Ganglioside GM3 inhibits hepatoma cell motility via down-regulating activity of EGFR and PI3K/AKT signaling pathway†
Xiaohua Huang and Ying Li contributed equally to this work.
Abstract
Two related sublines derived from murine ascites hepatoma cell lines Hca-F25, which were selected for their markedly different metastatic potential to lymph nodes, were found to be distinct in their ganglioside patterns. The low metastatic cell line (HcaP) contained a major ganglioside GM3, whereas the high metastatic cell line (HcaF) contained a major ganglioside GM2. Suppression of GM3 by P4 enhanced the mobility and migration of the low metastatic HcaP cells in vitro. Increase in GM3 content in high metastatic HcaF cells by addition of exogenous GM3 inhibited the mobility and migration. These results suggested that the differences in lymphatic metastasis potential between these two cell lines could be attributed to the differences in their ganglioside compositions, and GM3 could suppress the motility and migration of these cells. Further, we investigated the mechanism by which GM3 suppressed the cell mobility and migration. The results showed that suppression of GM3 synthesis by P4 in low metastatic HcaP cells promoted PKB/Akt phosphorylation at Ser473 and Thr308, and phosphorylation of EGFR at the Tyr1173. In contrast, increase in GM3 content in high metastatic HcaF cells by addition of exogenous GM3 into the culture medium suppressed phosphorylation of PKB/Akt and EGFR at the same residues. Taken together, these results suggested that the mechanism of GM3-suppressed cell motility and migration may involve the inhibition of phosphorylation of EGFR and the activity of PI3K/AKT signaling pathway. J. Cell. Biochem. 114: 1616–1624, 2013. © 2013 Wiley Periodicals, Inc.
Abbreviations:
P4, D-threo-1-phenyl-2-palmitoylamino-3-pyrrolidino-1-propanol; GM3, Neu5Acα2,3 Galβ1,4Glcβ1,1Cer; PI3K, phosphoinositol-3-kinase; EGFR, epidermal growth factor receptor; HPTLC, high performance thin-layer cromatocraphy.
Gangliosides are sialylated glycosphingolipids that are characteristic components of mammalian cell membranes and have been implicated in many biological functions of the cell [Van Echten and Sandhoff, 1993; Huwiler et al., 2000]. Based on their structural properties and location on the cell surface, gangliosides have been speculated to participate in many cell surface events, such as cell to cell adhesion, cell–extracellular matrix adhesion and modulation of transmembrane signaling mediated by plasma membrane receptor [Hakomori, 1981; Hakomori, 1996; Birkle et al., 2003; Regina Todeschini and Hakomori, 2008; Hakomori, 2010]. Among the gangliosides, the ganglioside GM3 (Neu5Acα2,3 Galβ1,4Glcβ1,1Cer) is the simplest member and a common precursor for more complex gangliosides [Prokazova et al., 2009]. The involvement of ganglioside GM3 in malignant behavior of tumor cells, such as cell proliferation, adhesion, invasion, and metastasis, has long been a subject of interest [Kojima and Hakomori, 1991; Iwabuchi et al., 1998; Ono et al., 2001; Toledo et al., 2004]. However, the exact mechanism of this effect remains unclear. One major problem is that GM3 exerts its actions in a tumor-type specific manner. Most studies have shown that ganglioside GM3 has the inhibitory activity on cell proliferation, motility, migration, invasiveness, and metastasis in several tumor models [Rebbaa et al., 1996; Kawamura et al., 2001; Noll et al., 2001; Satoh et al., 2001; Wang et al., 2003; Fujimoto et al., 2005]. However, there is an inverse relation between cell proliferation and metastasis properties and GM3 content in some types of tumor. For instance, GM3 was found to be abundant in highly metastatic lines of B16 melanoma and treatment with GM3 enhanced metastatic potential of mouse B16 melanoma cells to lung [Yogeeswaran et al., 1978; Saha and Mohanty, 2003]. Moreover, overexpression of ganglioside GM3 synthase in nonmetastatic breast cancer 67NR cells significantly increased cell migration. On the contrary, silencing of GM3 synthase in metastatic breast cancer 4T1 cells significantly inhibited cell migration, invasion, and lung metastasis in vivo [Gu et al., 2008]. These results indicated that GM3 might exert different or even opposite actions on different tumor types. However, until recently, the underlying mechanism of GM3 cell-type specific remains unclear and would require further work to clarify.
The metastatic spread of tumor cells can occur via either lymphatic or vascular vessels [Carr, 1983]. The lack of appropriate model tumor cells that metastasize specifically to lymph nodes has resulted in a lack of understanding of the mechanism of lymphatic metastasis [Yokoyama et al., 2006]. HcaF and HcaP are two related sublines derived from the same parental cell line, but each has different lymphatic metastasis ability [Li et al., 1998]. HcaF is a high metastatic cell line, with about 80% lymph node metastatic rate. HcaP is a low metastatic cell line, with zero to about 20% lymph node metastatic rate. Both cell lines do not exhibit vascular metastasis. Hence, HcaF and HcaP can be used as a good cell model to study the mechanism of lymphatic metastasis of tumor cells. In order to investigate the roles of gangliosides in lymph node metastasis, the ganglioside compositions of HcaF and HcaP cells were comparatively analyzed in the present study. The mechanism by which gangliosides influence cell motility and migration was also examined by inhibiting the synthesis of gangliosides with P4 as well as by adding exogenous GM3 to the culture medium.
MATERIALS AND METHODS
Cells and Cell Culture
HcaF and HcaP cells were derived from murine ascites hepatoma cell line Hca-F25 [Li et al., 1998]. The cells were grown in 10-cm cell culture dishes or in multi-well plates in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 mg/ml streptomycin at 37°C in a 5% CO2 atmosphere.
Antibodies and Reagents
Antibodies
Anti-EGFR rabbit IgG, anti-P-EGFR (Tyr-1173) rabbit IgG, anti-P-EGFR (Tyr-1086) goat IgG, anti-Akt rabbit IgG, anti-P-Akt (Ser473) rabbit IgG, anti-P-Akt (Ser308) rabbit IgG, anti-β-actin mouse IgG, goat anti-rabbit IgG-horseradish peroxidase, rabbit anti-goat IgG-horseradish peroxidase, and goat anti-mouse IgG-horseradish peroxidase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); mouse anti-GM3 IgM was obtained from Wako Pure Chemical Industries Ltd. PE anti-mouse IgM was obtained from BD Biosciences Pharmingen.
Reagents
LY294002 was from Sigma; EGF was obtained from ProSpec-Tany TechnoGene Ltd; protease inhibitor cocktail was obtained from Sigma–Aldrich, USA; D-threo-1-phenyl-2-palmitoyl -amino-3-pyrrolidino-1-propanol (P4) was purchased from Matreya. Ganglioside GM3 and mixture of gangliosides (GA Mix, Lot No:0313) were acquired from FIDIA Research Laboratories.
Comparative Analysis of Ganglioside Compositions of HcaF and HcaP Cells
Gangliosides were extracted and analyzed as previously described [Ladisch and Gillard, 1985]. Briefly, HcaF and HcaP cells were grown in 10-cm dishes until ∼90% confluence. The cells were trypsinized and washed three times with PBS. The cell pellet was extracted twice with chloroform/methanol (1:1, by volume), and the extracts, which contained the total lipids, were combined and dried under a stream of N2. The gangliosides were purified by partitioning the dried samples in di-isopropyl ether/1-butanol/17 mM aqueous NaCl followed by Sephadex G-50 gel filtration and lyophilization. Individual gangliosides was separated on silica gel 60 HPTLC plates (Merck, Darmstadt) with a solvent system consisting of chloroform/methanol/0.25% aqueous CaCl2·2H2O (60:40:9, by volume). The gangliosides were visualized as purple bands by spraying with resorcinol–HCI reagent and heating it at 120°C.
Regulation of Ganglioside Content in HcaF and HcaP Cells
For the suppression of ganglioside synthesis, HcaF and HcaP cells were seeded into 12-well plates in RPMI 1640 medium supplemented with 10% FBS and different concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 µM) of P4, a racemic threo-1-phenyl-2-hexadecanoyl-amino-3-pyrrolidinopropan-1-propanol, respectively. After 48 h of incubation, the cells were harvested and the inhibition of gangliosides synthesis was monitored by dot blot assay using anti-GM3 antibody or by HPTLC.
For the enhancement of ganglioside content in cells, cells were seeded into 12-well plates in RPMI 1640 medium supplemented with 10% FBS, and treated with 50µM of GM3 (for HcaF cells) or a mixture of gangliosides (including GM2, GM1, GD1a, GD1b, and GT1b; for HcaP cells). The cells were incubated for 2 days before harvesting.
In Vitro Migration Assay
Migration assay was performed using Boyden chamber (Costar, Cambridge, MA) with 8 µm pore polycarbonate filters (BD Biosciences, Franklin Lakes, NJ). Cells (1 × 105) treated as described above were resuspended in 300 µl of 0.1% FBS medium containing 5.0 ng/ml of EGF and the desired agents and placed in the top compartment of the chamber. Six hundred microliters of 10% FBS medium were placed in the bottom chamber. After 6 h of incubation at 37°C in 5% CO2 incubator, the cells on the top membrane surface were mechanically removed. The cells that migrated to the lower surface of the membrane were fixed and stained with 0.1% DAPI. Photographs were taken and stained cells from five randomly chosen fields were counted under a microscope. For the blocking of PI3K/Akt pathway with LY294002, the high metastatic HcaF cells or low metastatic HcaP cells treated with 1.0 µM of P4 were exposed to 15 µM of LY294002 for 4 h. The cells were then harvested and used for migration assay.
SDS/PAGE and Immunoblotting
HcaF and HcaP cells treated as described above were seeded into 12-well plates (Falcon) and treated with the desired agents described in the figure legends in serum-free medium overnight. The medium was changed and the cells were stimulated with 5.0 ng/ml EGF for 10 min at room temperature or left unstimulated (control). The cells were harvested and lysed in 200 µl of RIPA buffer (1% Triton X-100, 150 mM NaCl, 25 mM Tris, pH 7.5, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM pyrophosphate, 50 mM NaF) containing 1 mM Na3VO4, 1 mM DTT, 1% protease inhibitor kocktail and 1% phosphatase inhibitor cocktail. The lysate was subjected to Western blot analysis. β-Actin antibody was used as a control. All bands were detected using ECL Western blot kit (Amersham Biosciences, UK). The relative amount of protein on the blots was determined by densitometry using LabWorks software (UVP, Upland, CA).
Statistical Analysis
SPSS12.0 software was used in this study. Each assay was performed at least three times. The data were expressed as mean ± SD and analyzed by one-way ANOVA to determine the significance of differences in multiple comparisons. *P < 0.05 was considered to be statistically significant.
RESULT
Composition of Gangliosides in HcaF and HcaP Cells
HcaF and HcaP cells had different ganglioside compositions (Fig. 1). The low metastatic HcaP cells contained a major ganglioside GM3 (about 80% of total gangliosides), little GM2 and GD1a. While the high metastatic HcaF cells contained a major ganglioside GM2 (about 80% of total gangliosides), little GM3 and trace amounts of other gangliosides with complex structures.
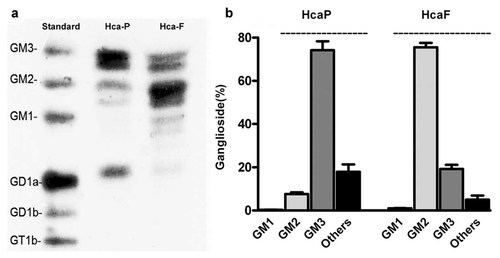
Composition of gangliosides in HcaF and HcaP cells. HcaF and HcaP cells were grown in 10-cm cell culture dishes in RPMI 1640 medium supplemented with 10% heat-inactivated FBS. Total lipids were extracted twice from the cell pellet with chloroform/methanol (1:1, by volume), dried, and then partitioned in di-isopropyl-ether/1-butanol/17 mM aqueous NaCl followed by Sephadex G-50 gel filtration and lyophilization. Individual gangliosides were separated on silica gel 60 HPTLC plates with a solvent system of chloroform:methanol:0.25% aqueous CaCl2·2H2O (60:40:9, by volume) and visualized by spraying the plate with resorcinol–HCI reagent following by heating at 120°C. Data are the mean intensity ± SD calculated from three independent experiments.
Suppression of Ganglioside Synthesis With P4 in HcaF and HcaP Cells
Ganglioside expression of HcaF and HcaP cells was inhibited with P4 and the inhibitory effect was monitored by dot blot assay using anti-GM3 antibody (Fig. 2a) and HPTLC (Fig. 2b). The dot blot assay showed that P4 could decrease the GM3 expression in a dose-dependent manner. About 90% GM3 expression was inhibited by 1.0 µM of P4 (Fig. 2a). Figure 2b showed that the expression of total ganglioside in HcaF and HcaP cells could be remarkably inhibited by 1.0 µM of P4 (Fig. 2b). Thus, 1.0 µM of P4 was used in each experiment.
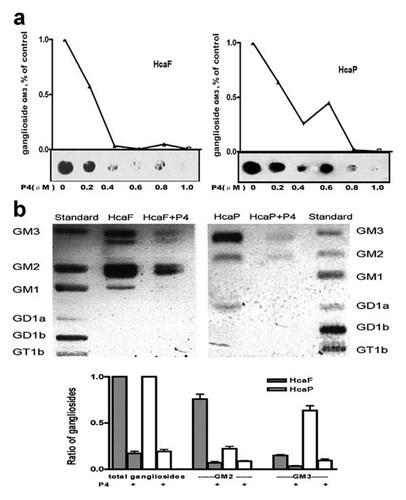
Inhibition of ganglioside synthesis in HcaP and HcaF cells by P4 (a) HcaF and HcaP cells were seeded into 12-well plates in RPMI 1640 medium supplemented with 10% FBS and different concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 µM) of P4, respectively. b: HcaF and HcaP cells were seeded into 10-cm cell culture dishes with RPMI 1640 medium containing 10% FBS and 1.0 µM of P4. After 48 h of incubation, the cells were harvested and inhibition of ganglioside synthesis was monitored by dot blot assay using anti-GM3 antibody (a) and HPTLC analysis (b) described as Materials and Methods Section. Data are the mean intensity ± SD calculated from three independent experiments.
Effect of Suppression of Ganglioside Synthesis on Motility and Migration of HcaF and HcaP Cell In Vitro
In order to understand whether the differences in ganglioside composition between HcaF and HcaP cells were associated with their differences in lymphatic metastasis ability, in vitro motility and migration assay were performed for these cells after suppression of ganglioside synthesis with P4. The depletion of gangliosides (mainly GM3) in low metastatic HcaP cells promoted cell motility and migration (Fig. 3a,b). In contrast, depletion of gangliosides (mainly GM2) in high metastatic HcaF cells had no effect on cell motility and migration (Fig. 3c,d). These results indicated that the differences in ganglioside composition between the HcaF and HcaP cells were associated with their lymphatic metastasis ability. It is possible that the ganglioside GM3 but not GM2 affected cell motility and migration ability and GM3 inhibited the cell lymphatic metastasis ability.
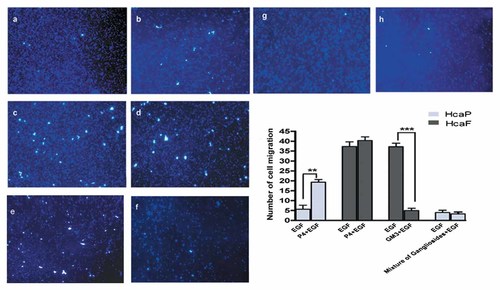
Effect of inhibiting ganglioside synthesis or addition of exogenous gangliosides on the migration ability of HcaP and HcaF cells in vitro HcaF and HcaP cells were treated with 1.0 µM of P4 for 48 h or 50 µM of GM3 or a mixture of gangliosids for 4 days. After starvation in serum-free medium overnight, the cells were stimulated with 5.0 ng/ml EGF for 10 min at room temperature or left unstimulated (control). Cells were collected and migration assays were performed as described in Materials and Methods Section. a: HcaP cells treated with EGF; (b) HcaP cells treated with EGF plus P4; (c) HcaF cells treated with EGF; (d) HcaF cells treated with EGF plus P4; (e) HcaF cells treated with EGF; (f). HcaF cells treated with EGF plus GM3; (g) HcaP cells treated with EGF; (h) HcaP cells treated with EGF plus mixture of gangliosides. The average number of cells that migrated through the filter was counted. Data are the mean ± SD calculated from three independent experiments. *P < 0.05 (n = 3); **P < 0.01 (n = 3); ***P < 0.001 (n = 3).
Effect of Exogenous Gangliosides on HcaF and HcaP Cell Motility and Migration
To confirm that GM3 but not other ganglioside involved in the effect on cell motility and migration in this cell model, the high metastatic HcaF cells were also treated with exogenous GM3 whereas the low metastatic HcaP cells were treated with a mixture of exogenous gangliosides (including GM2, GM1, GD1a, GD1b, and GT1b). HcaF cells exhibited a decrease in cell motility and migration in the presence of exogenous GM3 (Fig. 3e,f), but HcaP cells exhibited no change in both cell motility and migration in the presence of exogenous gangliosides (Fig. 3g,h). These results further demonstrated that the differences in lymphatic metastasis ability between HcaF and HcaP cells were associated with the differences in their GM3 content.
Effect of Ganglioside on the Activity of PI3K/Akt Signaling Pathway
The above data indicated that ganglioside GM3 may exert an inhibitory effect on cell motility and migration in this cell model. Thus the mechanism by which GM3 inhibited cell motility and migration was further investigated by determining the effect that alteration of ganglioside content would have on the activity of PI3K/Akt signaling pathway in HcaF and HcaP cells stimulated with EGF. The results showed that depletion of gangliosides (mainly GM3) in low metastatic HcaP cells increased the EGF-induced phosphorylation of PKB/Akt at both Ser-473 and Thr-308 sites (Fig. 4a). However, depletion of gangliosides (mainly GM2) in high metastatic HcaF cells did not change the level of the EGF-induced phosphorylation of PKB/Akt at neither Ser-473 nor Thr-308 (Fig. 4b). The effect of exogenous GM3 on the phosphorylation of PKB/Akt in high metastatic HcaF cells was also determined. Addition of exogenous GM3 reduced the EGF-induced phosphorylation of PKB/Akt at both Ser-473 and Thr-308 (Fig. 4c). These results were consistent with those of motility and migration assay, indicating that the activity of PI3K/Akt signaling pathway had a positive association with the motility and migration potential of HcaF and HcaP cells.
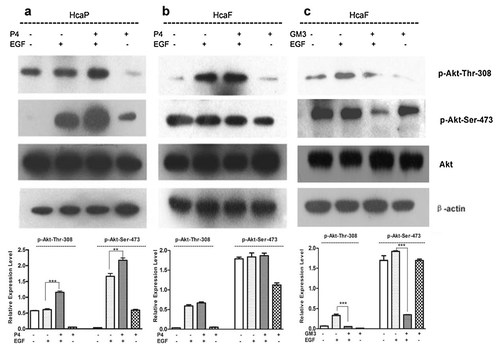
Effect of inhibition ganglioside synthesis or addition of exogenous GM3 on EGF-induced phosphorylation of Akt in HcaP and HcaF cells HcaF and HcaP cells were treated with 1.0 µM of P4 for 48 h or 50 µM of GM3 for 4 days. After starvation in serum-free medium overnight, the cells were stimulated with 5.0 ng/ml EGF for 10 min at room temperature or left unstimulated (control). The cells were harvested and lysed and the lysate was resolved by 10% SDS–PAGE. The phosphorylation of Akt was detected with anti-P-Akt (Thr308) and anti-P-Akt (Ser473) monoclonal antibody, respectively. Thr308 and Ser473 phosphorylations were normalized against β-actin as shown by the bar graph. a: Western blot analysis of AKT phosphorylation in HcaP cells treated with P4. b: Western blot analysis of Akt phosphorylation in HcaF cells treated with P4. c: Western blot analysis of Akt phosphorylation in HcaF cells treated with GM3. Data are the mean intensity ± SD calculated from three independent experiments. *P < 0.05 (n = 3); **P < 0.01 (n = 3); ***P < 0.001 (n = 3).
Effect of Blocking of PI3K/Akt Pathway on Cell Mobility and Migration
To validate that motility and migration of HcaF and HcaP cells were regulated by the PI3K/Akt signaling pathway, the activity of PI3K in HcaF cells was inhibited with LY294002, an inhibitor of PI3K. Inhibition of PI3K activity led to reduced motility and migration of HcaF cells in vitro (Fig. 5d,e). In addition, the enhancement effect on motility and migration of HcaP cells caused by P4-suppressed ganglioside synthesis could be reversed by treating the cells with LY294002 (Fig. 5a–c), suggesting that blocking the PI3K/Akt signaling pathway in these cells could effectively weaken their motility and migration ability. It proved that PI3K/Akt pathway was involved in the modulation of HcaF and HcaP cells motility and migration in vitro.
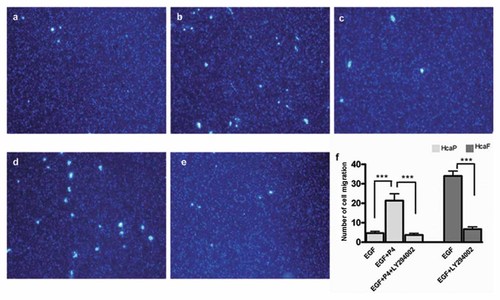
Effect of blocking the PI3K/Akt pathway on HcaP and HcaF cells migration in vitro. The high metastatic HcaF cells or low metastatic HcaP cells treated with 1.0 µM of P4 were treated with 15 µM of LY294002 for 4 h and then subjected to migration assay as described in Materials and Methods Section. a: HcaP cells treated with EGF; (b) HcaP cells treated with EGF plus P4; (c) HcaP cells treated with EGF plus P4 and LY294002; (d) HcaF cells treated with EGF; (e) HcaF cells treated with EGF plus LY294002; (f) the average number of cells that migrated through the filter was counted. Data are the mean ± SD calculated from three independent experiments. *P < 0.05 (n = 3); **P < 0.01 (n = 3); ***P < 0.001 (n = 3).
Effect of Ganglioside Content on EGF-Induced EGFR Autophosphorylation in HcaF and HcaP Cells
To investigate the exact molecular mechanism underlying the inhibition of HcaF and HcaP cell migration by GM3 in vitro, the effect of GM3 on EGF-induced autophosphorylation of EGFR was next evaluated by inhibition of ganglioside synthesis with P4 or addition of exogenous GM3. In the case of HcaP cells treated with P4, phosphorylation of EGFR was significantly enhanced at Tyr-1173 in response to EGF stimulation (Fig. 6a), whereas, phosphorylation of Tyr-1086 was reduced by 30%. In the case of HcaF cells treated with P4, there was no effect on the phosphorylation of EGFR at neither Tyr-1173 nor Tyr-1086 (Fig. 6b). Pretreatment of high metastatic HcaF cells with exogenous GM3 resulted in dramatically inhibited phosphorylation of EGF receptor at the Tyr-1173 residue, but enhanced phosphorylation of EGF receptor at Tyr-1086 residue (Fig. 6c). In summary, GM3 could suppress phosphorylation of EGF receptor at the Tyr-1173 residue, enhance the phosphorylation of EGF receptor at Tyr-1086. These results indicated that among the two phosphorylable tyrosine residues, only the change in phosphorylation status of Tyr-1173 residue was consistent with that of activity of PI3K/Akt pathway.
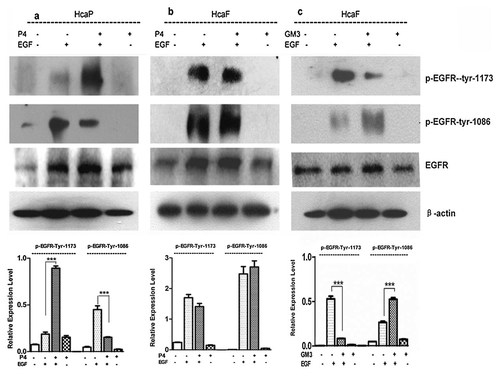
Effect of inhibition ganglioside synthesis or addition of exogenous GM3 on phosphorylation of EGFR in HcaP and HcaF cells HcaF and HcaP cells were treated with 1.0 µM of P4 for 48 h or 50 µM of GM3 for 4 days. After starvation in serum-free medium overnight, the cells were stimulated with 5.0 ng/ml EGF for 10 min at room temperature or left unstimulated (control). The cells were harvested and lysed. The lysate was subjected to western blot analysis using anti-EGFR, anti-P-EGFR (Tyr-1173), anti-P-EGFR (Tyr-1086). The phosphorylation of each tyrosine was normalized against the β-actin as shown by the bar graph. a: Western blot analysis of EGFR phosphorylation in HcaP cells treated with P4. b: Western blot analysis of phosphorylation of EGFR in HcaF cells treated with P4. c. Western blot analysis of EGFR phosphorylation in HcaF cells treated with GM3. Data are the mean intensity ± SD calculated from three independent experiments. *P < 0.05 (n = 3); **P < 0.01 (n = 3); ***P < 0.001 (n = 3).
DISCUSSION
HcaF and HcaP are two related sublines derived from the same parental cell line, but each has different lymphatic metastasis ability with no vascular metastasis ability. In the present study, we first compared the compositions of gangliosides in HcaF and HcaP cells, and found that these two cell lines had different compositions. About 80% of the total gangliosides in HcaF cells consisted of GM2 with a similar proportion of gangliosides in HcaP cells consisted of GM3.
Cells motility and migration ability are major factors that influence tumor metastasis. To study the role of gangliosides in the lymphatic metastasis of HcaF and HcaP cells, the effect of gangliosides on the motility and migration of these cells was determined following the alteration of GM3 content by treatment with P4 or exogenous GM3. In the case of HcaP cells, suppression of GM3 synthesis by P4 led to increased cell motility and migration, but suppression of GM2 synthesis by P4 in HcaF cells did not alter the motility and migration of these cells. In addition, increasing the content of GM3 in HcaF cells by addition of exogenous GM3 resulted in the suppression of cell motility and migration, whereas increasing the GM2 content in HcaP cells by addition of exogenous gangliosids (mainly GM2) did not influence cell motility and migration. The result indicated that the different content of GM3 between the HcaF and HcaP cells was the crucial factor responsible for the different lymphatic metastasis abilities of these tumor cells, and GM3 but not GM2, appeared to be the principle component that affected the motility and migration ability of these two tumor cell lines.
PI3K/Akt signaling pathway is considered as the most important pathway involved in modulation of tumor metastasis [Davies, 2012; De Luca et al., 2012]. We have shown here that by changing the content of GM3 in HcaF cells through adding exogenous GM3 or in HcaP cells by adding P4, the level of Akt phosphorylation could be altered, whereby an increase in GM3 content in the cells could cause a reduction of Akt phosphorylation at both Thr-308 and Ser-473 and vice versa. GM3 content also negatively regulated the motility and migration of HcaF and HcaP cells. Since the status of Akt phosphorylation is closely linked to the activation of the PI3K/Akt signaling pathway, the consistency between the effect of GM3 on cell motility and migration and the effect of GM3 on the phosphorylation of Akt suggested that the PI3K/Akt signaling pathway may be associated with the motility and migration ability of metastatic tumor cells. This was verified by the result showing that blocking of the PI3K/Akt signaling pathway with the PI3K specific inhibitor LY294002 suppressed cell motility and migration.
It is well known that phosphorylation of EGFR is an essential step for EGFR signaling, which results in phosphorylation at tyrosine residues on the intracellular domain of the receptor, thereby triggers the corresponding signaling pathways. Hence, to elucidate the precise molecular mechanism by which GM3 influences the phosphorylation of EGFR, it is essential to examine the phosphorylation status of each of the known phosphorylatable tyrosin residues of EGFR. In this study, we have determined the effect of GM3 on the Tyr-1173 and Tyr-1086 residues phosphorylation of EGFR. We found that decreasing of GM3 content in low metastatic HcaP cells with P4 promoted EGF-induced phosphorylation of EGF receptor at Tyr-1173, increasing of GM3 content in high metastatic HcaF cells with exogenous GM3 can suppress EGF-induced phosphorylation of EGF receptor at the Tyr-1173 residue, and decreasing of GM2 content in high metastatic HcaF cells with P4 had no effect on the phosphorylation of EGF receptor at the Tyr-1173 residue. But GM3 had an opposite effect on the phosphorylation of Tyr-1086 residue. These results indicated that GM3 suppressed the phosphorylation of Tyr-1173 residue, enhanced the phosphorylation of Tyr-1086 residue. Both Tyr-1173 and Tyr-1086 residues which located at EGFR's C-terminal are important EGFR autophosphorylation sites. Phosphorylated Tyr-1173 and Tyr-1086 residues can serve as a docking site for SH2-domain containing signaling molecules and leads to activation of the downstream signaling pathways. In this cell model, the effect of GM3 on the phosphorylation of Tyr-1173 residue of EGFR was consistent with that of GM3 on the phosphorylation of Akt and cell motility and migration. It was suggested that the inhibition of EGF-induced phosphorylation of Akt and Tyr-1173 residue of EGFR was, at least in part, involved in the mechanism by which GM3 influence the lymphatic metastasis potential of HcaF/HcaP cells. Tyr-1086 residue is also an important EGFR autophosphorylation site. However, in this cell model, the changes of phosphorylation level of Tyr-1086 were contrary to those of phosphorylation of PKB/Akt and cell motility and migration. Thereby, we speculated that Tyr-1086 may be not very important in activation of PI3K/Akt pathway and cell migration, or involved in other events of metastasis, such as cell proliferation, and adhesion in this model cell. From these data, it seemed that the mechanism of GM3 modulation activity of EGFR was rather complex. GM3 can regulate the activity of EGFR by different mechanisms. GM3 can directly influence the activity of EGFR by interaction with N-linked GlcNAc termini of the receptor [Yoon et al., 2006]. GM3 also indirectly regulates EGFR activity by influencing the interaction between EGFR and membrane proteins such as integrins and CD9 [Ono et al., 2001; Kawakami et al., 2002] or by affecting the intracellular Src kinase and protein tyrosine phosphatase activity [Suarez Pestana et al., 1997; Biscardi et al., 1999]. In this paper, we only investigated the role of EGFR. Other GFRs that involved in the regulation cell motility and migration in this cell model should not be excluded. It was reported that GM2/GM3 complex affixed on silica nanospheres strongly inhibits cell motility through CD82/cMet-mediated pathway [Todeschini et al., 2008]. If HGFR/cMet is involved in the regulation of cell motility and migration in this cell model, it would require further work to clarify.
Acknowledgements
This work was supported by Grant from the National Program on Key Basic Research Project (973 Program) (No. 2012CB822103) and Grant 2009T022 from Liaoning Province education bureau.




