N-acetyl-L-cysteine inhibits bleomycin induced apoptosis in malignant testicular germ cell tumors
Abstract
Antioxidants may prevent apoptosis of cancer cells via inhibiting reactive oxygen species (ROS). However, to date no study has been carried out to elucidate the effects of strong antioxidant N-acetylcysteine (NAC) on Bleomycin induced apoptosis in human testicular cancer (NTERA-2, NT2) cells. For this reason, we studied the effects of Bleomycin and NAC alone and in combination on apoptotic signaling pathways in NT2 cell line. We determined the cytotoxic effect of bleomycin on NT2 cells and measured apoptosis markers such as Caspase-3, -8, -9 activities and Bcl-2, Bax, Cyt-c, Annexin V-FTIC and PI levels in NT2 cells incubated with different agents for 24 h. Early apoptosis was determined using FACS assay. We found half of the lethal dose (LD50) of Bleomycin on NT2 cell viability as 400, 100, and 20 µg/ml after incubations for 24, 48, and 72 h, respectively. Incubation with bleomycin (LD50) and H2O2 for 24 h increased Caspase-3, -8, -9 activities, Cyt-c and Bax levels and decreased Bcl-2 levels. The concurrent incubation of NT2 cells with bleomycin/H2O2 and NAC (5 mM) for 24 h abolished bleomycin/H2O2-dependent increases in Caspase-3, -8, -9 activities, Bax and Cyt-c levels and bleomycin/H2O2-dependent decrease in Bcl-2 level. Our results indicate that bleomycin/H2O2 induce apoptosis in NT2 cells by activating mitochondrial pathway of apoptosis, while NAC diminishes bleomycin/H2O2 induced apoptosis. We conclude that NAC has antagonistic effects on Bleomycin-induced apoptosis in NT2 cells and causes resistance to apoptosis which is not a desired effect in eliminating cancer cells. J. Cell. Biochem. 114: 1685–1694, 2013. © 2013 Wiley Periodicals, Inc.
Testicular cancer is a malignant, highly aggressive neoplasm in young males. The molecular mechanisms operative in this malignancy have not been fully understood [Cheung et al., 2011; Winter and Albers, 2011]. Testicular germ cell tumor (TGCT) is an invasive germ cell tumor histologically classified as seminomatous and non-seminomatous. Few seminomatous cell lines have been identified to date. Non-seminomatous tumors can be further sub-classified into embryonal carcinoma, teratoma, choriocarcinoma, and yolk-sac tumor. Most of the non-seminomatous tumors include multiple cell types. Embryonal carcinoma is the most frequent type and represents ∼87% of non-seminomatous tumors [Bosl and Motzer, 1997]. Several embryonal carcinoma cell lines have been established and shown to be useful for pathobiological and clinical studies [Andrews et al., 2005]. Ntera2 (NT2) is one of the established pluripotent human testicular embryonal carcinoma cell lines. This cell line has been extensively used in research on TGCT [Burger et al., 1998; Koch et al., 2003; Skotheim et al., 2005]. In this study, we used NT2 as a cell model to study the effects of bleomycin and NAC on apoptosis.
Reactive oxygen species (ROS) have been identified as important mediators regulating signal transduction [Pani et al., 2000]. ROS can induce injury in a variety of mammalian cells [Farber et al., 1990; Kim et al., 2010] and play crucial roles in the signaling pathway of drug-induced preconditioning [Pani et al., 2000]. ROS generation has been demonstrated following administration of chemotherapeutic agents [Kalivendi et al., 2001]. In many studies, it has been reported that ROS generation is followed by downstream release of Cyt-c from the mitochondria [Gottlieb et al., 2000]. Numerous in vitro studies have demonstrated that a wide range of anticancer agents induce apoptosis in malignant cells by generating ROS which is one of the important therapeutic interventional approaches in cancer. Oxidative stress has been shown to decrease the LD50 (the dose killing 50% of the cancer cells) doses of several types of antineoplastic agents and induce apoptosis in cancer cells [Papet et al., 2011].
Bleomycin is a polypeptide antibiotic and is used in combination with other antineoplastic agents to effectively treat lymphomas, testicular carcinomas, and squamous cell carcinomas of cervix, head, and neck [Aouida et al., 2010]. Bleomycin is an essential component of cisplatin-based chemotherapy regimens used in the treatment of testicular cancer. Bleomycin is approved by FDA to be used alone or with other drugs for palliative treatment of testicular cancer. Bleomycin is used in combination with etoposide and cisplatinum (BEP therapy) for the treatment of adult and childhood testicular germ cell tumors. It is known that drug combinations usually work better than single drugs because different drugs kill cancer cells in different ways. The major side effects of this BEP regimen is damage to the spermatogenic system, resulting in a transient or permanent decrease in sperm production in patients shortly after treatment; and development of pulmonary toxicity which is the most serious dose-limiting side-effect [Izuhara et al., 2008; Marcon et al., 2010]. In our study, we did not use BEP, but applied bleomycin alone, because our aim was to search and elucidate the mechanism of action of bleomycin [de Wit et al., 1997]. Bleomycin generates oxygen radicals through its ferrous binding site, induces oxidative cleavage of DNA strand and induces apoptosis in cancer cells [Burger et al., 1981].
There is an intense argument on the concurrent use of antioxidants during the conventional cancer therapies. This argument is based on the fact that some chemotherapeutic drugs generate ROS which might be an alternative mechanism for their cytotoxic effect via inducing apoptosis and antioxidants may prevent cancer cells to be killed by ROS-induced apoptosis by scavenging ROS [Conklin, 2004]. There are numerous studies in the literature searching the effects of antioxidants on ROS-induced apoptosis in cancer [Bejarano et al., 2011; Kim et al., 2011]. So far, only three antioxidants, N-acetyl cysteine (NAC) with cisplatinum and doxorubicin; tangeretin with tamoxifen; and beta carotene with 5-fluorouracil have been shown to decrease the effectiveness of conventional cancer therapy in vivo [Akbas et al., 2006; Ozben, 2007]. Antioxidants may have diverse effects on apoptosis depending on the specific antioxidant and its dose; the chemotherapy drugs being used; and the type of cancer being treated. Small molecular weight antioxidant molecules are effective reducing agents, but some, including glutathione (GSH), NAC, and alpha-lipoic acid, are strong nucleophiles, because they possess a sulfhydryl group [Conklin, 2004]. NAC is a potent hydroxyl radical scavenger, a potent antioxidant, a source of cysteine for glutathione synthesis and increases levels of glutathione which is the major antioxidant of body, and can react directly with ROS [Millea, 2009]. As a precursor of GSH, NAC plays a key role in the protection of cells against oxidative stress by inhibiting formation of hydrogen peroxide (H2O2) under in vitro conditions [Raju et al., 1994; Gillissen et al., 1997]. NAC has been shown to have protective effects towards DNA damage and carcinogenesis [Wagner et al., 1989; De Flora et al., 2001]. Several studies have suggested that its protective effects could be due to scavenging ROS and stimulation of glutathione synthesis [De Vries and De Flora, 1993]. No study has been carried out to date to elucidate the effect of NAC on bleomycin-induced apoptosis in human testicular cancer cells, NT2 cells. The aim of our study was to clarify the molecular mechanisms and the effect of NAC on apoptosis induced by bleomycin and H2O2 in human testicular cancer (NT2) cells. For this reason, we determined the cytotoxic effect of bleomycin on NT2 cells and measured apoptosis markers such as caspase-3, -8, -9 activities, Bcl-2, Bax, Cyt-c, Annexin V-FTIC and PI (propidium iodide) levels in NT2 cells incubated with bleomycin, H2O2, NAC, bleomycin + NAC, H2O2 + NAC. We compared the effects of bleomycin on apoptotic signaling pathways with H2O2 which directly produces ROS.
MATERIALS AND METHODS
Cell Line
NT2 cells were obtained from the American Type Culture Collection (ATCC). Cells were grown to confluence at 37°C in a humidified atmosphere containing 5% CO2 in air in RPMI medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 100 IU/ml penicilin and 10 µg/ml streptomycin (catalog # 15140, Invitrogen). Cells were then incubated with bleomycin (Nippon Kayaku, Tokyo, Japan), and/or NAC (Sigma) for 24, 48, and 72 h.
Viability Assay
The viability of the cells was measured using the colorimetric 3-(4,5)dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Millipore) [Mosmann, 1983; Sladowski et al., 1993]. The cells were incubated with different concentrations of bleomycin (20–700 µg/ml) for 24, 48, and 72 h. The cells were incubated with different concentrations of H2O2 (50–500 µM) and NAC (1–10 mM) for 24 h. The control cells were prepared in plates containing only medium. A microplate reader (BioTek) was used to measure the absorbances at 570 nm. The average absorbance values of the cells incubated with different agents were compared with the average absorbance values of the control cells to calculate the percentage of the viable cells.
Caspase-3 Assay
This assay was performed using a commercial kit (ApoTarget kit, code #: KHZ0022; BioSource International, Inc., Camarillo, CA). The colorimetric protease assay of Caspase-3 provides a simple and convenient means of quantifying the enzyme activity that recognizes the amino acid sequence, DEVD (a synthetic tetrapeptide, the upstream amino acid sequence of the Caspase-3 cleavage site), coupled with p-nitroanilide, which is released upon substrate cleavage. Cells were incubated with bleomycin (400 µg/ml), H2O2 (400 µM), NAC (5 mM), bleomycin + NAC or H2O2 + NAC for 24 h, while control cells were incubated with no agent.
Caspase-8 Assay
This assay was performed using a commercial kit (ApoTarget kit, code: KHZ0061: BioSource International, Inc.).
Caspase-9 Assay
This assay was performed using a commercial kit (ApoTarget kit, code: KHZ0101: BioSource International, Inc.).
Bax Assay
Bax protein concentration was determined using the Human Bax Enzyme Immunometric Assay Kit (AssayDesigns, Ann Arbor, MI).
Bcl-2 Assay
The intracellular content of Bcl-2 was quantified using the human Bcl-2 ELISA kit (Bender Medsystems).
Cytosolic Cytochrome c (Cyt-c) Assay
The Cyt-c Release Apoptosis Assay Kit (Calbiochem) was used to obtain cytosolic cytochrome fraction. Cells were collected by centrifugation at 600g for 5 min at +4°C. The cell pellets were washed once with ice-cold phosphate buffer saline (PBS) and resuspended in Cytosol Extraction Buffer (20 mM Hepes–KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol, and 0.1 mM PMSF) containing 250 mM sucrose on ice for 10 min. The cells were homogenized with the grinder on ice. Passes (30–50) were performed and the homogenates were centrifuged at 700g for 10 min at +4°C. The supernatants were centrifuged at 10,000g for 30 min at +4°C. The supernatant collected containing the cytosolic fraction and also other subcellular constituents, for example, lysosomes was used for cytochrome c assay.
Flow Cytometric Analysis of Annexin V-FITC
Apoptosis was detected using a flow cytometric Annexin-V-FITC Apoptosis Detection Kit (Bender Medsystems) on FACS Canto II.
Statistical Analysis
All the data are presented as the mean ± standard error (mean ± SE). Statistical analysis was performed using SPSS Data Access Pack for Windows version 10.0 (SPSS, Inc., Chicago, IL). P < 0.05 was considered as a significant difference. Categorical and continuous variables were compared using the chi-square test, ANOVA, and Student's t-test. The Mann–Whitney U-test and Kruskal–Wallis test were used to compare non-parametric variables.
RESULTS
Half Maximal Lethal Dose (LD50) of Bleomycin on NT2 Cell Viability
We incubated NT2 cells with different concentrations of bleomycin (20–700 µg/ml) for 24, 48, and 72 h and measured cell viability using the MTT assay. The cell viability of the NT2 cells (control) incubated with no agent was arbitrarily set as 100% and the cell viability of the other groups was compared with the viability of the control cells. According to the results of these experiments, we found LD50 doses of bleomycin on NT2 cell viability as 400, 100, and 20 µg/ml after incubations for 24, 48, and 72 h, respectively (P < 0.01; Fig. 1). According to the result of our previous studies, we found the LD50 dose of H2O2 on NT2 cell viability as 400 µM. Out of various doses of NAC (1–10 mM), the dose of 5 mM NAC that maintained the highest cell viability at 24 h was used for 24 h incubations (Fig. 2). This is the NAC dose applied also in our previous studies [Akan et al., 2004; Timur et al., 2005]. We used LD50 doses of bleomycin (400 µg/ml), H2O2 (400 µM) and NAC (5 mM) for the experiments of Caspase-3, -8, -9, Bax, Bcl-2, Cytosolic Cyt-c and flow cytometric analysis of Annexin V-FITC.
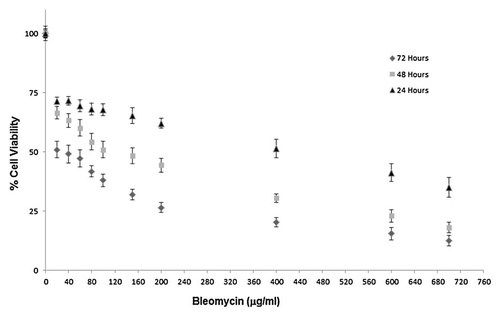
Dose and time dependent cytotoxicity of bleomycin. Different concentrations of bleomycin on NT2 Cell Viability after incubation for 24, 48, and 72 h. Each value represents mean ± SE (n = 6).
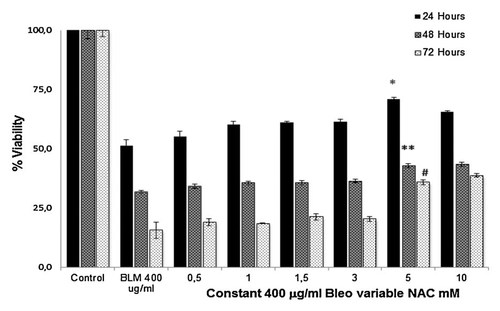
% cell viability of NT2 cells incubated for 24, 48, and 72 h with variable concentrations of NAC (0.5–10 mM) and a fixed concentration of bleomycin.(400 µg/ml). Each value represents mean ± SE (n = 6). *P < 0.01, 5 mM NAC + 400 µg/ml Bleo for 24 h versus 400 µg/ml Bleo for 24 h. **P < 0.01, 5 mM NAC + 400 µg/ml Bleo for 48 h versus 400 µg/ml Bleo for 48 h. #P < 0.01, 5 mM NAC + 400 µg/ml Bleo for 72 h versus 400 µg/ml Bleo for 72 h.
Caspase-3 Activity
NT2 cells were incubated with bleomycin, NAC, H2O2, bleomycin + NAC and H2O2 + NAC for 24 h and caspase-3 activity was determined using the caspase-3 assay kit. The absorbances of the cells incubated with different agents were compared to the absorbance of the control cells not incubated with any agent to determine the change in caspase-3 activity. The caspase-3 activity in the NT2 cells is shown in Figure 3. The lowest caspase-3 activity was found in the control cells and cells incubated with NAC compared to the other groups. There was no difference in caspase-3 activities between the control cells and the cells incubated with NAC. Incubation with bleomycin and H2O2 induced significant increases in caspase-3 activity compared to the control and NAC cells groups. Co-incubation of the cells with bleomycin + NAC and H2O2 + NAC significantly decreased caspase-3 activities compared to the cells incubated with bleomycin and H2O2 alone.
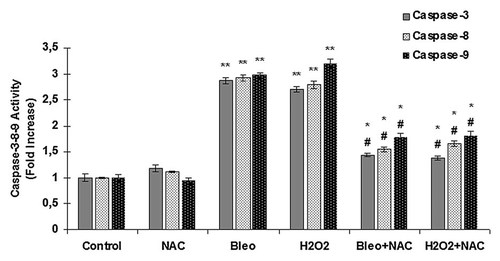
Caspase-3, -8, -9 activities. Caspase-3, -8, -9 activity in NT2 cells incubated with NAC, bleomycin, H2O2, bleomycin + NAC, and H2O2 + NAC for 24 h. Each value represents the mean ± SE (n = 6). *P < 0.005, H2O2 + NAC and bleomycin + NAC versus bleomycin and H2O2 for Caspase-3, -8, -9. **P < 0.001, bleomycin and H2O2 versus control and NAC for Caspase-3, -8, -9. #P < 0.01 bleomycin + NAC and H2O2 + NAC versus control and NAC for Caspase-3, -8, -9.
Caspase-8 Activity
NT2 cells were incubated with NAC, bleomycin, H2O2, bleomycin + NAC, and H2O2 + NAC for 24 h and caspase-8 activity was determined using the caspase-8 assay kit. The absorbances of the cells incubated with different agents were compared to the absorbance of the control cells not incubated with any agent to determine the change in caspase-8 activity. The caspase-8 activity in the NT2 cells incubated with different agents for 24 h is shown in Figure 3. There was no difference in caspase-8 activities between the control cells and the cells incubated with NAC. Incubation with bleomycin, bleomycin + NAC, H2O2, and H2O2 + NAC induced a marked increase in caspase-8 activity in comparison to the control and NT2 cells incubated with NAC. Co-incubation of the cells with bleomycin + NAC and H2O2 + NAC significantly decreased caspase-8 activity compared to the cells incubated with bleomycin and H2O2 alone.
Caspase-9 Activity
NT2 cells were incubated with NAC, bleomycin, H2O2, bleomycin + NAC and H2O2 + NAC for 24 h and caspase-9 activity was determined using the caspase-9 assay kit. The absorbances of the cells incubated with different agents were compared with the absorbance of the control cells not incubated with any agent to determine the change in caspase-9 activity. The caspase-9 activity in the NT2 cells incubated with different agents for 24 h is shown in Figure 3. There was no difference in caspase-9 activities between the control cells and the cells incubated with NAC. Incubation with bleomycin and H2O2 increased caspase-9 activity compared to the control and cells incubated with NAC. Co-incubation of the cells with bleomycin + NAC and H2O2 + NAC significantly decreased Caspase-9 activity compared to the cells incubated with bleomycin and H2O2 alone.
Bax Levels
NT2 cells were incubated with NAC, bleomycin, H2O2, bleomycin + NAC and H2O2 + NAC for 24 h and Bax concentration was determined using a Bax assay kit. Bax levels in the NT2 cells incubated with the different agents for 24 h are shown in Figure 4. There was no difference in Bax levels between the control cells and the cells incubated with NAC. Incubation with bleomycin and H2O2 increased Bax level significantly compared to the control and NAC groups. Co-incubation of the cells with bleomycin + NAC and H2O2 + NAC decreased Bax levels significantly compared to the cells incubated with bleomycin and H2O2 alone.
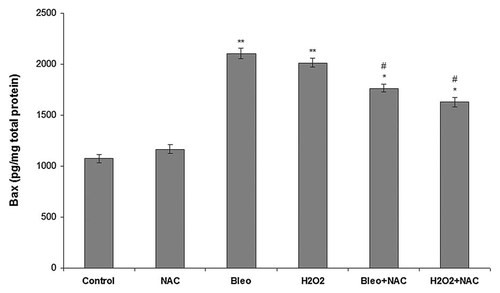
Bax levels in NT2 cells incubated with NAC, bleomycin, H2O2, bleomycin + NAC, H2O2 + NAC for 24 h. Each value represents the mean ± SE (n = 6). *P < 0.05, H2O2 + NAC and bleomycin + NAC versus bleomycin and H2O2. **P < 0.01, bleomycin and H2O2 versus control and NAC. #P < 0.05, bleomycin + NAC and H2O2 + NAC versus control and NAC.
Bcl-2 Level
NT2 cells were incubated with NAC, bleomycin, H2O2, bleomycin + NAC, and H2O2 + NAC for 24 h and anti-apoptotic protein Bcl-2 level was determined using a quantitative ELISA Bcl-2 assay kit. Bcl-2 levels in the NT2 cells incubated with the different agents or their combinations for 24 h are shown in Figure 5. Bcl-2 content in the NT2 cell lysates incubated with NAC increased significantly compared to the other groups. Incubation with bleomycin and H2O2 drastically decreased Bcl-2 level compared to the control cells and cells incubated with NAC. In contrast, co-incubation with NAC (bleomycin + NAC and H2O2 + NAC) significantly increased Bcl-2 level compared to the cells incubated with bleomycin and H2O2 alone.
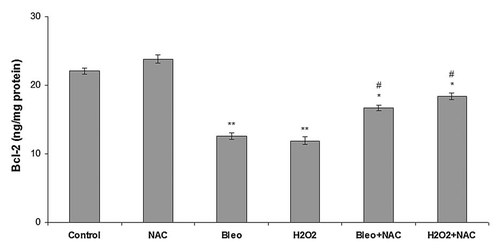
Bcl-2 levels in NT2 cells incubated with NAC, bleomycin, H2O2, bleomycin + NAC, and H2O2 + NAC for 24 h. Each value represents the mean ± SE (n = 6). *P < 0.01, H2O2 + NAC and bleomycin + NAC versus bleomycin and H2O2. **P < 0.005, bleomycin and H2O2 versus control and NAC. #P < 0.01, bleomycin + NAC and H2O2 + NAC versus control and NAC.
Cyt-c Level
NT2 cells were incubated with NAC, bleomycin, H2O2, bleomycin + NAC, and H2O2 + NAC for 24 h and cytoplasmic Cyt-c level was determined using the Cyt-c assay kit. Cyt-c levels in the NT2 cells incubated with the different agents or their combinations for 24 h are shown in Figure 6. Incubation with bleomycin, H2O2, bleomycin + NAC, and H2O2 + NAC increased Cyt-c levels significantly compared to the control cells and cells incubated with NAC. Incubation with NAC did not cause any change in the Cyt-c level compared to the control cells. Co-incubation with NAC remarkably decreased Cyt-c levels compared to the cells incubated with bleomycin and H2O2 alone indicating that the extent of Cyt-c release was decreased by incubation with NAC.
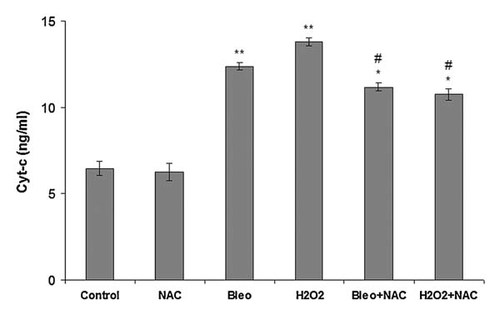
Cyt-c levels in NT2 cells incubated with NAC, bleomycin, bleomycin + NAC, H2O2, H2O2 + NAC for 24 h. Each value represents the mean ± SE (n = 6). *P < 0.05, H2O2 + NAC and Bleomycin + NAC versus bleomycin and H2O2. **P < 0.01, bleomycin and H2O2 versus control and NAC. #P < 0.01, bleomycin + NAC and H2O2 + NAC versus control and NAC.
FACS Analysis
NT2 cells were incubated with bleomycin, NAC and bleomycin + NAC for 6 h (pre-apoptotic time). In addition to the morphological evaluation, the protective effect of NAC against apoptosis was confirmed by the flow cytometric analysis using the Annexin V and the PI double-staining system. Figure 7 shows that incubation of the cells with 400 µg/ml bleomycin induced 48,6% apoptosis in the cells (Q1-1 pre-apoptotic side and Q2-2 post-apoptotic side) compared to the control cells. Incubation with 5 mM NAC did not cause any significant change in the percentage of apoptosis compared to the control cells. Co-incubation of the NT2 cells with bleomycin and NAC drastically reduced the proportion of apoptotic cell populations from 48,6% to 29,5% (Q1-1 and Q2-2; n = 6, *P < 0.001; Fig. 8).
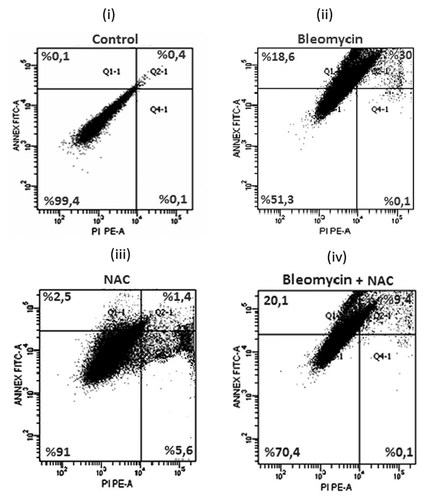
FACS analysis of apoptosis using annexin V/PI staining. (i) control cells; (ii) cells incubated bleomycin; (iii) cells incubated wit NAC; and (iv) cells incubated with Bleomycin + NAC for 6 h (mean ± SE, n = 3 measurements of 25,000 cells).
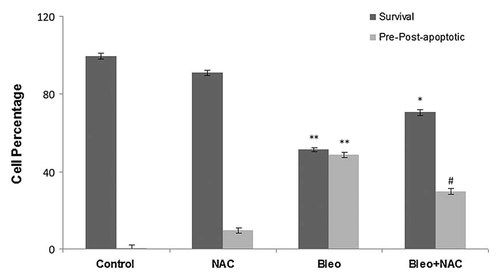
Percentage of survival or apoptotic cells using FACS analysis (mean ± SE, n = 3 measurements of 25,000 cells). *P < 0.01, survival of cells incubated with bleomycin + NAC versus survival of control cells and cells incubated with NAC and bleomycin. #P < 0.01, pre-post-apoptotic cells incubated with bleomycin + NAC versus pre-post-apoptotic cells incubated with bleomycin. **P < 0.001, survival or pre-post-apoptotic cells incubated with bleomycin versus survival or pre-post-apoptotic control cells.
DISCUSSION
In our cytotoxicity studies; we found LD50 doses of bleomycin on cell viability as 400, 100, and 20 µg/ml after incubations for 24, 48, and 72 h, respectively (Fig. 1). Our results indicate that the cytotoxic effects of bleomycin are dose and time dependent. Co-incubation of bleomycin with NAC for 24, 48, and 72 h decreased the cytotoxicity of bleomycin in the NT-2 cells (Fig. 2).
In our study, we quantified apoptosis flow cytometrically by labeling the NT2 cells undergoing apoptosis with annexin V conjugated with FITC. This assay is based on the fact that apoptotic cells lose membrane phospholipid asymmetry and expose phosphatidylserine on the outer leaflet of the plasma membrane. An increase in the apoptotic cells was observed in the bleomycin-treated group with a lower number of living cells, and the apoptotic process was accompanied by upregulation of the Bax protein. NAC administration led to a reproducible decrease in the rate of early apoptosis/late apoptosis in the cells compared to the cells exposed to Bleomycin. This negative effect of NAC was observed in the co-incubation with bleomycin compared to the cells incubated with bleomycin only (Figs. 7 and 8).
The impact of chemotherapy-induced oxidative stress and the role of ROS in drug-induced apoptosis have been demonstrated following the administration of many chemotherapeutic agents. ROS are implicated in apoptosis induced by a variety of anticancer agents [Antosiewicz et al., 2008]. ROS generation has important clinical significance to guide the treatment of testis cancers and has been reported to occur upstream of mitochondrial outer membrane permeabilization following exposure to hyperoxia and mediates mitochondrial dysfunction in bleomycin-induced apoptosis [Lin et al., 1979; Stohs and Bagchi, 1995; Buccellato et al., 2004]. It is well known that the action of bleomycin on tumor cells includes: (i) binding with iron after entering the cells, (ii) damaging DNA and RNA, (iii) determining cell fate response to the damage [Lazo, 1999; Chen and Stubbe, 2005]. The damage signals triggered by bleomycin lead to the generation of ROS and arrest cell cycle, finally leading to the initiation of the cell death program, namely apoptosis [Mungunsukh et al., 2010].
All the antioxidants cannot be viewed as equal when evaluating their potential impact on cancer chemotherapy, and an individual antioxidant cannot be anticipated to have the same impact on the activity of all the chemotherapeutic agents. It is expected that antioxidants may interfere with the antineoplastic activity of the chemotherapeutic agents if their cytotoxicity depends on the generation of ROS [Conklin, 2004]. In our previous studies, we observed diverse effects of curcumin on apoptosis in two testis cancer cell types (NCCIT and NT-2 cells). In NT-2 cells, curcumin showed synergistic effect with bleomycin on apoptosis and concurrent use of curcumin with bleomycin induced apoptosis to a greater extent in NT-2 cells than incubation with bleomycin alone [Cort et al., 2012a], while in NCCIT cells, curcumin diminished the apoptotic effects of bleomycin in press [Cort et al., 2012b]. Curcumin has been shown to selectively induce apoptosis in tumor cells at the G2 phase via the upregulation of p53 expression and initiation of the mitochondrial apoptotic pathway via Cyt-c release and increased Bax expression. Our observation suggests that the effects of curcumin and bleomycin on apoptosis are synergistic in wild-type p53-expressing NT-2 cells because of curcumin induced upregulation of p53. In contrast, co-incubation with bleomycin and curcumin diminished apoptosis in the NCCIT cells compared to the NCCIT cells incubated with bleomycin alone [Cort et al., 2012b]. Thus, the non-synergistic effect of bleomycin and curcumin on apoptosis in p53 mutant NCCIT cells might be due to the antioxidant effect of curcumin. Co-incubation with curcumin might inhibit ROS generated by bleomycin leading to decreased induction of mitochondrial pathway of apoptosis as indicated by decreased release of cytochrom c from mitochondria and diminished level of Bax and caspase activities in NCCIT cells [Cort et al., 2012c]. We repeated our experiments using NAC, another potent antioxidant instead of curcumin on bleomycin-induced apoptosis in NCITT cells. In the study published in Journal of Physiology And Biochemistry [Cort et al., 2012d], we found that NAC has negative effects on bleomycin-induced apoptosis in NCCIT cells. The effects of NAC were similar to the effects of curcumin on bleomycin-induced apoptosis in NCCIT cells [Cort et al., 2012b, d]. Co-incubation with bleomycin and curcumin or bleomycin and NAC diminished apoptosis in the NCCIT cells compared to the NCCIT cells incubated with bleomycin alone [Cort et al., 2012b, d]. The aim of this recent study submitted to Journal of Cellular Biochemistry was to clarify the effects of NAC on bleomycin-induced apoptosis in NT-2 cells. In contrast to curcumin, co-incubation with NAC diminished bleomycin-induced apoptosis in NT-2 cells compared to the cells incubated with bleomycin alone and the effects of NAC on bleomycin-induced apoptosis were not different in both cell types (NCCIT and NT-2 cells). The diminishing effect of NAC on bleomycin-induced apoptosis in two different testis cancer cell types might be due to the strong antioxidant effect of NAC [Cort et al., 2012e]. Co-incubation with NAC might inhibit ROS generated by bleomycin leading to decreased induction of mitochondrial pathway of apoptosis as indicated by decreased release of cytochrom c from mitochondria and diminished level of Bax and caspase-3, -9 activities and increased level of anti-apoptotic Bcl-2 level [Cort et al., 2012e]. The factors responsible for the diverse effects of NAC and curcumin in NTera-2 cells, but not in NCCIT cells might be related to their different anti-oxidative potentials and the difference in the characteristics of the testis cancer cells [Cort et al., 2012c, e].
The release of Cyt-c from mitochondria into cytosol is regulated by Bcl-2 family members via multiple molecular mechanisms. Many studies have indicated that members of the Bcl-2 family are the mediators of cell survival and apoptosis [Llambi and Green, 2011]. Bcl-2 inhibits apoptosis by preventing Cyt-c release, while Bax promotes apoptosis by triggering Cyt-c release. ROS increases Bax levels and decreases anti-apoptotic Bcl-2 levels [Mungunsukh et al., 2010]. The activation of caspase-8 and caspase-9 has been documented to play central roles in mediating apoptosis signaled by death receptors and mitochondria, respectively [Yang et al., 2011]. In our study, bleomycin and H2O2 induced caspase-8 activity indicates activation of the extrinsic pathway of apoptosis, because caspase-8 is a well-known initiator caspase of the extrinsic apoptotic pathway [Fulda and Debatin, 2006]. Similar to our results, in other studies, it was reported that bleomycin caused activation of caspase 8 [Wallach-Dayan et al., 2006; Mungunsukh et al., 2010]. The mechanisms of caspase-8 activation by bleomycin, might be a death receptor-independent pathway. Caspase-8 activation [Strasser et al., 2000] can be achieved independently from death receptor induction [Zhuang et al., 2000; Lee et al., 2002; Zhang et al., 2003]. Caspase-8 may help to amplify mitochondria-dependent apoptosis through a feed forward loop on which initial mitochondrial damage by ROS causes caspase-3 activation [Wallach-Dayan et al., 2006]. Caspase-3 then activates caspase-8, which further leads mitochondrial release of Cyt-c and activation of caspase-3 [Tang et al., 2000]. Co-incubation of the cells with bleomycin + NAC and H2O2 + NAC significantly decreased caspase-8 activity indicating that co-incubation with NAC suppresses death receptor pathway of apoptosis induced by bleomycin and H2O2.
Our study demonstrated that NAC diminishes bleomycin induced mitochondrial and death receptor pathways of apoptosis. This antiapoptotic behavior of NAC on bleomycin induced apoptosis is most probably due to its strong antioxidant characteristic which scavenges ROS generated by bleomycin. Antioxidants such as NAC having negative effects on apoptosis should be consumed carefully, or even concurrent use of them with the chemotherapeutic agents should be avoided during the conventional cancer therapy.
CONCLUSIONS
Our results indicate that bleomycin induces apoptosis in NT2 cells by increasing mitocondrial and death receptor pathways of apoptosis and NAC diminishes bleomycin induced apoptosis via inhibiting both apoptosis pathways. We conclude that NAC has negative effects on bleomycin induced apoptosis in NT2 cells and causes resistance to apoptosis which is not a desirable effect in the fight against cancer.




