Relative intensities of β-catenin signal from at least three independent measurements are indicated at the top (fold-induction, means). β-cat, β-catenin; α-tub, α-tubulin. B: Changes in the expression of Wnt/β-catenin pathway target genes in MC3T3-E1 cells were assessed by real-time-PCR. L-Glucose (25 mM) was used as an osmotic control. C: MC3T3-E1 cells were treated or not with different concentrations of each PTHrP peptide in the presence of HG for 24 h, and Lef-1 gene expression was analyzed by real-time-PCR. Values are mean ± SEM of three experiments in duplicate. *P < 0.05; **0.01 versus Co; #P < 0.05; ##0.01 versus HG value.
As an attempt to characterize the possible intracellular pathways involved in the stimulatory effect of PTHrP (1–36) and PTHrP (107–139) on the Wnt/β-catenin signaling, pharmacological inhibitors of PKA and PKC pathways were used. We found that the increased TOPFLASH-luciferase reporter activity by PTHrP (1–36) and PTHrP (107–139) was abrogated by the PKA inhibitor Rp-cAMPS, at 25 µM, and the PKC inhibitor calphostin C, at 200 nM, respectively, in MC3T3-E1 cells (Fig. 6).
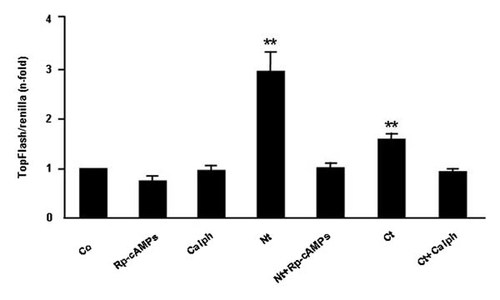
The N- and C-terminal domains of PTHrP activate β-catenin signaling through different mechanisms in MC3T3-E1 cells. MC3T3-E1 cells were incubated with or without (control; Co) PTHrP peptides (each at 100 nM), in the presence or absence of Rp-cAMPS (25 µM) or calphostin C (Calph; 200 nM), for 24 h. When present, these inhibitors were added 2 h prior to the PTHrP peptides. TOPFLASH-luciferase reporter activity was determined as described in the text. Values are mean ± SEM of at least three experiments in triplicate. **P < 0.01 versus all other conditions.
DISCUSSION
It has been demonstrated by us and others that chronic exposure of cells of the osteoblastic lineage to a diabetic environment hampers osteoblastic function and promotes dedifferentiation towards acquisition of an adipocyte phenotype [Mody et al., 2001; Botolin and McCabe, 2006; Wan et al., 2008; Lozano et al., 2009; Barbagallo et al., 2010; Lozano et al., 2011; Motyl et al., 2012]. Furthermore, previous studies using various osteoblastic cell preparations have shown a variable effect of HG concentrations on cell growth and viability, from being deleterious, inefficient, or even stimulatory [Terada et al., 1998; Balint et al., 2001; Botolin and McCabe, 2006; Wang et al., 2010; Zhen et al., 2010]. Thus, this effect of HG appears to depend on experimental conditions such as the type of osteoblastic cell culture and exposure scheme. In a previous study using MC3T3-E1 cells, we showed that exposure for 5 days to 25 mM glucose reduced the cell number by about 25% [Lozano et al., 2009].
The recognized role of the Wnt/β-catenin cascade as a key modulator of osteoblast growth and function provides a rationale for its investigation in diabetic osteopenia. In a recent study, using immunohistochemistry, we observed a depletion of total and nuclear β-catenin immunostaining in osteoblasts and osteocytes in the tibia of STZ-induced T1D mice [Portal-Núñez et al., 2010]. Moreover, by using PCR arrays, significant changes in some Wnt pathway-related genes—which were not, however, the classical components of this pathway—were also found in the femur of these mice [Portal-Núñez et al., 2010]. The observed pattern of these changes precluded to conclude a clear status of the Wnt pathway in T1D mice. This prompted us to here examine the putative interaction of HG—characterizing the diabetic condition—with major activator components of canonical Wnt pathway in mouse osteoblastic cell cultures.
The present in vitro studies show that HG can target different components of the canonical Wnt pathway to induce β-catenin destabilization in mouse osteoblastic cells. These seem to include downregulation at the gene level of classic upstream Wnt pathway activators, Wnt3a and co-receptors LRP-5 and 6, and downstream components such as GSK3β phosphorylation and β-catenin degradation. Our findings also indicate that PTHrP—through its N- and C-terminal domains—interacts with the canonical Wnt pathway to trigger its activation in osteoblastic cells even in this HG setting.
Wnt3a is an important member of the Wnt protein family whose homozygous deletion has dramatic effects in the mouse axial skeleton [Takada et al., 1994]. This protein activates canonical Wnt/β-catenin pathway [Ling et al., 2010], and its expression has been shown to occur related to osteoblast differentiation [Bain et al., 2003; Cho et al., 2009]. Wnt proteins signal by interacting with a cell membrane receptor complex comprising the Fzd family of receptors and co-receptors LRP-5 and LRP-6 [Mao et al., 2001a, b; Glass and Karsenty, 2007]. A clear relationship exists between these co-receptors and the maintenance of bone mass as revealed by gain- or loss-of-function mutations affecting LRP-5 and LRP-6 genes in both mice and humans [Bodine and Komm, 2006]. LRP-5−/− mice have a decreased number of osteoblasts and low bone formation, and combined LRP-5−/− and LRP-6−/− mice have a dramatic osteopenia which mimics that observed in β-catenin−/− mice, suggesting the role of both LRP-5 and LRP-6 to fully activate β-catenin in osteoblasts [Holmen et al., 2004, 2005]. Hence, the observed concomitant decrease in Wnt3a, as well as LRP-5 and 6 gene expression could have contributed to β-catenin inactivation in osteoblastic cells under diabetic conditions. These in vitro results contrast with our previous observations in STZ-induced T1D mice and those recently reported in a similar model in rats, showing unaltered expression levels of these genes in the diabetic rodent tibia [Portal-Núñez et al., 2010; Hie et al., 2011]. The reason for these apparent discrepancies could be related to the fact that changes in the aforementioned genes in osteoblasts might be more difficult to detect using total RNA from whole bone tissue than in that directly isolated from osteoblastic cells in culture.
However, our present findings indicate that the inhibitory effect of HG on the Wnt pathway lies mainly downstream to Wnt3a, and this has an impact on osteoblastic differentiation-related markers, namely Runx2 and OPG, in the osteoblastic MC3T3-E1 cell line. Of interest in this respect, present findings suggest that inhibition of the canonical Wnt pathway by HG depends at least in part on p38 MAPK activation, coherent with the reported role of the latter in several alterations of osteoblast function elicited by a diabetic environment [Zayzafoon et al., 2002; Alikhani et al., 2007]. Our data herein also demonstrate that HG appears to act downstream of GSK-3β to promote β-catenin destabilization in these osteoblastic cells. Consistent with previous observations in other systems in diabetic conditions [Mody et al., 2001; Peiró et al., 2001; Hamada et al., 2009], present data suggest that oxidative stress and, as a consequence, FoxO activation might contribute to the observed decrease in β-catenin signaling in osteoblasts under HG exposure.
In addition, our findings show that both N- and C-terminal domains of PTHrP can counteract the HG-induced decrease of β-catenin signaling. Our present in vitro data cannot rule out that endogenous Wnt pathway inhibitors, like DKK1 and SOST/sclerostin, might be putative PTHrP targets to activate this pathway in osteoblasts in a diabetic setting. In fact, previous reports have shown that PTH and PTHrP downregulate these inhibitors in rat osteoblastic osteosarcoma UMR-106 cells grown in normal glucose [Kulkarni et al., 2005; de Castro et al., 2012]. However, although some previous studies have shown that some MC3T3-E1 cell preparations can express DKK1 [Guo et al., 2010], we have been unable to detect this gene in our MC3T3-E1 cell preparation (subclone 4), using an Assay-by-Design™ system with TaqMan MGB probes (Applied Biosystems) and primers covering the exons 3 and 4 of the mouse DKK1 cDNA. Thus, DKK1 is unlikely to contribute to the observed PTHrP-induced β-catenin activation in these cells used in the present study. Moreover, our previous findings indicate that SOST gene and its protein product, sclerostin, which are mainly expressed in terminally differentiated osteocytes [Papapoulos, 2011], were not up-regulated but decreased in the tibia of T1D mice—in contrast to T1D rats [Hie et al., 2011]—as a consequence of diminished osteocyte viability; an effect which was reversed by PTHrP administration [Portal-Núñez et al., 2010].
Recent reports also indicate that activation of the PTH1R can elicit β-catenin stabilization in a Wnt-independent manner [Wan et al., 2008; Romero et al., 2010]. Whether such a mechanism might play a role in the increased β-catenin signaling by PTHrP (1–36), which interacts with the PTH1R with the same affinity than PTH in osteoblastic cells [Datta and Abou-Samra, 2009], is presently unknown. Of interest in this respect, we here found that PTH (1–34) was as efficient as PTHrP (1–36) in stimulating several well characterized β-catenin target genes in MC3T3-E1 cells exposed to HG. In addition, activation of β-catenin signaling (assessed by estimating Lef-1 mRNA levels) by each PTHrP peptide in these cells occurred in a dose-dependent manner. This effect elicited by PTHrP (107–139) was even observed at sub-nM doses, in agreement with the observed stimulation of another β-catenin target gene, OPG, by this peptide in these cells [Lozano et al., 2011].
Our findings herein also suggest that cAMP may act as a mediator for the stimulatory effect of PTHrP (1–36) on β-catenin activation in mouse osteoblastic cells, consistent with previous data using PTH as agonist [Kulkarni et al., 2005]. On the other hand, PKC appears to be a key mediator for PTHrP (107–139)-induced β-catenin signaling. In this regard, PKC activation has been shown to trigger β-catenin stabilization and activation in a non-osteoblastic cell system [Garrido et al., 2002]. Also in support of these findings, cAMP/PKA and PKC pathways have been demonstrated to be involved in various osteoblastic actions elicited by the N- and C-terminal domains of PTHrP, respectively [Esbrit et al., 2000; Valín et al., 2001; Guillén et al., 2002; de Gortázar et al., 2006].
In conclusion, these in vitro findings show different mechanisms whereby HG can interact with the canonical Wnt pathway to impact osteoblast function. Our results also demonstrate the ability of the N- and C-terminal domains of PTHrP to target this pathway and thereby counteract these deleterious effects of HG in osteoblastic cells.
Acknowledgements
We thank A.F. Stewart, M.D. and A. García-Ocaña, Ph.D. (University of Pittsburgh School of Medicine, Pittsburgh, PA) for generously providing PTHrP (1–36), and F. Roncal, Ph.D. (Centro Nacional de Biotecnología-CSIC, Madrid) for human PTHrP (107–139) synthesis. We are also indebted to M. M. González (IIS-Fundación Jiménez Díaz) and M.T. Seisdedos-Domínguez (Centro de Investigaciones Biológicas-CSIC, Madrid) for her technical support with confocal microscopy. This work was supported by grants from the Spanish Ministerio de Educación y Cultura (SAF2005-05254), Instituto de Salud Carlos III (PI050117, PI080922, PI11/00449, RD06/0013/1002, and RD12/0043/0008), Fundación de Investigación Médica Mutua Madrileña, FEIOMM, and Consejería de Educación de la Junta de Castilla-La Mancha (JCCM, POII10-0274-3182). A.L.-H. was supported by Fundación Conchita Rábago and Ministerio de Educación-FPU program (AP2009-1871). S.P.-N., D.L., and A.G.-M. are recipients of a post-doctoral research contract from RETICEF (RD06/0013/1002 and RD12/0043/0008), Comunidad Autónoma de Madrid (S-2009/Mat-1472), and the Spanish Ministerio de Ciencia e Innovación (MICINN)-Juan de la Cierva program (JCI-2009-04360), respectively. F.C.P.-M. was recipient of a Torres Quevedo contract from MICINN and NanoDrugs, S.L.




