Effect of mesenchymal stem cells on inhibiting airway remodeling and airway inflammation in chronic asthma†
The authors have no financial conflicts of interest.
Abstract
Previous studies proved that bone marrow-derived mesenchymal stem cells (BMSCs) could improve a variety of immune-mediated disease by its immunomodulatory properties. In this study, we investigated the effect on airway remodeling and airway inflammation by administrating BMSCs in chronic asthmatic mice. Forty-eight female BALB/c mice were randomly distributed into PBS group, BMSCs treatment group, BMSCs control group, and asthmatic group. The levels of cytokine and immunoglobulin in serum and bronchoalveolar lavage fluid were detected by enzyme-linked immunosorbent assay. The number of CD4+CD25+regulatory T cells and morphometric analysis was determined by flow cytometry, hematoxylin-eosin, immunofluorescence staining, periodic-acid Schiff, and masson staining, respectively. We found that airway remodeling and airway inflammation were evident in asthmatic mice. Moreover, low level of IL-12 and high levels of IL-13, IL-4, OVA-specific IgG1, IgE, and IgG2a and the fewer number of CD4+CD25+regulatory T cells were present in asthmatic group. However, transplantation of BMSCs significantly decreased airway inflammation and airway remodeling and level of IL-4, OVA-specific IgE, and OVA-specific IgG1, but elevated level of IL-12 and the number of CD4 + CD25 + regulatory T cells in asthma (P < 0.05). However, BMSCs did not contribute to lung regeneration and had no significant effect on levels of IL-10, IFN-Y, and IL-13. In our study, BMSCs engraftment prohibited airway inflammation and airway remodeling in chronic asthmatic group. The beneficial effect of BMSCs might involved the modulation imbalance cytokine toward a new balance Th1–Th2 profiles and up-regulation of protective CD4 + CD25 + regulatory T cells in asthma, but not contribution to lung regeneration. J. Cell. Biochem. 114: 1595–1605, 2013. © 2013 Wiley Periodicals, Inc.
Asthma is a chronic inflammatory disorder, affecting approximately 300 million individuals globally, with a prevalence range from 1% to 18% varying in different countries [Beasley, 2004; Masoli et al., 2004]. Although 80% patients can obtain good control of the disease by regular adequate medication, persistent or intermittent episodes can inflict huge burden for patients and their relatives due to direct medical costs and non-medical cost [Weinstein and Stason, 1977; Clark, 1990; Weiss et al., 1992; Weiss and Sullivan, 1993]. Asthma is an immune-mediated disease, pathologically characterizes as airway inflammation and airway remodeling which results in subepithelial fibrosis, airway smooth muscle hypertrophy and hyperplasia and mucous gland hyperplasia and hypersecretion. Airway remodeling is currently suboptimal control and the main death reason in chronic asthma patients. Therefore, it is vital to find a useful method to suppress the development of airway remodeling in asthma.
Because of low expression of HLA class I and non-expression of MHC II and costimulatory molecule, bone marrow-derived mesenchymal stem cells (BMSCs) have strong immunosuppressive properties which can be used in immune-mediated diseases and organ transplantation. The available evidence suggests that BMSCs can evade the recognition by T cells [Krampera et al., 2003] and NK cells [Rasmusson et al., 2003], thus inhibits proliferation of B-cell and T-cell and prohibit dendritic cells maturation and its antigen presentation function [Krampera et al., 2003; Rasmusson et al., 2003; Aggarwal and Pittenger, 2005; Jiang et al., 2005; Corcione et al., 2006; Spaggiari et al., 2006; Uccelli et al., 2006]. In the lung, several reports in the literature demonstrate that BMSCs can ameliorate acute lung injury on account of its anti-inflammatory activity [Gupta et al., 2007; Ortiz et al., 2007; Xu et al., 2007]. Moreover, recent studies have indicated that BMSCs can improve other autoimmune diseases in animal models, such as encephalomyelitis [Zappia et al., 2005] and arthritis [Augello et al., 2007] as well as diabetes [Lee et al., 2006]. To date, only a few paper report that engraftment of BMSCs can relieve inflammatory response in asthma [Lee et al., 2011]. However, its beneficial mechanism in asthma is seldom involved. To investigate the effect of BMSCs on the development of airway remodeling and airway inflammation in chronic asthma, BMSCs were administered intratracheally to ovalbumin-sensitized mice. The results in our study showed that early transplantation of BMSCs in asthma inhibited the development of airway remodeling and reduced the airway inflammation. Moreover, the beneficial effect was likely to be mediated by modulating the imbalance cytokine toward a new balance Th1–Th2 profile and up-regulation of CD4+CD25+regulatory T cells in chronic asthmatic mice, but not related to lung regeneration.
MATERIALS AND METHODS
Animals
Expressing EGFP gene transgenic Balb/c mice were purchased from Cyagen Biosciences, Inc., Guangzhou, P.R. China and Balb/c mice were purchased from Shanghai Laboratory Animal Center. Mice were maintained in a specific pathogen-free animal facility at the Second Military Medical University, Shanghai, P.R. China. All animal studies were conformed to National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Second Military Medical University, Shanghai.
Culture Bone Marrow-Derived Mesenchymal Stem Cells
EGFP–BMSCs were obtained from murine bone marrow cells as described previously [Phinney, 2008]. To isolate EGFP–BMSCs, bone marrow cells were harvested by flushing the medullary cavity of mouse femurs with Dulbecco's modified Eagle's medium (DMEM; Gibco, Manassas, MD) containing 1% penicillin–streptomycin (Gibco, BRL, Grand Island, NY). Marrow cells were washed once with DMEM and plated in plastic dishes at 2 × 106/ml in DMEM media containing 10% fetal calf serum (Gibco) supplemented with 2 mM L-glutamine (Gibco), non-essential amino acids (Gibco), and pyruvate (Gibco), and cultured at 37°C in 5% CO2. The non-adherent cells were removed by changing the medium every 3 days and colonies were established 1 week later. Passage 3–5 cells were assessed and used for in vivo experiments. Check the cells using an inverted phase contrast microscope and fluorescence microscope.
Differentiation Assays
Adipogenic differentiation of BMSCs
Briefly, confluent cell cultures in 6- well plates were induced with adipogenic differentiation medium containing (10 ug/ml insulin [Sigma, St. Louis, MO], 0.5 mM/L 3-Isobutyl-1-methylxanthine, 0.1 mM/L indomethacin (Sigma), 1 µmol/L dexamethasone (Sigma) in low-glucose DMEM (Gibco) with 10% FBS). After 3 weeks, treated cells were fixed with 4% paraformaldehyde at −20°C for 2 min and washed with 50% ethanol, then incubated with fresh Oil-red O for 15 min at room temperature and removed excess stain by washing with 50% ethanol again and stained with hematoxylin for 2 min.
Osteogenic differentiation of BMSCs
Osteogenic differentiation was induced by incubating the cells with 0.2 mM/L ascorbate (Sigma), 10 mM/L-Glycerophosphate (Sigma), 0.1 µmol/L dexamethasone and 2 mM L-glutamine in 1% penicillin–streptomycin DMEM (Gibco) with 10% FBS (Gibco). The media was changed 2–3 times a week. The cells were cultured continuously for up to 3 weeks. Deposition of calcium mineral was visualized by staining with von Kossa. Briefly, cells were fixed with 4% paraformaldehyde for 5 min and washed with distill three times, then were incubated with 5% silver nitrate and irradiated by ultraviolet for 1 h. After washed with distill wash for several times, 5% sodium hyposulfite was added to neutralize silver nitrate. Finally, abandon residue and dried at room temperature.
Phenotypic characterization of BMSCs
Cellular antigen phenotype was detected by a BD Biosciences FACS machine as described previously [Phinney, 2008]. Passages 5 BMSCs were harvested by trypsinization, washed in PBS, and stained by fluorescein isothio-cyanate (FITC) or phyco-erythrin (PE)-coupled antibodies against CD117 (0.5 mg/ml; BioLegend, San Diego, CA), CD44 (0.5 mg/ml; Biolegend) and SCA-1(0.2 mg/ml; Biolegend), CD29 (0.2 mg/ml; Biolegend) CD34 (0.2 mg/ml; Biolegend), CD14 (0.5 mg/ml; Biolegend), CD45(0.5 mg/ml; Biolegend), CD11b (0.5 mg/ml; Biolegend).
Chronic asthmatic mice model
Chronic asthmatic mice model were made as described previously [Temelkovski et al., 1998]. Mice were sensitized by intraperitoneal injection of 100 µg OVA (Sigma) emulsified in 1.3 mg aluminum hydroxide (Sigma) in a total volume of 200 µl on Days 0, 7, and 14. Mice were challenged via the airways with OVA (2.5% in PBS) for 30 min on 3 days/week for up to 8 weeks by ultrasonic nebulization (air-compressing nebulizer; Jiangsu Yuyue Medical Equipment, Inc.). Mice were sacrificed 24 h after final challenge.
Experimental Design
Forty-eight female BALB/c mice at 7–8 week of age were equally randomized into four groups as following: PBS group were sensitized, treated, and challenged with PBS; asthmatic model group were sensitized and challenged with OVA, but pretreated with PBS; BMSCs treatment group were sensitized and challenged with OVA and pretreated with BMSCs; BMSCs control group were sensitized and challenged with PBS and pretreated with BMSCs. Two mice in each group were sacrificed at 10 day after transplantation to determine whether EGFP–BMSCs stay in lung and immigrate to liver, spleen, and heart. Forty mice were killed and assessed at 50 day after transplantation (Fig. 1).
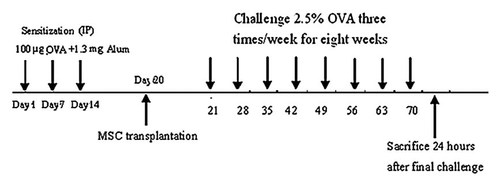
The experimental protocol. The mice were sensitized on Days 0, 7, and 14 by intraperitoneal injection OVA. Before the first challenge, BMSCS were transplanted intratracheally in asthmatic mice model. On Day 21, mice were challenged with 2.5% OVA for 30 min on 3 days/week for up to 8 weeks by ultrasonic nebulization. On Day 78, mice were sacrificed and then samples were collected 24 h after final challenge.
Intratracheal transplantation of BMSCs
Each experimental mouse intratracheally received 5 × 105 BMSCs in 30 µl PBS. Control animals received equal volume of PBS. The following was the detail method of intratracheal injection. MSCs were trypsinized and washed with PBS, and a cell suspension of 16,666 cells/µl was prepared in PBS. The cell suspension was put on ice until the Balb/c mice were anesthetized with pentobarbital sodium (50 mg/kg i.p., Sigma). Under sterile conditions, the trachea was exposed through a midline incision, and 30 µl of cell suspension were injected into trachea with a 1-ml syringe and a 0.5-inch 23-gauge needle. The intratracheal injection was made during a deep inspiration after compression of the thorax to aid in the distribution of BMSCs to distal airspaces. A total of 5 × 105 allogeneic BMSCs were injected into the trachea.
Bronchoalveolar lavage
On Day 78, mice were anesthetized and blood samples were collected by cardiac punctures. Bronchoalveolar lavage fluid (BALF) was collected in left lung by cannulating the trachea and lavaging with three 0.3-ml sterile PBS. The aliquots were pooled and the volume was measured. Total cell number for each animal was determined. After centrifugation at 1,000g for 5 min, the BALF supernatant was stored at −80°C for subsequent cytokine assays and the cell pellet was resuspended and cytospined on microscope slides. Differential cell analysis was performed on slides stained with Wright and Giemsa solutions and at least 400 cells on each preparation were counted.
Measurement of cytokines
OVA-specific IgE, OVA-specific IgG1 and OVA-specific IgG2a in serum were determined according to manufacturer's instructions (PDGF; R&D Systems, Minneapolis, MN). Moreover, BALF and serum samples were collected and analyzed with commercially available mouse Quantikine kits IL-4, IL-10, IL-13, IFN-Y, and IL-12 (R&D Systems).
Analysis of regulatory CD4+CD25+T lymphocytes
Pulmonary lymph node were disassociated and red blood cells were lysed. Cells were then fixed in Paraformaldehyde and stored at 4°C overnight until analysis. The monoclonal antibodies used were anti-CD25-APC (0.2 mg/ml; BD Biosciences, San Jose, CA), anti-CD4-FITC (0.5 mg/ml; Bioscience), anti- Foxp3 0.2 mg/ml; Bioscience), Appropriate isotype controls were performed for each experiment.
Immunohistochemistry
The immunofluorescence staining was performed using a protocol described previously [Xie et al., 2010]. Briefly, the right mouse lung tissue was perfusion fixed with 4% paraformaldehyde followed by overnight post-fixation in the same fixative, cryoprotected in 30% sucrose and frozen in OCT. Sections were boiled in 10 mM citrate buffer (pH 6.0) to improve antigen-retrieval and immersed in 0.3% H2O2 in methanol for 15 min to inactivate endogenous peroxidase activity. Sections were then incubated overnight with primary antibodies followed by secondary antibodies conjugated to horseradish peroxidase (HRP). The signal was further amplified using a tyramide amplification kit (Perkin-Elmer, TSA Biotin system) and visualized with streptavidin-Alexa Fluor 594 (Invitrogen). DAPI was used as a nuclear counterstain. The antibodies used in this study, source and dilution were as follows: mouse anti-CK antibody (Sigma), 1:1,000; Rabbit polyclonal to alpha smooth muscle Actin, (Abcam), 1:1,000; rabbit polyclonal anti-SP-C antibody (Santa Cruz), 1:1,000; goat-anti-rabbit IgG-HRP, Vector, 1:200 and horse-anti-mouse-HRP, Vector, 1:2,000.
Morphometric analysis
After collecting BALF, the left lung was filled with 0.5 ml 4% paraformaldehyde, removed, and fixed in 4% paraformaldehyde, then sectioned, and stained with periodic-acid schiff (PAS) for the enumeration of mucin-secreting cells and hematoxylin-eosin for the identification of cellular inflammation and masson trichrome staining for the subepithelial collagenisation. Image Pro-Plus software 6.0 (IPP 6.0) was used to analyzed inflammatory cells infiltration and airway remodeling among four groups. The numerical scores for each view field were determined as follows [Ford et al., 2001]: 0 = normal; 1 = few cells; 2 = a ring of inflammatory cells one cell layer deep; 3 = a ring of inflammatory cells 2–4 cells deep; 4 = a ring of inflammatory cells of >4 cells deep. In sections stained with PAS, medium-sized airways were assessed. For determination of collagen deposition around bronchioles, the ratio of the area of trichrome staining to the basement membrane perimeter (Pbm) was measured. The numerical scores for the abundance of PAS-positive goblet cells in each airway were determined as follows: 0: <5% goblet cells; 1: 5–25%; 2: 25–50%; 3: 50–75%; 4: >75%.
Statistical Analysis
Statistical analysis was performed with the statistical SPSS 15.0 software (SPSS, Inc., Chicago, IL). Data were expressed as Medians and interquartile ranges. Morphometry and cytokine and total cells in BALF were compared statistically among the groups by using Kruskal–Wallis test followed by nemenyi multiple comparison procedure. Statistical significance was defined as P < 0.05.
RESULTS
BMSCs Have Multipotentiality
Morphologically, the cells had a spindled, fibroblast appearance under light microscopy (Fig. 2a) and were shown green by using fluorescent microscopy (Fig. 2b). Under lineage-specific induction medium, BMSCs differentiated into adipocytes (Fig. 2c) and osteoblasts (Fig. 2d), which indicated its multilineage differentiation capacity.
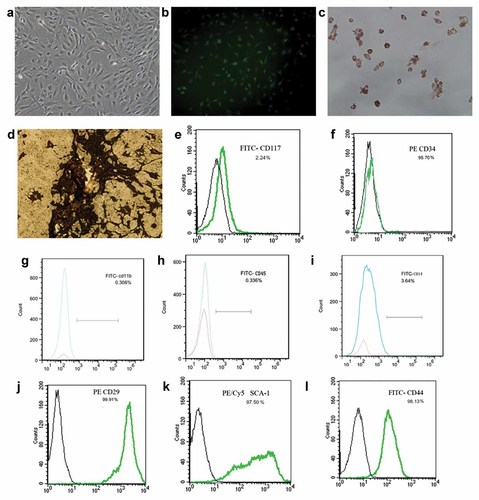
Characterization of EGFP–BMSCs: EGFP–BMSCs from expressing EGFP gene transgenic Balb/c mice showed spindled, fibroblast morphology by light microscope(a, magnification ×200) and green cells under fluorescence microscope(b, magnification ×200). BMSCs differentiated into adipocytes with adipogenic induction medium (c, Oil Red staining, magnification ×200) and osteoblasts with osteo-inductive medium (d, Von Kossa staining, magnification ×200). Flow cytometry of MSC demonstrated that cells did not express the cell surface markers CD117, CD34, CD14, CD45, and CD11b,but expressed the cell surface markers Sca-1, CD29, and CD44 (e–l). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcb]
Cellular markers of BMSCs were analyzed by flow cytometry and exhibited the expression of Sca-1, CD29, and CD44, but not CD117, CD34, CD14, CD45, and CD11b, which confirmed to cellular antigen phenotype of mouse BMSCs (Fig. 2e–l).
Effect of BMSCs Administration on the Numbers of Inflammatory Cells in BALF in Asthma
To assess pulmonary inflammation, eosinophils, neutrophil, monocyte, and total cells in BALF were counted after the last challenge. There was no obvious inflammatory cells in PBS group (Fig. 3a) and BMSCs control group, but a great deal of eosinophil (Fig. 3b,e), neutrophil (Fig. 3f), and monocyte (Fig. 3g) in asthmatic group (P < 0.01). However, BMSCs engraftment in asthmatic mice significantly decreased the numbers of inflammatory cells (Fig. 3c,d), including eosinophils (Fig. 3c,e), neutrophil (Fig. 3f), and monocyte (Fig. 3g) in BALF (P < 0.05). These results indicate that BMSCs engraftment prohibit BALF-inflammatory cells recruitment in asthmatic mice.
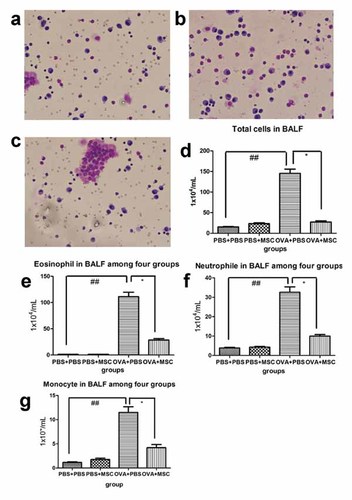
BMSCs engraftment significantly inhibited OVA-induced BAL-eosinophilia in asthmatic mice. There was no obvious inflammatory cells in PBS group in BAL (a), but a great number of eosinophil (b,e), neutrophil (f) and monocyte (g) infiltration in asthmatic mice(d), especially eosinophil. However, BMSCs engraftment significantly reduced eosinophil, neutrophil, and monocyte in BAL (c–g; Giemsa and Wright's staining; magnification ×200). Kruskal–Wallis test followed by nemenyi multiple comparison procedure was used for comparing the total cells in BALF (* represent the comparison between BMSCs treatment group and asthma group. *, P < 0.05; **, P < 0.01. # represent Asthma group compare to PBS control and BMSCs control group. #P < 0.05; ##P < 0.01). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcb]
Effect of BMSCs Administration on Numbers of Inflammatory Cells, Goblet Cell Metaplasia and Subepithelial Fibrosis in Mouse Airway
There was no obvious inflammatory cells infiltration (Fig. 4a), hyperplasia of Goblet Cell (Fig. 4k) and subepithelial fibrosis (Fig. 4f) in the airway in PBS group. Similarly, lack of inflammatory cells infiltration (Fig. 4b) and goblet Cell hyperplasia (Fig. 4l) and subepithelial fibrosis (Fig. 4g) was evident in BMSCs control group. However, accumulation of inflammation cells, particularly eosinophils around airway and its adjacent vessel was evident in OVA-induced chronic mouse model (Fig. 4c), accompanied with mucus hypersecretion (Fig. 4d, arrow) and hyperplasia of Goblet Cell (Fig. 4m) and subepithelial fibrosis in airway (Fig. 4h). BMSCs engraftment significantly reduced airway inflammation (Fig. 4e,p) and hyperplasia of Goblet Cell (Fig. 4n,o) and subepithelial fibrosis in airway (Fig. 4i,j) in asthmatic group.
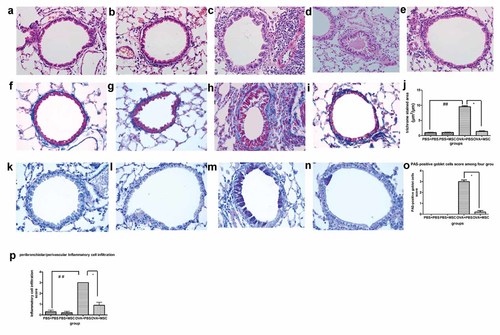
BMSCs administration reduced the extent of airway inflammation, goblet cell hyperplasia and subepithelial fibrosis in asthmatic mice model. a–p: Representative airway inflammation and airway remodeling for each group. There were a great number of inflammatory cells infiltration around airway and its adjacent vessel in chronic asthma model, particularly eosinophil (c), in conjunction with patchy airway occlusion by hyperviscous mucus (d, arrowhead). In chronic asthma model, Goblet cells shown by arrowhead were remarkably increased in the epithelium of the intrapulmonary airways (m) and the marked deposition of collagen were evident in masson trichrome staining (h). In PBS group, there was no obvious inflammatory cells infiltration (a) and goblet cell hyperplasia (k) and subepithelial fibrosis (f). Similarly, lack of inflammatory cells infiltration (b) and goblet Cell hyperplasia (l) and subepithelial fibrosis (g) was evident in BMSCs control group. BMSCs treatment relieved the extent of inflammatory cells recruitment around airway and its adjacent vessel (e,p) and the number of cells staining positively for PAS (n,o) as well as the deposition of collagen beneath epithelium (i,j; HE staining, PAS staining and masson staining; magnification ×400, bar size = 20 µm). BMSCs engraftment inhibited inflammatory cell infiltration around vasculars and airways in asthmatic mice (p) and reduced the deposition of collagen beneath epithelium (j) and hyperplasia of Goblet Cell (o). Quantitative determination of airway inflammatory and Goblet Cell and collagen deposition were compared among the groups by using Kruskal–Wallis test followed by nemenyi multiple comparison procedure (* represent the comparison between BMSCs treatment group and asthma group. *, P < 0.05; **, P < 0.01. # represent Asthma group compare to PBS control and BMSCs control group. #P < 0.05; ##P < 0.01). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcb]
EGFP–BMSCs Were Not Related to Lung Regeneration in Chronic Asthmatic Mouse Model
To detect donor cells among four groups, two mice in each group were sacrificed at 10 day after EGFP–BMSCs transplantation. EGFP–BMSCs were determined by endogenous GFP fluorescence and DAPI-nuclear-counterstaining in frozen lung sections. In our study, there was no green cell in the PBS group (Fig. 5g), BMSCs control group (Fig. 5h) and asthmatic mouse group (Fig. 5i) at10 day after EGFP–BMSCs transplantation, but only a few of EGFP+BMSCs could be detected by fluorescence microscope in BMSCs treatment group (Fig. 5a–c) and much less EGFP–BMSCs was detected in lung tissue at 50 day after transplantation (Fig. 5d–f). Furthermore, there was no EGFP+BMSCs in hear (Fig. 5k), liver (Fig. 5j), and spleen (Fig. 5l) in asthmatic mouse model at 10 day after transplantation.
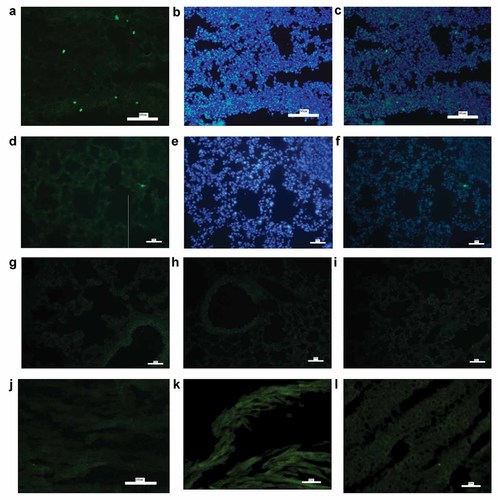
EGFP–BMSCs were detected in lung tissue at 10 day after EGFP–BMSCs transplantation in asthmatic mouse model. To localize EGFP–BMSCs, frozen lung sections were directly observed under fluorescence microscope. Then Nuclei was counterstained with DAPI. Colocalization of EGFP and nuclear blue cells were regard as donor EGFP+ BMSCs. At 10 day after EGFP–BMSCs transplantation, two mice in each group were sacrificed to detect donor cells among four groups. As shown in this figure, there was no green cell in the PBS group (g), BMSCs control group (h) and asthmatic mouse group (i), but only a few of green cells could be detected in BMSCs treatment group (a). Then this section were counterstained with DAPI (b) and a small number of donor EGFP+ BMSCs were determined as colocalization of EGFP and nuclear blue cells (c). However, green cells in lung tissue was detected much less at 50 day after transplantation (d) than that at 10 day after transplantation in BMSCs treatment group. Further counterstaining with DAPI (e) showed much less EGFP+ BMSCs at 50 day after transplantation (f). Similarly, Frozen hear (k), liver(j), and spleen (l) sections were directly observed under fluorescence microscope. In our study, there was no green cell in hear (k), liver (j), and spleen (l) in asthmatic mouse model at 10 day after transplantation (all sections: 5 µm. Size bar = 50 µm). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcb]
At 50 day after EGFP–BMSCs transplantation, immunofluorescence staining was performed to detect whether EGFP–BMSCs differentiate into lung tissue in chronic asthmatic mouse model. Similarly, there was no green cell in the PBS group (Fig. 6a,e,i) and BMSCs control group (Fig. 6b,f,j) and asthmatic mouse group (Fig. 6c,g,k) at 50 day after transplantation, but much less EGFP+BMSCs could be detected in BMSCs treatment group (Fig. 6d,h,l) at 50 day after transplantation. To further determine whether EGFP–BMSCs had acquired the molecular phenotype of type II pneumocytes(SPC) or epithelial cells (cytokeratin, CK) or smooth muscle cells (α-SMA) in lung tissue, we performed immunofluorescent staining for SP-C,CK and α-SMA. However, a small number of EGFP–BMSCs in BMSCs treatment group did not express CK (Fig. 6d), α-SMA (Fig. 6h) and SPC (Fig. 6l) in lung tissue, which indicated that a small number of EGFP–BMSCs did not differentiate into lung tissue in chronic asthmatic mouse after transplantation.
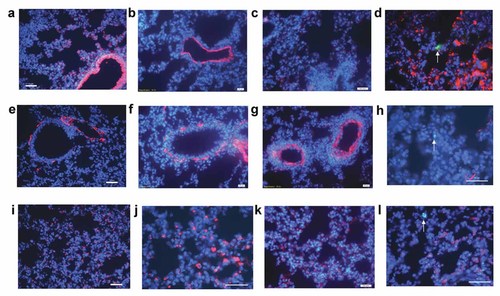
Immunofluorescent (IF) was performed on lungs from mice at 50 day after transplantation. Similarly, a small number of green cells shown by arrowhead could be detected in BMSCs treatment group (d,h,l), which accounted for less than 1% of lung population. However, no EGFP positive cells was observed in the PBS group (a,e,i) and BMSCs control group (b,f,j) and asthmatic model group (c,g,k). To determine whether EGFP–BMSCs contributed to lung tissue, IF against CK (a–d), α-SMA (e–h), and SPC(i–l) were examined. CK expression was observed mainly in airway and alveolar regions(a–d), but coexpression of green fluorescent protein and CK was not detectable in BMSCs treatment group (d). Similarly, α-SMA expression (red) were observed mainly around airway (e–h) and we found no α-SMA expression in green donor cells in BMSCs treatment group (h). Red staining type II pneumocytes were observed extensively in alveolar among four groups (i–l). In the composite image, there was no co-localization of the green cell and red SP-C cell in BMSCs treatment group (l; all sections: 5 µm. Size bar = 50 µm). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcb]
Effect of BMSCs Administration on Levels of IL-4 and IL-12 in BALF and Serum in Asthma
Compared to the PBS group and BMSCs control group, low levels of IL-12 and high levels of IL-13 and IL-4 were present in asthmatic mice group (P < 0.05). Transplantation of BMSCs significantly (P < 0.05) elevated levels of IL-12 (Fig. 7c,d) and decreased levels of IL-4 (Fig. 7a,b) in serum and BALF, but had no significant effect on levels of IL-10, IFN-Y, and IL-13 (Fig. 7e,f).
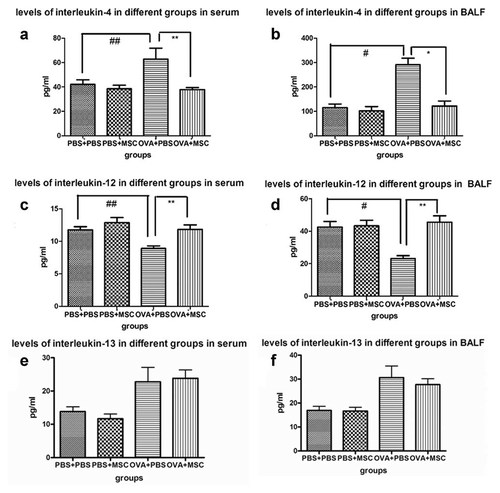
Analysis of the cytokine production in serum and BALF. To evaluate the effect of BMSCs on asthmatic mice, cytokines in different groups of mice were determined. Compared to the PBS group and BMSCs control group, low levels of IL-12 and high levels of IL-13 and IL-4 were present in asthmatic mice group (P < 0.05). Mice treated with BMSCs showed a significant reduction of IL-4 in serum (a) and BALF (b) and increase IL-12 in serum (c) and BALF (d), but no significant effect on levels of IL-10, IFN-Y and IL-13 (e,f; there were 10 mice per group.* represent the comparison between BMSCs treatment group and asthma group. *, P < 0.05; **, P < 0.01. #represent Asthma group compare to PBS control and BMSCs control group. #P < 0.05; ##P < 0.01).
Effect of BMSCs Administration on OVA-Specific Immunoglobulin in Asthma
Immunoglobulin were measured by ELISA. The levels of OVA-specific IgG1, OVA-specific IgE, and OVA-specific IgG2a were higher than that in PBS group and BMSCs control group (P < 0.01), whereas BMSCs transplantation remarkably decreased the levels of OVA-specific IgE, OVA-specific IgG1 (P < 0.05) and the ratio of OVA-specific IgG1 to IgG2a (P < 0.01) and increased the level of OVA-specific IgG2a in asthma (Fig. 8).
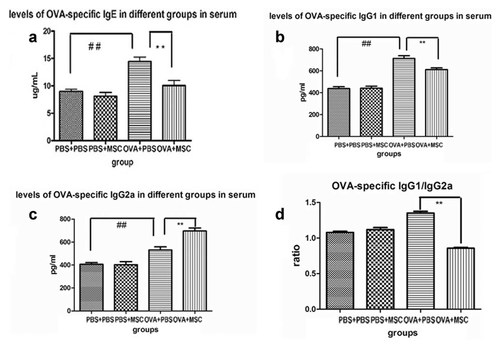
Analysis of OVA-specific IgE, IgG and IgG2a subtypes in serum. Concentrations of OVA-specific immunoglobulin were measured among groups of mice. Concentrations of Th2-specific IgE (a) and IgG1(b) were found significantly higher in asthmatic group, whereas BMSCs transplantation significantly decreased the levels of OVA-specific IgE, IgG1 and the ratio of IgG1 to IgG2a (d) in asthma. Compared to PBS group and BMSCs control group, OVA-specific IgG2a was higher in asthmatic mice group. However, BMSCs engraftment increased the level of OVA-specific IgG2a in asthma (c; there were 10 mice per group. * represent the comparison between BMSCs treatment group and asthma group. *, P < 0.05; **, P < 0.01. # represent Asthma group compare to PBS control and BMSCs control group. #P < 0.05; ##P < 0.01).
Effect of BMSCs Administration on CD4+CD25+regulatory T Cells in Asthma
To study the mechanism of BMSCs effect, CD4+CD25+regulatory T cells were determined. Compared to the PBS group and BMSCs control group, the ratio of CD4+CD25+regulatory T cells to lymphocyte in pulmonary lymph node was significantly lower in asthmatic model group (P < 0.05). Moreover, BMSCs transplantation significantly increased the ratio of CD4+CD25+regulatory T cells in pulmonary lymph node in asthma (P < 0.05; Fig. 9).
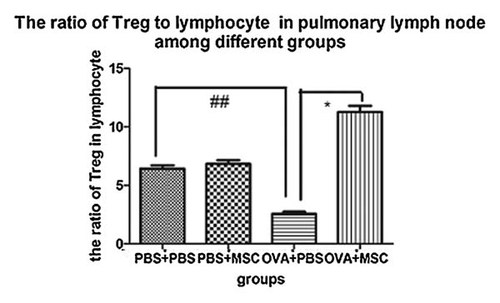
Analysis of CD4+CD25+regulatory T cells in pulmonary lymph node among different groups. The ratio of CD4+CD25+regulatory T cells to lymphocyte were determined in pulmonary lymph node among four groups of mice. Compared to the PBS group and BMSCs control group, the ratio of CD4+CD25+regulatory T cells to lymphocyte was significantly lower in asthmatic model group (P < 0.05). However, the ratio of CD4+CD25+regulatory T cells in pulmonary lymph node was significantly increased after BMSCs transplantation in asthma (P < 0.05; there were 10 mice per group. * represent the comparison between BMSCs treatment group and asthma group. *, P < 0.05; **, P < 0.01. # represent Asthma group compare to PBS control and BMSCs control group. #P < 0.05; ##P < 0.01).
DISCUSSION
In our study, prolonged exposure to OVA induced airway remodeling in OVA-sensitized mice, resulting in subepithelial fibrosis and mucous gland hyperplasia and hypersecretion. However, administration of BMSCs prior to challenge in OVA-sensitized mice inhibited the development of airway remodeling and airway inflammation. In BMSCs treatment group, inflammatory cells infiltration around airway and vessel and in BALF were remarkably decreased, moreover, subepithelial deposition of collagen and hyperplasia of goblet cells were alleviated greatly in asthmatic mice. Our findings indicated that BMSCs blocked recruitment of inflammatory cells into the airway and BALF and prohibited airway remodeling in OVA-sensitized mice, the present data were in keeping with previous reports for MSCs [Bonifield et al., 2010].
As reported previously, BMSCs could immigrate to and stay in damaged tissue for long time. In our study, EGFP–BMSCs was detected in BMSCs treatment group, but not in the PBS group and BMSCs control group and asthmatic group. Furthermore, EGFP–BMSCs was detected in lung tissue but not in liver, spleen and heart in asthmatic mouse at 10 day after transplantation, which indicated that EGFP–BMSCs immigrated to and stayed in impaired lung tissue for long time. However, EGFP–BMSCs in lung tissue was detected much more at 10 day after transplantation than that at 50 day after transplantation in BMSCs treatment group and the ratio of EGFP–BMSCs to lung tissue cells was extremely low at 50 day after transplantation (<1%), which confirm to previous report [Gupta et al., 2007].
In our study, EGFP +BMSCs in further immunofluorescent(IF) staining did not show expression of CK, α-SMA, and SPC in lung tissue, which indicated that the beneficial effect of BMSCs was not related to lung regeneration in chronic asthmatic mouse model. Previous studies proved that amelioration acute lung injury by BMSCs engraftment was not due to contribution to lung tissue, but its anti-inflammatory activity. [Gupta et al., 2007; Ortiz et al., 2007; Xu et al., 2007]. To date, few paper report that BMSCs administration contribute to lung tissue in asthmatic mouse model. We therefore mainly explore its immunomodulatory activity in chronic asthmatic mouse model.
As reported previously, the present data also indicated that IL-12 was decreased and IL-4 was increased in chronic asthmatic model [Naseer et al., 1997]. In the study of Onari et al. [2009], endogenous IL-12p40 was likely to inhibit airway hyperresponsiveness (AHR) and peribronchial fibrosis in prolonged antigen exposure to asthmatic mice model. Furthermore, Intranasal IL-12 was notably useful in preventing from AHR and enhancing grass pollen allergen-specific IgG2a antibody and IL-5/IFN-gamma ratio in GAL-sensitized mice [Matsuse et al., 2003]. IL-4 was associated with differentiation of airway epithelial cells into mucous glycoconjugate-containing goblet cells [Dabbagh et al., 1999]. In addition, antagonists of IL-4 mutant inhibit airway eosinophilia and AHR in the treatment of allergic asthma [Tomkinson et al., 2001]. In brief, the protective cytokine IL-12 was decreased and inflammatory cytokine IL-4 was increased in asthma, which associated with airway inflammation and airway remodeling.
In our study, TH1-derived cytokine IL-12 and TH2-derived cytokine IL-4 was imbalanced in asthma. Compared to the PBS group, TH2-derived OVA-specific IgG1 and OVA-specific IgE levels were higher in chronic asthmatic mice group. However, BMSCs transplantation significantly decreased the levels of TH2-derived OVA-specific IgG1 and the ratio of IgG1 to IgG2a and increased the levels of TH1-derived IgG2a. Moreover, BMSCs administration induced up-regulation of IL-12 and down-regulation of IL-4 in asthma. The present results indicated BMSCs might induce the imbalance of cytokines toward a new balanced Th1–Th2 profiles.
T regulatory cells was a unique T cell population with strong immunosuppressive properties. Previous studies indicate BMSCs preferentially activate CD4+CD25+T cell subsets which are the main underlying mechanisms for immunosuppressive activity of MSCs. In previous study, allogeneic BMSCs in murine rheumatoid arthritis model prevented the occurrence of severe irreversible damage by inducing production of antigen-specific Tregs [Maccario et al., 2005; Boudousquié et al., 2006; Augello et al., 2007; Selmani et al., 2008]. However, effect of BMSCs on Tregs in asthma was still unknown. We detected Tregs in Pulmonary lymph node and the data indicated that the ration of CD4+CD25+regulatory T cells to lymphocyte in pulmonary lymph node was reduced in asthmatic model group, whereas BMSCs transplantation significantly increased the ratio of CD4+CD25+regulatory T cells in pulmonary lymph node. Therefore, beneficial effect of BMSCs in asthma might involve up-regulation of CD4+CD25+regulatory T cells to suppress abnormal immune response in asthma.
In conclusion, BMSCs engraftment in OVA-sensitized mice inhibited the development of airway remodeling and airway inflammation. The mechanism underlying its immunosuppressive activities might involve of modulating the imbalance cytokine toward a new balance Th1–Th2 profiles and up-regulation of CD4+CD25+regulatory T cells, but not contribution to lung regeneration in chronic asthmatic mice.
Acknowledgements
We thank professor Yiping Hu and Pu You for their technical assistance.




