Muscle glycogen synthase isoform is responsible for testicular glycogen synthesis: Glycogen overproduction induces apoptosis in male germ cells
Abstract
Glycogen is the main source of glucose for many biological events. However, this molecule may have other functions, including those that have deleterious effects on cells. The rate-limiting enzyme in glycogen synthesis is glycogen synthase (GS). It is encoded by two genes, GYS1, expressed in muscle (muscle glycogen synthase, MGS) and other tissues, and GYS2, primarily expressed in liver (liver glycogen synthase, LGS). Expression of GS and its activity have been widely studied in many tissues. To date, it is not clear which GS isoform is responsible for glycogen synthesis and the role of glycogen in testis. Using RT-PCR, Western blot and immunofluorescence, we have detected expression of MGS but not LGS in mice testis during development. We have also evaluated GS activity and glycogen storage at different days after birth and we show that both GS activity and levels of glycogen are higher during the first days of development. Using RT-PCR, we have also shown that malin and laforin are expressed in testis, key enzymes for regulation of GS activity. These proteins form an active complex that regulates MGS by poly-ubiquitination in both Sertoli cell and male germ cell lines. In addition, PTG overexpression in male germ cell line triggered apoptosis by caspase3 activation, proposing a proapoptotic role of glycogen in testis. These findings suggest that GS activity and glycogen synthesis in testis could be regulated and a disruption of this process may be responsible for the apoptosis and degeneration of seminiferous tubules and possible cause of infertility. J. Cell. Biochem. 114: 1653–1664, 2013. © 2013 Wiley Periodicals, Inc.
Abbreviations:
GS, glycogen synthase; GYS1, muscle glycogen synthase gene; GYS2, liver glycogen synthase gene; Poly-Ub, polyubiquitinated/polyubiquitination; TUNEL, terminal desoxynucleotidyl transferase dUTP nick end labelling; TdT, terminal desoxynucleotidyl transferase.
Glycogen, a branched glucose polymer, acts as an intracellular carbon and energy reserve in many cell types [Preiss and Walsh, 1981; Stalmans et al., 1987]. Many studies of glycogen metabolism have focused on its cytosolic synthesis and degradation, though surprisingly little is known of its regulation and function in tissues other than liver or muscle [Stalmans et al., 1987; Öz et al., 2007; Graham et al., 2010; Stapleton et al., 2010; Roach et al., 2012]. Glycogen synthase (GS) is a key enzyme in glycogen synthesis. It is encoded by two genes, GYS1, expressed in muscle (muscle glycogen synthase, MGS) and other tissues, and GYS2, primarily expressed in liver (liver glycogen synthase, LGS) [Browner et al., 1989; Nuttal et al., 1994]. Glycogen is a dynamic and vital metabolic fuel that has a very limited energetic capacity. Thus, glycogen regulation is highly complex and multifaceted, but when imbalances occur or homeostasis lost, glycogen is toxic and may then be an apoptotic signal to cells. In many glycogen storage diseases, tissues are finally destroyed by an over-accumulation of glycogen [Zerban et al., 1994; Cavanagh, 1999; Wender et al., 2000; Kuijpers et al., 2003; Lee et al., 2004; Vilchez et al., 2007]. Expression of GS and its activity have been widely studied in many tissues. To date it is not clear which isoform of GS is responsible for glycogen production in mammalian testes. Regulation of testicular glycogen synthesis as well as the role of glycogen in spermatogenesis is also unclear [Arzac, 1953]. Spermatogenesis is a complex process in which stem spermatogonia become mature spermatozoa through a series of events involving mitosis, meiosis and cell differentiation. Germ cell death, as well as being involved in cell proliferation and differentiation, is also conspicuous during normal spermatogenesis of various mammalian species, including humans, and plays a critical role in determining the quantitative degree of sperm output. However, the mechanism regulating the rate of spontaneous apoptosis during the first spermatogenic wave is unknown. In most mammals, testicular glycogen undergoes many changes during normal testis development, from being very abundant in the intratubular portion of seminiferous tubules in the prepuberal stage but diminished at the onset of puberty [Arzac, 1950; Ewing et al., 1966; Fabbrini et al., 1969; Fouquet and Guha, 1969]. Studies in rat indirectly suggest that an imbalance in glycogen homeostasis in adult testes could induce apoptosis and degeneration of germ cells [Thakur et al., 2003; Kuramori et al., 2009]. Thus, glycogen could be a molecule that modulates the survival of germ cells and also affects fertility in mammals. The goal of this study was to examine the regulation of glycogen synthesis during testis development, by considering glycogen to be an active molecule in spermatogenesis and germ cell selection by apoptosis.
MATERIALS AND METHODS
Animals
All OF1 mice were obtained from the Biosafety Laboratory Facility for Animal Experimentation (Veterinary Science Faculty, Universidad Austral de Chile, Valdivia, Chile). All experiments were performed in accordance with the standards given by the Manual for Biosafety and Bioethics (CONICYT, Chilean Government) and according to guidelines established by the Animal Protection Committee of the Universidad Austral de Chile. Animals were maintained in a 12:12 light:dark cycle environment and supplied with commercial food pellets and water ad libitum.
Transgenic Animals
Targeting vector construction and knock-in strategy was designed and performed by genOway (Lyon, France) and previously described in Duran et al. [2012]. In brief, the superactive MGS (saMGS) was introduced into the Hprt locus by homologous recombination in 129/Ola (E14). Embryonic stem cell (ES) clones were then injected into C57BL/6 blastocysts and implanted into OF1 pseudopregnant females. Highly chimaeric males were selected for breeding with wild-type C57BL/6J females. The saMGS was performed by site-specific mutation of serine residues to alanine. All procedures were approved by the Barcelona Science Park's Animal Experimentation Committee and were carried out in accordance with the European Community Council Directive and National Institutes of Health guidelines for the care and use of laboratory animals.
Cell Culture and Transfections
Sertoli cell line 42GPA9 widely validated as isolated Sertoli cells by the group that isolated this cell line, Georges Pontis and Dominique Segretain at the Université Paris 5, Paris, France [Bourdon et al., 1998] and spermatogonium-like cell line GC-1 were cultured in DMEM/F12 medium (Thermo Scientific HyClone) supplemented with 10% FBS medium (Thermo Scientific HyClone) [Bourdon et al., 1998; Angulo et al., 2008]. Primary Sertoli cells were cultured as previously described [Angulo et al., 2008]. Transfections were performed with Lipofectamine™ 2000 reagent (Invitrogen) using 1 µg of plasmidial DNA and following the manufacturer's instructions. The plasmidial DNA that contained the mouse PTG fused to GFP (pEGFP-C1 vector, Clontech) was a gift from Dr. Guinovart and has been described previously [Vilchez et al., 2007]. All cells were incubated at 37°C with 5% CO2.
Electrophoresis and Immunoblotting
Tissue and cell-culture plates were processed for protein extract preparation as has been previously reported [Vilchez et al., 2007]. Proteins were resolved by 10% SDS–PAGE, transferred onto a nitrocellulose membrane (Bio-Rad) and probed with the following antibodies: rabbit antibody to human MGS (Cell Signaling) that recognizes MGS independent of its phosphorylation state; rabbit antibody to rabbit LGS (a gift from Dr. Joan J. Guinovart); rabbit antibody to MGS with phosphorylation of Ser640 (Cell Signaling); mouse antibody to beta-actin (Santa Cruz Biotechnology); mouse antibody to ubiquitin (Cell Signaling).
MGS-Polyubiquitination Assay
Cells seeded on 60 mm plates were cultivated in DMEM/F12 medium for 12 and 24 h in the presence of cycloheximide (1 µg/ml), proteosomal inhibitor MG-132 (10 µM) or DMSO (0.1% w/w). They were then processed for protein extract preparation and separated into two fractions. One fraction was used for isolation of the total polyubiquitinated proteins with the Ubiquitinated protein enrichment kit (Calbiochem–Merck) and the other fraction was used to analyse the total protein fraction. Both protein fractions were probed by Western blot method as described previously.
Immunofluorescence and Histochemical Assays
Cells seeded on poly-L-lysine-coated coverslips were fixed for 30 min in PBS containing 4% (w/v) paraformaldehyde. After fixation, cells were permeabilised for 20 min with PBS containing 0.3% (v/v) Triton X-100. Blocking and incubation with the primary and secondary antibodies were carried out in PBS containing 3% (w/v) BSA and 0.15% (v/v) Triton X-100. Coverslips were washed in PBS and 0.3% (v/v) Triton X-100, and mounted onto glass slides using fluorescence mounting medium from Dako. We used primary antibodies to MGS3 and LGS3 (from Dr. Guinovart's laboratory), antibody to cleaved caspase3 (Cell Signaling) and a monoclonal antibody against glycogen (a gift from O. Baba, Tokyo Medical and Dental University). In some cases, nuclei were stained with Hoechst 33342 or TO-PRO®-3 (Molecular Probes). Fluorescence images were obtained with an Olympus FV-1000 confocal microscope (Universidad Austral de Chile). The light source was an argon/krypton laser (75 mW), and optical sections (0.1 mm) were obtained. For immunohistochemistry, tissue sections were fixed for 48 hr in PBS containing 4% (w/v) paraformaldehyde or BOUIN, paraffin-embedded and treated as described previously. The fibrosis studies performed in testis from wild-type and transgenic mice were carried out with Masson's trichrome and Periodic Acid-Schiff (PAS) stains and with haematoxylin-eosin analysis.
Apoptosis Assays
Tissue sections (0.4 µM) were deparrafinised in a graded alcohol series, washed in sterile PBS and processed for TUNEL or active caspase 3 staining. TUNEL assays were carried out using the Strept-Fluo-tag in situ Apoptosis Detection Kit (R&D Systems), by following the manufacturer's instructions. Active caspase 3-positive cells were visualized by immunocytochemistry using the cleaved caspase3 antibody (Cell Signaling). The TUNEL- and active caspase 3-positive cells were photographed with a Nikon Eclipse E-600 microscope using a 40× objective. The percentage of positive cells was estimated in 8–14 fields from each of three coverslips (two independent experiments) for each treatment condition (100 seminiferous tubules, knock-in or wild-type, respectively). Total number of cells was demonstrated after staining nuclei with Hoechst 33342 or TO-PRO3®.
RNA Purification and Retrotranscription
Total RNA was isolated from mouse tissue using Trizol® reagent (Invitrogen) according to the manufacturer's instructions. RNAs were purified for a second time using RNAeasy (Qiagen), and 5 µg total RNA was retrotranscripted with SuperScript™ III First-Strand Synthesis Supermix (Invitrogen). A series of specific primers were designed to specifically amplify a fragment of the coding sequence of mouse MGS, LGS, malin (Emp2b), laforin (Emp2a) and beta-actin for conventional RT-PCR analyses [Vilchez et al., 2007].
Quantitative RT-PCR
We followed the standard RT-PCR protocol of the ABI Prism® 7700 Sequence Detection System, together with the ready-made TaqMan primer and probe sets (Applied Biosystems; #Mm00472712_m1, #Hs99999901_s1 and #Mm03302249_g1). Each sample was analysed in triplicate wells with 100 ng of first-strand cDNA in a total reaction volume of 20 µl. The temperature profile consisted of 40 cycles, of 15 s, at 95°C and of 1 min at 60°C. Data was analysed with the comparative 2-ΔΔCt method using ribosomal 18S as an endogenous control [Vilchez et al., 2007].
Glycogen Analysis
Total homogenates, supernatants and sediments were boiled at 100°C in 30% KOH and glycogen content was determined by an amyloglucosidase-based assay as previously reported [Vilchez et al., 2007; Duran et al., 2012].
Determination of MGS Activity
Tissue sections were processed as described previously [Vilchez et al., 2007]. Protein concentration was measured with the Bradford Method. MGS activity was measured in homogenates in the presence of 4.4 mM UDP-glucose, and in the presence or absence of 6.6 mM G6P as described previously. The activity measured in the absence of G6P represents the active form of MGS (I or a form), whereas the activity measured in the presence of G6P represents total MGS activity.
GST-Pull Down
E. coli BL21 was transformed with a vector contained the malin-ring domain construct [Gentry et al., 2005]. One hundred microgram total proteins bacterial extract contained the malin-ring overexpressed as protein fusion to GST was immobilized to gluthation-sepharosa beads (GE Healthy) following the manufacturing instructions. The malin-ring immobilized was used as bait to demonstrate its interaction with MGS in testicular cells. 500 µg total proteins extract from GC-1 or 42GPA9 cell lines were incubated with beads overnight at 4°C and continuous stirred. None bound fraction was washed using phosphate buffered saline and 0.1% w/w Tween-20, and the proteins specifically bounded were eluted using Laemmli buffer [Laemmli, 1970]. Equal volumes from each fraction were fractionated by 10% SDS–PAGE and immunodetected using commercial antibody, previously described.
Statistical Analysis
Student's t-test was applied or non-parametric statistical analyses were performed using Prism version 3.0 for Microsoft software.
RESULTS
Muscle Glycogen Synthase Is Expressed in Testis, Is Active and Is Responsible for the Glycogen Store
It is almost 60 years ago that glycogen content and glycogen fluctuations were detected in the testis of different mammals [Arzac, 1950, 1953; Ewing et al., 1966; Fabbrini et al., 1969; Fouquet and Guha, 1969; Fawcett and Dym, 1976; Datta et al., 1988]. However, which isoform of GS was expressed in this tissue was unknown. Using immunofluorescence, RT-PCR and immunoblotting techniques, we found only the muscle isoform of glycogen synthase (MGS) and not the liver isoform in whole testis extracts (Fig. 1, images a–c). MGS was localized in seminiferous epithelium from rat adult testis, as well as at different stages of male germ cell differentiation, from the basal to the adluminal compartment of tubules (Fig. 1, images b and c). The inset shows a magnified portion of germ epithelium that is immunoreactive to MGS (Fig. 1, image c). The MGS-positive signal was very granular, implying many sites of glycogen synthesis, as has been reported in other cell types and tissues [Graham et al., 2010; Roach et al., 2012]. By conventional RT-PCR (Fig. 1B) we only detected the expression of MGS and by using Western blot analysis (Fig. 1C), we confirmed the presence of this enzyme to reveal a main protein band that migrated with an apparent molecular mass of approximately 85 kDa in adult tissue. In addition, we evaluated by RT-PCR (Fig. 1D) and Western blot (Fig. 1E) the presence of MGS in isolated seminiferous epithelial cells. Sertoli cells showed higher levels of MGS than germ cells pool, suggesting important differences between somatic cells and male germ cells respect to glycogen metabolism. In contrast to previously published results by Ballester et al. [2000], we detected the muscle isoform of GS but not the liver isoform in mammalian spermatozoa. In addition, we confirmed the presence of MGS in spermatozoa from human, stallion, boar, dog, rat, and mouse using immunofluorescence (data not shown). We also examined GS activity during testis development (Fig. 2A) and the glycogen store (Fig. 2B) in mice. We detected higher levels of total activity, measured in the presence of glucose-6P, which represents the total amount of enzyme in the first days after birth, specifically at day 5. GS activity then decreases progressively with testis development. We discovered essentially the same result for glycogen levels, an initial increase and then a major decrease after day 5. By Western blot analysis, we confirmed the MGS presence in the same samples used for enzymatic assays (Fig. 2C). At short exposure times, the immunoreactive signal was positive only for the first days after birth; however, at longer exposure times MGS was detected in all ages.
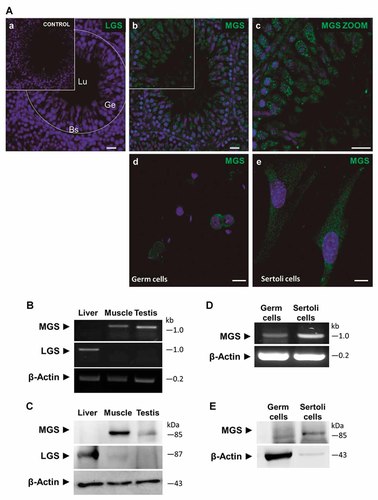
Muscle isoform glycogen synthase (MGS) is expressed in testis. Glycogen synthase (MGS or LGS) was analysed by immunofluorescence in testis (A). Images (a–c) show immunodetection of LGS or MGS (green) in whole testis from rat. In addition, images (d, e) show the immunodetection of MGS in pool of germ cells and Sertoli cells primary culture from mouse. Nuclei were stained with TO-PRO-3®. Seminiferous tubule was drawn schematically in dashed line, Lumen (Lu); germinal epithelium (Ge); Basal segment (Bs). Scale bar: 20 µm. Using conventional RT-PCR (B) and Western blot analysis (C) in whole testis (T) from adult mouse, we detected only the muscle isoform of glycogen synthase. As a control we used both total RNA and total protein extracted from liver (L) and skeletal muscle (M). MGS was detected in germ cells pool and Sertoli cells primary culture by RT-PCR (D) and Western blot (E). Inset in (A) show the control without primary antibody.
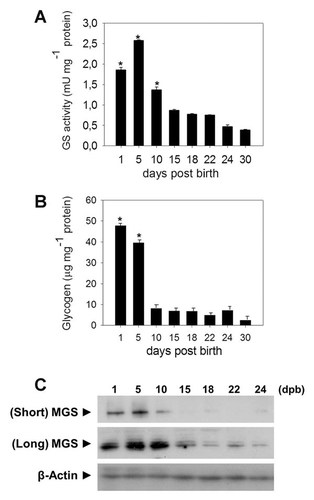
Muscle glycogen synthase is active and responsible for testicular glycogen synthesis. The glycogen synthase activity of testicular MGS (A) and glycogen storage (B) were analyzed at different ages (days post birth, dpb). Total glycogen synthase activity was measured by incorporation of UDP-glucose (14C) in the presence of the allosteric activator, glucose-6-phosphate in the enzymatic reaction mix. Glycogen storage was quantified as glucose units and represented as micrograms of glycogen per milligram of total protein. Glycogen was precipitated by applying ethanol to whole testis extract and precipitated glycogen was then digested with alpha-amyloglucosidase. Both results were expressed as a function of total protein fraction. The presence of MGS in whole proteins extract used to enzymatic assays was verified by Western blot (C). The immunoreactive signal was analysed at two exposure times, 10 s (short) or 10 min (long). The results were expressed as the mean ± SEM. Asterisks indicate significantly different means (P < 0.001).
Sertoli Cells as the Main Source of Intratubular Glycogen
Many authors have reported that Sertoli cells are able to synthesise glycogen, which is the main source of glycogen in the seminiferous tubules and considered as a cell maturation signal [Arzac, 1950; Ewing et al., 1966; Fabbrini et al., 1969; Fouquet and Guha, 1969]. By immunofluorescence and confocal microscopy, we analyzed the subcellular localization of MGS and glycogen in 42GPA9 Sertoli cell line (Fig. 3A). MGS signal was granular while glycogen presented a homogeneous distribution within the whole cytoplasm (images a and b). The merged fluorescence pattern, showed partial colocalization of MGS and glycogen, suggesting active glycogen synthesis sites (image c). MGS was also detected in Sertoli primary culture (Fig. 1A, images d and e), confirming the validation of cell line 42GPA9 as previously demonstrated by transferrin, sulfated glycoprotein-2, vitamin C and glucose transporters (SVCTs and GLUTs), and these cells also retained FSH receptors as suggested by their specific responsiveness to the gonadotropin and the detection of FSH receptor mRNAs [Bourdon et al., 1998; Angulo et al., 2008, 2011]. MGS amount present in Sertoli and male germ cell lines was estimated by an enzymatic assay (Fig. 3B). Total GS activity was measured in the presence of G6P showing that Sertoli cell line 42GPA9 (black bar) has nearly 30% more activity than spermatogonium cell line GC-1 (gray bar), suggesting that Sertoli cells have higher levels of the enzyme.
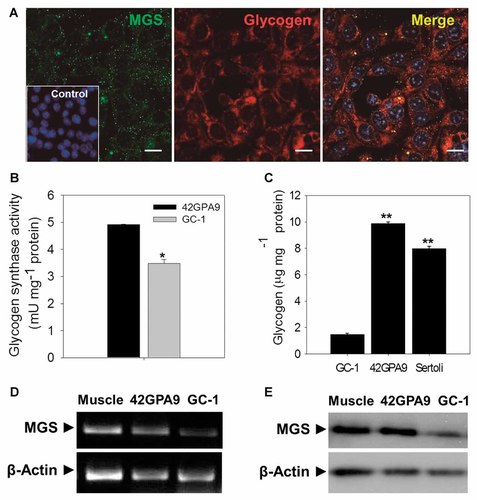
Sertoli cells are the main source of testicular glycogen. Cell lines GC-1 (spermatogonium type B-like cell) and 42GPA9 (Sertoli cell) were cultured in DMEM-F12 medium and used to estimate the main source of glycogen to the seminiferous tubules. MGS and glycogen were estimated in Sertoli cell by immunofluorescence and confocal microscopy (A). The total glycogen synthase activity of testicular MGS (B) were analysed in both cell line (GC-1 and 42GPA9). Glycogen synthase (GS) activity was measured by incorporation of UDP-glucose (14C) in the presence (total activity, T) of the allosteric activator, glucose-6-phosphate in the enzymatic reaction mix. Glycogen was estimated in normal culture conditions both cell lines and Sertoli cell primary culture (C). Glycogen storage was quantified as glucose units and represented as micrograms of glycogen per milligram of total protein. Glycogen was precipitated by applying ethanol to whole proteins extract and precipitated glycogen was then digested with alpha-amyloglucosidase. The presence of MGS in whole proteins extract in cell lines GC-1 and 42GPA9 was verified by Western blot (D). The results were expressed as the mean ± SEM. Asterisks indicate significantly different means (*P < 0.01; **P < 0.001). In (A) nuclei were stained with TO-PRO® and inset show the control without primary antibody.
We estimated glycogen stores by biochemical assays in cell line GC-1, representing 10% of the glycogen store in Sertoli cells (42GPA9 cell line and primary culture; Fig. 3C). The glycogen content was very different between germ cells and Sertoli cells, which confirms the contribution of Sertoli cells to the intratubular glycogen storage. MGS detection in each cell line was confirmed by RT-PCR (Fig. 3D) and immunoblotting (Fig. 3E); Sertoli cells (cell line 42GPA9) showed higher levels of MGS than germ cells (cell line GC-1), confirming our results in isolated cells (germ cells pool and Sertoli cells primary culture).
Glycogen Synthesis in Testis Could Be Regulated by the Malin–Laforin Complex
The malin–laforin complex has been described in brain, specifically in neurons, where it regulates MGS availability and activity [Vilchez et al., 2007; Solaz-Fuster et al., 2008; Worby et al., 2008; Roach et al., 2012]. To determine whether this complex might be present in mammalian testis, we evaluated malin and laforin expression in testis from adult mice with real time and convetional RT-PCR and immunodetection of malin and laforin proteins (Fig. 4). The Real-time RT-PCR results show a differential expression of malin and laforin (Fig. 4A, B), suggesting that laforin is present during the first days of testis development. On the contrary, malin transcript is detected after 10 days post-birth, at the same time in which GS and glycogen store decreased. By conventional RT-PCR, we detected the respective transcripts in whole testis, Sertoli cells (cell line 42GPA9) and isolated germ cells with the exception of spermatids, suggesting that these cells do not express the malin gene at that particular differentiation stage (Fig. 4C). With immunoblotting, laforin was detected in testis (Fig. 4D), confirming the expression of this protein as suggested by other authors [Roach et al., 2012].
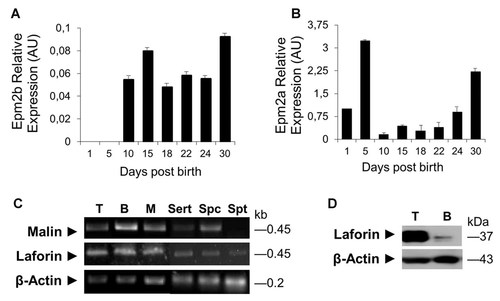
Testicular expression of malin and laforin. Relative expression levels of Epm2b or malin (A) and Epm2a or laforin (B) were estimated by real time RT-PCR. One hundred nanogram total cDNA from whole mouse testis at different ages were used to amplify each mRNA using taqman® (Applied Biosystems) probe. The relative expression levels of each messenger were expressed as changes fold respect to day 1, and previously normalized using 18S rRNA expression levels. Malin and laforin expression in adult testis (T) was verified by conventional RT-PCR (C) and Western blot analysis (D). As a control, we used muscle (M) or Brain (B) total RNA or total protein extract. The results were expressed as the mean ± SEM. The results are representative of three independent experiments. Sert, Sertoli cell line 42GPA9, Spc, isolated spermatocytes, Spt, isolated spermatids.
The presence of laforin and the putative presence of malin in testicular tissue could be an additional mechanism to regulate glycogen synthesis in the seminiferous tubules. To support this hypothesis, we performed GST-pull down using the malin-ring domain as bait and detected the interaction between malin and endogenous MGS in germ cell (cell line GC-1), Sertoli cell (cell line 42GPA9) and whole testis (Fig. 5A) [Gentry et al., 2005]. In all cases, MGS in protein extracts from different cell types were able to interact and bind to the catalytic domain of malin (as E3 ubiquitin ligase), suggesting that MGS in testicular tissue is substrate to malin and confirming that ubiquitination is one mechanism to modulate the activity and availability of GS for testicular glycogen synthesis (Fig. 5A). This regulatory mechanism may involve the malin–laforin complex, as for neurological tissues and could be a common mechanism to other cell types. As it has been previously found that Sertoli cells could be the main source of intratubular glycogen in adult testis and from our own experimental evidence (Fig. 3), we choose the Sertoli cell line 42GPA9 to demonstrate that the malin–laforin complex is able to polyubiquitinate MGS and indirectly regulate glycogen synthesis in testis. After treatment with cycloheximide and MG-132 (a proteosomal inhibitor), we detected accumulation of a polyubiquitinated form of MGS by Western blot analysis (Fig. 5B). The polyubiquitinated-MGS present in the total protein extract has a molecular weight that varies by 20–40 kDa over its normal migration, showing an evident slip in its electrophoretic profile when the MGS was immunoprecipitated and immunodetected using anti-ubiquitin antibody (data not shown). Despite this, the polyUb-MGS is mainly found in a 110 kDa form. To confirm MGS polyubiquitination, we isolated the total polyubiquitinated-protein fraction (polyUb fraction) and investigated the presence of MGS with immunodetection. Results showed a specific immunoreactivity for this protein both in cells treated with MG-132 alone or with MG-132 plus cycloheximide. The molecular weight of MGS in the polyUb fraction is the same as that detected in the total protein extract, suggesting that ubiquitination of MGS and eventually proteosomal degradation in Sertoli cells might be the mechanism by which glycogen synthesis in testis is regulated. Other studies have investigated the malin–laforin complex using several other experimental methods, such as transfection and overexpression or silencing of genes in different cell models. In this work, we present for the first time evidence of an endogenous malin–laforin complex and its activity, specifically in Sertoli cells under normal conditions.
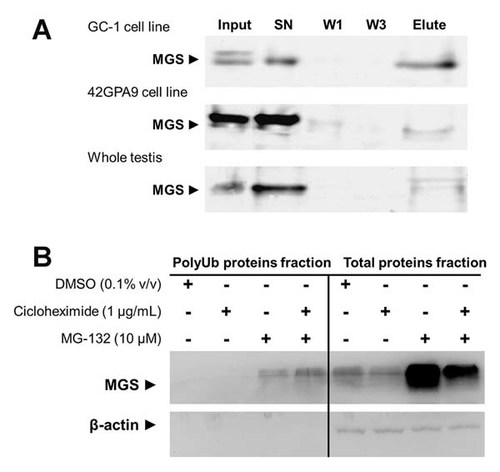
Malin and laforin form an active complex that regulates MGS by polyubiquitination in testis. By GST-pull down using malin-ring domain as bait, we detected the interaction between MGS and malin in total protein fraction from germ cell (cell line GC-1), Sertoli cell line 42GPA9 or whole testis (A). Malin-ring was expressed as fusion protein to GST and immobilized on gluthation-sepharose beads. Five hundred microgram of total protein fraction from each cell line or tissue were used to pray the MGS protein. Equal volumes of different fractions were fractionated by SDS–PAGE and immunodetected using commercial antibody. W1 and W3, correspond to washed fractions 1 and 3, respectively. SN or supernatants corresponds to the proteins none bound to the bait. Elute fraction, was obtained using the Laemmlli buffer directly. To see whether the malin–laforin complex may regulate MGS quantity in male reproductive tissue, we studied the accumulation of polyubiquitinated MGS (polyUb) using the proteosomal inhibitor MG-132 (B). Immunoprecipitation of polyUb protein fractions was performed, followed by immunodetection of MGS (B). Sertoli cells (cell line 42GPA9) were cultured in DMEM-F12 in the presence or absence of pharmacological stimuli at the concentration indicated for 24 h. MGS and actin were immunodetected using a commercial antibody and the polyUb protein fractions were obtained as described in the Materials and Methods Section.
Overproduction of Glycogen Triggers Germ Cell Apoptosis and Seminiferous Tubule Degeneration
To induce male germ cells glycogen overproduction, we transfected spermatogonia-like cell line GC-1 with PTG (protein targeting to glycogen), which targets PP1 to glycogen synthesis sites, inducing MGS activation [Ingebritsen et al., 1983; Graham et al., 2010; Roach et al., 2012]. Overexpression of PTG in GC-1 cells should promote glycogen accumulation by reducing levels of phosphoSer640-MGS (or the less active form), and thus synthesising more glycogen. In addition, PTG overexpression could induce simultaneously an inactivation of glycogen phosphorylase activity promoting more glycogen storage. The PTG effect was measured by immunodetection of phosphoSer640-MGS (pMGS) and cleaved caspase 3 (Fig. 6A), with a decrease in pMGS levels detected. However, cleaved caspase3 and glycogen stores increase (Fig. 6B, C), which suggests a negative effect on cell viability induced by an imbalance on glycogen synthesis.
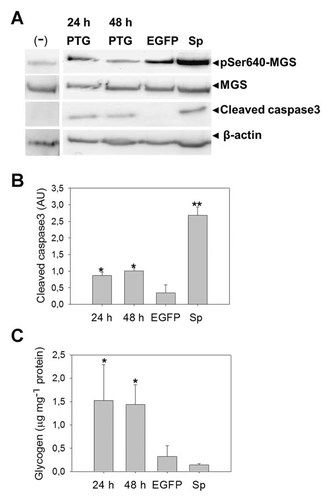
Male germ cells are sensitive to the glycogen overproduction by PTG overexpression. GC-1 cell line (spermatogonium type B-like cells) were transfected with PTG-EGFP and cultured for 24, 48 h. Empty EGFP vector was used as a control. We applied 10 nM staurosporine (Sp) for 12 h to induce apoptosis and form cleaved caspase3. After treatment, total proteins were extracted and subjected to Western blot analysis. (A) shows MGS and pSer640MGS detection using specific antibodies. In the same samples, cleaved caspase3 was mainly detected in PTG-transfected cells. Levels of cleaved caspase3 were measured by densitometry analysis and expressed as arbitrary units (AU) in the graph (B) with respect to transfection with the empty vector at 48 h. Glycogen was estimated in the transfected cells (C). Glycogen storage was quantified as glucose units and represented as micrograms of glycogen per milligram of total protein. Glycogen was precipitated by applying ethanol to whole proteins extract and precipitated glycogen was then digested with alpha-amyloglucosidase. The results were expressed as the mean ± SEM. Asterisks indicate significantly different means (*P < 0.001; **P < 0.05). The results are representative of three independent experiments.
To better understand the role of glycogen during testis development and spermatogenesis, we investigated the effects of the loss in glycogen homeostasis during testis development (supplementary data). Glycogen overproduction was induced in nestin-positive cells by expression of the superactive form of MGS (saMGS) [Duran et al., 2012], enzyme which is able to synthesise higher amounts of glycogen, unbalancing the normal synthesis rate (elongation-branch and store balance). Histological analysis from Kin testes suggested an infantile phenotype of transgenic testis, characterized as an involution or cell cycle arrest like in many pathological processes of male reproductive tissues (cryptorchidism or varicocele). PAS staining in saMGS Kin testes revealed the presence of strongly PAS-positive structures in the seminiferous tubules (data not shown), confirming the hyperactivity of saMGS and glycogen overproduction [Duran et al., 2012]. The PAS staining showed many apparently dense structures, which suggested the formation of polyglucosan bodies in the seminiferous epithelium. These results were confirmed by immunodetection of MGS and polyglucosan. Immunolocalization of MGS was evaluated in saMGS knock-in (Kin) animals and was found to be specifically over-expressed in nestin-positive cells (supplementary data). Strong glycogen labelling seen in the germinal epithelium from saMGS Kin testis, but not in the control littermate. Glycogen has been previously associated with cell death in neurons and other cell types by an unknown mechanism [Vilchez et al., 2007; Vallés-Ortega et al., 2011; Duran et al., 2012]. To verify germ cell apoptosis, we detected formation of cleaved caspase 3 and we readily found specific labeling for caspase 3 in spermatocytes and spermatogonia Using TUNEL assay, we estimated an increase in the apoptotic rate of approximately eightfold with respect to control animals (supplementary data).
DISCUSSION
In this study, we have evaluated the GS isoform responsible for glycogen synthesis during testicular development and spermatogenesis and its possible participation in apoptosis of male germ cells. We showed, first of all, that the muscular isoform of GS is expressed in testicular tissue, revealing that it is this isoform and not the hepatic isoform that is the enzyme responsible for intratesticular glycogen synthesis, even in spermatozoa from different mammalian species. Many studies have shown changes in enzyme activity and glycogen content in whole testis [Arzac, 1950, 1953; Ewing et al., 1966; Fabbrini et al., 1969; Fouquet and Guha, 1969; Fawcett and Dym, 1976; Datta et al., 1988], but we demonstrate localization and distribution of the enzyme in male germ cell epithelium and also observe glycogen content at different stages of cell differentiation. We have established that GS activity and glycogen content reach a maximum point during testicular development that coincides with the onset of sexual maturity [Ewing et al., 1966; Austin and Short, 1972; Amann, 2008]. This stage is characterized by a massive increase in cell proliferation, which is accompanied by an increased cell and testicular mass. A gradual decrease in both GS activity and glycogen content occurs later in development, perhaps highlighting the importance of these parameters during the formation of male germinal epithelium. Glycogen is considered as an energy source and an intermediary for steroidogenesis and for other important metabolites [Arzac, 1953]. Regulation of GS activity is generally considered to be the main mechanism by which glycogen availability is modulated. Regulation of the activity of this enzyme requires the presence of allosteric activator G6P, as well as covalent modifications mediated by kinases and phosphatases, which in most models studied results in a change in subcellular localization of GS [Graham et al., 2010; Roach et al., 2012]. On the contrary, we have not been able to establish such a change in subcellular localization of this enzyme in testis cells, in particular in Sertoli cells, where it is mainly found in the cytosol. This enzyme shows a low intrinsic activity (without the allosteric activator G6P in the reaction mixture, data not shown), but is able to synthesise glycogen. Most recently, a new regulatory mechanism for GS activity, and even for availability of the enzyme, has been described. A heterodimeric malin–laforin complex would regulate the amount of active enzyme, and with it, glycogen synthesis, via polyubiquitination and proteosomal degradation processes [Vilchez et al., 2007]. The presence of both proteins, and the polyubiquitination of GS in the Sertoli cell line 42GPA9, confirm that this machinery functions in different tissues of the central nervous system and that it is perhaps a strategy for maintenance of glycogen homeostasis that is widely distributed throughout the organism. Our study reveals for first time, at an endogenous level, a role for the malin–laforin complex in GS availability. Previous reports have shown that glycogen accumulation in neurons triggers conditions of stress resulting in apoptotic cell death [Vilchez et al., 2007; Vallés-Ortega et al., 2011; Duran et al., 2012]. PTG overexpression induces glycogen synthesis and its accumulation in spermatogonium-like cell line GC-1, resulting in caspase3 activation, suggesting the proapoptotic role of glycogen overproduction. In addition, in our own transgenic model, glycogen accumulation occurs in nestin-positive cells, such as Leydig cells, Sertoli cells and male stem cells; however, the negative effect induced by glycogen overproduction occurs mainly in spermatogonia and premitotic spermatocytes, essential components of normal testicular architecture [Austin and Short, 1972; Fröjdman et al., 1997; Lobo et al., 2004; Mruk and Yan Cheng, 2004; Nakanishi and Shiratsuchi, 2004; Amann, 2008; Hutchison et al., 2008; Shaha et al., 2010]. The observed histology may be explained by a loss in stability of the blood–testis barrier, where glycogen accumulation in Sertoli cells interferes with remodeling of the cytoskeleton and with existing tight junction unions with the basal membrane of the seminiferous tubule, thus disorganizing the germinal epithelium [Yazama et al., 1997; Mruk and Yan Cheng, 2004; Mruk and Cheng, 2010; Mruk and Cheng, 2011]. Previous studies have also shown that administration of lithium carbonate in adult rats, a known activator of GS activity, induces desquamation of the male germinal epithelium and electron microscopy studies revealed that this was due to a loss of integrity of junctions between Sertoli cells and the basal lamina [Yazama et al., 1997; Thakur et al., 2003]. Other studies have shown that interruption of the glycogen catabolic route produces a major increase in the rate of apoptosis, suggesting that there exists a tolerance threshold for this macromolecule. It has been previously reported that PTG simultaneously inactivates glycogen phosphorylase and activates GS, suggesting a possible dual role of this protein during glycogen accumulation and cell viability. [Greenberg et al., 2003; Lee et al., 2004; Greenberg et al., 2006; Vilchez et al., 2007; Kuramori et al., 2009; Roach, 2011; Tagliabriacci et al., 2011; Vallés-Ortega et al., 2011; Duran et al., 2012; Roach et al., 2012].
To date, the true role of glycogen during spermatogenesis is not clear. It may be a source of energy and structural components, but under certain conditions, such as during the overproduction of the poorly branched form of glycogen we are studying glycogen may trigger new signals and induce cell degeneration or death (see model, Fig. 7) [Gentry et al., 2012; Roach et al., 2012]. The next challenge we face will be to establish the mechanism by which glycogen participates in physiological selection of male germ cells. An improved knowledge of the role of glycogen and its homeostasis in testicular development and spermatogenesis in mammals may prove to be an important tool for further understanding the processes responsible for male infertility.
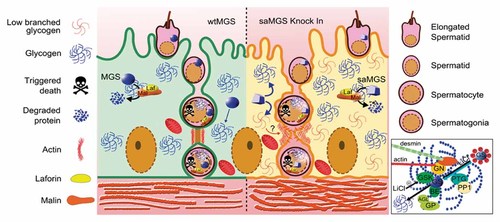
Glycogen overproduction induces degeneration in seminiferous tubules and apoptosis in male germ cells. In normal conditions, muscle glycogen synthase (wtMGS) activity in Sertoli cells and germ cells is regulated by phosphorylation and a malin–laforin complex. In Sertoli cells, the glycogen homeostasis is used to support the energetic balance to the germinal epithelium development. However, in germ cells (mainly spermatogonium) the glycogen storaged could be a pro-apoptic signal during the first wave spermatogenic by unknown mechanisms. By other hand, when glycogen homeostasis is unbalance by overactivation of glycogen synthase (saMGS) or higher amount of PTG, the glycogen synthesized could be anomalously branched and able to induce alterations in blood-testis barrier and architecture of the male germ epithelium by interactions with the cytoskeletal and other proteins. At same time, the higher levels of glycogen synthesized could supply metabolic substrates to extracellular matrix and induce a fibrosis condition. The catalytic core to the glycogen synthesis (inset) is a macromolecular complex, in which glycogen and its main proteins involved are attached to cytoskeletal and where many external stimuli (e.g., LiCl) or endogenous modulators are present, which can modify the enzymatic activity, storage or structural conformation of glycogen. GSK, glycogen synthase kinase; GN, glycogenin; GS, glycogen synthase; BE, branching enzyme; AGL, debranching enzyme; GP, glycogen phosphorylase; PTG, protein targeting to glycogen; PP1, protein phosphatase 1.
Acknowledgements
We thank Dr. Dominique Segretain for providing 42GPA9 Sertoli cell line (Groupe d'Etude des Communications Cellulaires, INSERM U895, Université Paris 5, Paris, France) and Dr. Joan Enric Rodriguez-Gil for the gift of spermatozoa from different experimental animals (Unitat de Reproducció Animal, Facultat de Veterinària, Universitat Autònoma de Barcelona, Bellaterra, Barcelona, Spain). This study was supported by FONDECYT (Fondo Nacional de Desarrollo Científico y Tecnológico) Grant 1110508 (to I.I.C.) and 1090740 (to J.C.S); DID-UACh (Dirección de Investigación y Desarrollo, Universidad Austral de Chile), J.J.G.'s laboratory was funded by grants from the Ministerio de Economía y Competitividad (BFU2011-30554), the Generalitat de Catalunya (2009 SGR 01176), and the CIBER de Diabetes y Enfermedades Metabólicas Asociadas (ISCIII, Ministerio de Economía y Competitividad). Grant S-201-14; Dirección de Estudios Postgrados and Escuela de Graduados, Facultad de Ciencias, Universidad Austral de Chile; CONICYT (Comisión Nacional de Investigación Científica y Tecnológica) Scholarship for doctoral study (to F.V.E) and AT24100011 (to F.V.E.); MECESUP (Programa de Mejoramiento de la Calidad y Equidad de la Educación Superior) Scholarship grant UCO0606 and AUS0704 (to F.V.E).




