Cell fate conversion: Direct induction of hepatocyte-like cells from fibroblasts
Abstract
One of the essential features of stem cells is their cellular plasticity to differentiate into daughter cells with defined functions. Recently, induction of pluripotent stem cells from somatic cells by defined transcription factors led to the focus on cellular plasticity of terminally differentiated cells. This approach is adopted by other studies to demonstrate the cell fate conversion between different lineages of terminally differentiated cells. We and others showed that induced hepatocyte-like (iHep) cells are directly converted from mouse fibroblasts by overexpression of liver-enriched transcription factors. iHep cells as well as pluripotent stem cell- or mesenchymal stem cell-derived hepatocyte-like cells provide potential cell sources for disease modeling, transplantation, and tissue engineering independent of donor organs. Here, we review the latest advances in generating hepatocyte-like cells and summarize general criteria for evaluating these cells. In addition, we propose a possible role of the p19Arf/p53 pathway in cell fate maintenance, which apparently limits the formation of induced pluripotent stem (iPS) cells and iHep cells. J. Cell. Biochem. 114: 256–265, 2013. © 2012 Wiley Periodicals, Inc.
Embryonic stem cells of vertebrates have the potential to develop into all types of terminally differentiated cell lineages, which was thought to be an irreversible process. The emergence of somatic cell nuclear transfer (SCNT) and cell fusion makes it possible to reprogram the epigenetic status of somatic genome and reverse somatic cells to the pluripotent status in vitro [Gurdon, 1962; Blau et al., 1983]. Remarkably, recent advances show that somatic cells can be dedifferentiated to induced pluripotent stem (iPS) cells by introducing four transcription factors [Takahashi and Yamanaka, 2006; Takahashi et al., 2007], thus providing a new way to obtain patient-specific pluripotent stem cells.
With the success of reprogramming somatic cells to pluripotent cells, a general idea of direct cell fate conversion between terminally differentiated lineages has been under the spotlight. It was found that forced expression of C/EBPα and C/EBPβ successfully converts B cells into macrophages [Xie et al., 2004], while combination of C/EBPα or C/EBPβ with PU.1 induces fibroblasts to macrophage-like cells [Feng et al., 2008]. Lately, it has been demonstrated that cardiomyocytes and blood cells are induced from mouse fibroblasts by overexpression of specific transcription factors [Ieda et al., 2010; Szabo et al., 2010]. Furthermore, it was shown that cells could be directly converted to lineages with different germ layers of origin. For example, mouse fibroblasts are converted into functional hepatocyte-like cells simultaneously by two laboratories [Huang et al., 2011; Sekiya and Suzuki, 2011]. Mouse- and human-induced neuron (iN) cells are both generated by expression of neuron-specific transcription factors in fibroblasts, although the factors required are not identical for different species [Vierbuchen et al., 2010; Pang et al., 2011]. Markedly, cell fate conversion was even demonstrated in vivo using mouse models. Enforced expression of Ngn3, Pdx1, and Mafa converts differentiated pancreatic exocrine cells into β-cell like cells in vivo [Zhou et al., 2008]. Intriguingly, deletion of Pax5 also allows B cells to dedifferentiate back to progenitors, which give rise to T lymphocytes in vivo [Cobaleda et al., 2007]. These findings suggest that terminally differentiated cells from both mouse and human can be converted into other cell lineages. Cell fate conversion as well as lineage-specific differentiation of pluripotent cells provides new technologies to generate surrogate cells for disease modeling and regenerative medicine without a prerequisite of donor organs.
The liver plays important roles in regulating many physiological processes, such as metabolism control, glycogen storage, albumin (ALB) secretion, low-density lipoprotein (LDL) uptake, and xenobiotics detoxification [Si-Tayeb et al., 2010a]. End-stage liver diseases such as cirrhosis and hepatocellular carcinoma are important causes of death in East Asia. While the incidence of these liver diseases is relatively low in Western countries, non-alcoholic steatohepatitis is rapidly emerging and predicted to become one of the leading diseases in the future. Liver transplantation is currently the only treatment for some of these diseases at the terminal stages. However, the demand for liver transplantation far exceeds the supply of cadaveric livers or liver tissues from living donors.
To that end, hepatocyte transplantation has been evaluated to reduce the dependence on donor liver organs [Puppi et al., 2012]. The feasibility of hepatocyte transplantation is demonstrated in treatment of patients with acute liver failure and liver-based metabolic diseases [Fox and Roy-Chowdhury, 2004]. It is worth mentioning that besides the in vivo application for transplantation, hepatocytes are widely used for in vitro disease modeling such as hepatitis B and C virus infection and for drug metabolism and pharmacokinetics analysis to evaluate hepatobiliary disposition, metabolism and toxicity of drug candidates. For these applications, hepatocytes need to be obtained primarily from human donor organs. Moreover, primary hepatocytes should be immediately used, since these cells are not expandable in vitro and may lose part of hepatic functions after cryopreservation [Sahi et al., 2010]. Thus, generation of donor organ-independent hepatocytes with proliferative capacity and hepatic functions holds great promise for clinical and research purposes. Here, we summarize currently available techniques to obtain donor organ-independent hepatocyte-like cells (Fig. 1).
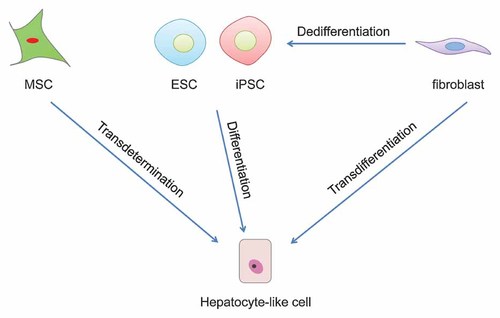
Strategies to generate hepatocyte-like cells. Hepatocyte-like cells can be generated through differentiation of embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC) or through transdetermination from mesenchymal stem cells (MSC) in vitro. Latest findings demonstrate that hepatocyte-like cells can also be obtained through transdifferentiation from fibroblasts by overexpression of defined factors.
EMBRYONIC STEM (ES) CELL-DERIVED HEPATOCYTE-LIKE CELLS
Several groups have successfully differentiated human and mouse ES cells into hepatocyte-like cells in vitro using lineage-specific differentiation protocols (Table I). The general strategy for hepatic differentiation is a stepwise culture with the addition of growth factors and cytokines, which mimics in vivo micro-environmental cues during liver development [Snykers et al., 2009]. Addition of Activin A is applied to efficiently induce the formation of definitive endoderm from ES cells [Kubo et al., 2004; D'Amour et al., 2005]. Bone morphogenetic proteins (BMPs) in combination with basic fibroblast growth factors (FGFs) and Activin A were later demonstrated to be required for the determination of hepatic fate from definitive endoderm [Gouon-Evans et al., 2006]. At the final step, hepatic growth factor (HGF) together with oncostatin M (OSM) and dexamethasone (Dex) facilitate the maturation of definitive endoderm-derived hepatocyte-like cells [Cai et al., 2007; Agarwal et al., 2008; Shiraki et al., 2008].
| Refs. | Origina | In vitro characterization | In vivo characterization | ||
|---|---|---|---|---|---|
| Expression of hepatic genes | In vitro function | Mouse model | In vivo function | ||
|
Cai et al. [2007 ] |
hESC | AFP, ALB, CK8, CK18, AAT, PEPCK, TAT, TDO2, HNF4α, CYP2B6, CYP3A4, CYP7A1 | Glycogen storage, ALB secretion, ICG uptake and release, inducible CYP activity | CCl4-induced liver injury in scid mice | 500 Human AAT positive cells in 150 foci in host livers |
|
Duan et al. [2007 ] |
hESC | AFP, ALB, AAT, CK18, CYP2B6, CYP2C9, CYP3A4, G6P, FOXA2, HNF4α, CEBPα/β, GATA4 | Glycogen storage, ALB secretion, ICG uptake, inducible CYP1A2 activity | Injection into livers of NOD-scid mice | Scattered human ALB and AAT positive cells in host livers; human ALB in sera |
|
Hay et al. [2008 ] |
hESC | ALB, AFP, HNF4α, TAT, TO, APOF, CYP3A4, CYP7A1 | Gluconeogenesis, urea synthesis, secretion of ALB, AFP, fibrinogen etc. | Intrasplenic injection to NOD-scid mice | Clusters of human CK18, CK19 and ALB positive cells in host spleens |
|
Agarwal et al. [2008 ] |
hESC | AFP, ALB, AAT, CYP3A4, CYP7A1, HNF4α, GATA4, CD26 | Glycogen storage, ALB secretion, ICG uptake and release | hES-derived DE cells injected to CCl4-induced liver injury in scid mice | Sporadic engraftment of human CD26 and AAT positive cells in host livers |
|
Shiraki et al. [2008 ] |
hESC | AFP, ALB, CYP7A1, CK7, CK18, SOX17, CYP3A4, OATP1B1 | Glycogen storage | Not tested | Not tested |
|
Basma et al. [2009 ] |
hESC | AFP, ALB, CFVII, TAT, AAT, G6P, ASGPR1, CYP1A1, CYP1A2, CYP2B6, CYP3A4, CYP7A1 | ALB and AAT secretion, urea synthesis, inducible CYP1A activity | Alb-µPA, scid mice | Clusters of human ALB and CK18 positive cells throughout liver parenchyma; human ALB in sera |
|
Haridass et al. [2009 ] |
hESC | Not shown | Not shown | Alb-µPA, Rag2−/−, IL2Rγ−/− mice | No engraftment |
|
Touboul et al. [2010 ] |
hESC | AFP, ALB, CK19, AAT, APOA-II, TAT, TDO2, FIX, CYP3A7, CYP7A1, ASGPR1, C-MET, HNF4α, FOXA2, HNF6 | Glycogen storage, ALB secretion, ICG and LDL uptake, CYP3A5/3A7 activity | Alb-µPA, Rag2−/−, IL2Rγ−/− mice | Clusters of human ALB, AAT positive cells in host livers; human ALB in sera |
|
Jozefczuk et al. [2011 ] |
hESC | ALB, AFP, SOX17, FOXA2, AAT, TDO2 | Glycogen storage, ICG uptake, urea synthesis | Not tested | Not tested |
|
Teratani et al. [2005 ] |
mESC | Alb, Ck8, Foxa2, Hnf4α, G6p, Cps1, Pepck, Lst-1, Tdo2, Tat, Ttr, Cyp1a1 | Glycogen storage, urea synthesis | DMN-induced liver cirrhosis | 1 × 106 engrafted cells in host liver per mouse, significant suppression of cirrhosis, increase of plasma fibrinogen and ALB |
|
Heo et al. [2006 ] |
mESC | Alb, Afp, G6p, Aat, Tdo, Hnf4α | Not shown | MUP-µPA, scid mice | 1.94% repopulation rate in host livers |
|
Gouon-Evans et al. [2006 ] |
mESC | Afp, Alb, Cps1, Tat, Cyp7a1, Cyp3a11, Ck19 | Glycogen storage, ALB secretion | Dpp4−/−, Rag2−/− mice with retrorsine and CCl4 treatment; Fah−/− mice | Scattered DPP4 positive cells in host livers Clusters of Fah positive cells in host livers |
|
Soto-Gutierrez et al. [2006 ] |
mESC | Alb, Tat, G6p, Ck18, Foxa2, Hnf4α, Cyp7a1, Ck19, Afp | ALB secretion, ammonia clearance | 90% hepatectomized scid mice treated with a subcutaneously implanted bioartificial liver seeded with ES cell-derived hepatocytes | Improvement of liver function and prolonged survival of 90% hepatectomized scid mice |
|
Shiraki et al. [2008 ] |
mESC | Afp, Alb, Aat, Sult2a1, Ugt1a1, Cyp7a1, Cyp2b10, Cyp3a11, Cyp3a13, Slco1a4, Abcb11 | Glycogen storage, ALB secretion, CYP3A4 activity | Not tested | Not tested |
|
Haridass et al. [2009 ] |
mESC | Not shown | Not shown | Alb-µPA, Rag2−/−, IL2Rγ−/− mice | No engraftment |
|
He et al. [2012 ] |
mESC | Alb, Aat, Hnf4α, Ttr, Tat, G6p, Cyp7a1 | Glycogen storage, ALB secretion, LDL uptake, ICG uptake and release, ammonia clearance | Fah−/− mice | 0.001–12.5% repopulation in primary recipients; 2.8–31.6% repopulation in secondary recipients; decrease of plasma tyrosine, bilirubin, ALTb |
- a h, human; m, mouse.
- b ALT, alanine aminotransferase.
These hepatocyte-like cells resemble typical epithelial morphology of primary hepatocytes and express several genes specific for hepatocytes such as ALB, TAT, and cytochrome P450 (CYP) genes (Table I). ES cell-derived hepatocyte-like cells also possess hepatic functions, including glycogen storage, ALB secretion, urea production, indocyanine green (ICG) uptake, and inducible CYP enzyme activity (Table I). On the other hand, the in vivo functions of ES cell-derived hepatocyte-like cells seem to be limited. After transplantation into the mice with acute liver failure or liver metabolism diseases, ES cell-derived hepatocyte-like cells only show some scattered engraftment in livers (Table I).
The hepatic differentiation efficiency of human ES cells has been optimized to 70–80% in vitro [Agarwal et al., 2008]; however, it is still a mixed culture of cells at various differentiation status including undifferentiated ES cells. Undifferentiated ES cells have the potency to form teratoma upon transplantation, thus largely preventing the application of ES cell-derived hepatocyte-like cells in regenerative medicine. To that end, asialoglycoprotein receptor (ASGPR) a surface marker of mature hepatocytes has been used to enrich human ES cell-derived hepatocyte-like cells [Basma et al., 2009]. After transplantation into immunodeficient mice with severe liver disease (Alb-µPA, scid mice), ASGPR positive cells engrafted into livers without forming apparent teratomas or liver tumors [Basma et al., 2009]. Nevertheless, the application of human ES cell-derived hepatocyte-like cells is limited by immune rejection and ethical problems.
iPS CELL-DERIVED HEPATOCYTE-LIKE CELLS
Generation of iPS cells from terminally differentiated cells provides a novel strategy to obtain patient-specific cells for autologous cell-based therapies without ethical problems. It was first reported that human iPS cells are differentiated into hepatocyte-like cells through the stage of definitive endoderm and hepatoblast by stepwise addition of growth factors and cytokines [Song et al., 2009; Sullivan et al., 2010; Si-Tayeb et al., 2010b]. Human iPS cell-derived hepatocyte-like cells express hepatic genes and exhibit hepatic functions, as in the case with ES cell-derived hepatocyte-like cells (Table II). Similar findings were reported to generate mouse iPS cell-derived hepatocyte-like cells [Gai et al., 2010; Sancho-Bru et al., 2011]. Transplantation of iPS cell-derived hepatocyte-like cells into mouse models results in cell engraftment; however, these cells do not expand in host liver parenchyma and fail to rescue mice with severe liver diseases (Table II). Recently, it was reported that human iPSC-derived hepatocyte-like cells repopulate the liver of highly immunodeficient NOD-scid, IL2Rγ−/− mice and repair dimethylnitrosamine (DMN)-induced liver cirrhosis in these mice [Liu et al., 2011]. Despite the efficient inoculation of the iPSC-derived hepatocyte-like cells in livers, human ALB levels in sera of recipient mice were extremely low, indicating that a large fraction of these cells were likely not fully matured. On the other hand, the mixture of cells at various differentiation stages also brings the concern of tumorigenesis for the application of iPS cell-derived hepatocyte-like cells.
| Refs. | Origin | In vitro characterization | In vivo characterization | ||
|---|---|---|---|---|---|
| Expression of hepatic genes | In vitro function | Mouse model | In vivo function | ||
|
Song et al. [2009 ] |
hiPSC | AFP, ALB, CK8, CK18, CK19, AAT, TDO2, CYP3A4, CYP2A6 | Glycogen storage, ALB secretion, urea synthesis, inducible CYP4 activity | Not tested | Not tested |
|
Liu et al. [2010 ] |
hiPSC | ALB, AFP, AAT, CYP3A4 | Glycogen storage, CYP1A2 and CYP3A4 activity | Not tested | Not tested |
|
Si-Tayeb et al. [2010b ] |
hiPSC | ALB, AFP, HNF4α, FOXA2, GATA4 | Glycogen storage, ALB secretion, ICG and LDL uptake | Injection into livers of newborn mice | Foci of human ALB positive cells in injected lobes |
|
Sullivan et al. [2010 ] |
hiPSC | ALB, AFP, E-CAD, HNF4α, CYP7A1, CYP3A4 | Fibrinogen, fibronectin, transthyretin, and AFP secretion, CYP1A2 and CYP3A4 activity | Not tested | Not tested |
|
Inamura et al. [2011 ] |
hiPSC | ALB, AFP, CK18, CYP3A4, CYP7A1, CYP2D6 | Inducible CYP3A4 activity | Not tested | Not tested |
|
Jozefczuk et al. [2011 ] |
hiPSC | ALB, AFP, SOX17, FOXA2, AAT, TDO2 | Glycogen storage, ICG uptake and release | Not tested | Not tested |
|
Liu et al. [2011 ] |
hiPSC | ALB, AFP, AAT, TTR, Fibrinogen, CYP2E1 | ALB secretion, CYP activity | DMN induced liver cirrhosis in NOD-scid, IL2Rγ−/− mice | 8–15% repopulation rate in host liver, human ALB, AAT and Fibrinogen in sera |
|
Nakamura et al. [2012 ] |
hiPSC | ALB, AFP, AAT, CYP3A4, TDO2, HNF4α | Glycogen storage, ICG uptake and release, inducible CYP3A4 activity | Not tested | Not tested |
|
Gai et al. [2010 ] |
miPSC | Afp, Alb, Aat, Ck19, E-Cad, Hnf4α, Tdo, Ttr, Cyp1a1 | Glycogen storage, LDL uptake, ICG uptake and release | Injection into mouse livers via portal veins | Integration into host livers |
|
Sancho-Bru et al. [2011 ] |
miPSC | Afp, Alb, Ck18, Ck19, Hnf4α, Pepck, Aat, Ttr, G6p, Cyp3a7, Cyp7a1 | Glycogen storage, urea synthesis, ALB secretion, inducible Cyp1a2 activity | Injection into Fah−/− mouse livers | Engrafted but failed to expand in livers |
MESENCHYMAL STEM CELL (MSC)-DERIVED HEPATOCYTE-LIKE CELLS
MSCs, which exist in many tissues such as bone marrow, adipose tissues and umbilical cord blood, have been extensively studied due to their easy accessibility and feasibility for autologous transplantation with low tumorigenic potential [English et al., 2010]. The long-term self-renewal and the capacity to differentiate into diverse mature cell types make MSCs a promising cell source for treatment of various diseases. MSCs are able to not only differentiate into bones, cartilages, or adipocytes [English et al., 2010], but also transdeterminate to hepatocytes in vitro (Table III). It was shown that addition of a cocktail of cytokines including FGF, HGF, and OSM together with Dex induces mouse and human MSCs to transdeterminate into epithelial-like cells which display hepatic functions and gene expression at low efficiency [Schwartz et al., 2002; Lee et al., 2004]. By exposing MSCs to cytokines in a manner that reflects their temporal expression during liver embryogenesis, hepatic differentiation of MSCs are significantly enhanced with increased hepatic functions, including inducible CYP enzyme activity [Snykers et al., 2007]. Furthermore, addition of trichostatin A or 5-azacytidine considerably improves endodermal differentiation of MSCs towards functional hepatocyte-like cells [Snykers et al., 2007; Seeliger et al., 2012]. Nonetheless, transplantation of human MSC-derived hepatocyte-like cells to mouse models only showed small clusters of ALB positive cells with little improvement of liver functions in vivo (Table III). It is also worth noting that MSC-derived hepatocyte-like cells show limited capability to proliferate in vitro, which largely prohibits these cell for the application in regenerative medicine.
| Refs. | Origin | In vitro characterization | In vivo characterization | ||
|---|---|---|---|---|---|
| Expression of hepatic genes | In vitro function | Mouse model | In vivo function | ||
|
Schwartz et al. [2002 ] |
hBM-MSCa | ALB, CK19, CK18, CYP1B1, CYP2B6, FOXA2, C-MET | Glycogen storage, ALB secretion, urea synthesis, LDL uptake, inducible CYP activity | Not tested | Not tested |
|
Lee et al. [2004 ] |
hBM-MSC | AFP, ALB, CK18, TAT, TO, G6P, CYP2B6, HNF4α | Glycogen storage, urea synthesis, LDL uptake, Inducible CYP activity | Not tested | Not tested |
|
Snykers et al. [2007 ] |
hBM-MSC | ALB, AFP, CK18, MRP2, HNF1α, HNF3β, C/EBPα | Glycogen storage, ALB secretion, urea synthesis, inducible CYP activities | Not tested | Not tested |
|
Aurich et al. [2007 ] |
hBM-MSC | CK18, CK19, CK7, AFP, TF, ALB, PCK1, CPS, CYP3A4 | Glycogen storage, urea synthesis | Intrasplenic injection into Pfp, Rag2−/− mice treated with partial hepatectomy and propranolol | Engrafted into host liver |
|
Banas et al. [2007 ] |
hAD-MSCb | ALB, AFP, TTR, TDO2, CYP7A1, HNF4α | Glycogen storage, ALB secretion, LDL uptake, ammonia clearance | Intrasplenic injection into BALB/c nude mice with CCl4-induced acute liver failure | Incorporated into host liver; decrease of ammonia concentration |
|
Talens-Visconti et al. [2007 ] |
hAD-MSC | ALB, TTR, CYP2E1, C/EBPβ | Not shown | Not tested | Not tested |
|
Yoshida et al. [2007 ] |
hUCB-MSCc | ALB, CEBPα/β, CYP1A1, CYP1A2, PEPCK | Glycogen storage, urea synthesis | Not tested | Not tested |
|
Campard et al. [2008 ] |
hUCM-MSCd | ALB, AFP, CNX-32, CK19, DPP4 | Glycogen storage, CYP3A4 activity | UCM-MSCs injected to spleens of partially hepatectomized scid mice | Small clusters of human ALB positive cells in host liver |
|
Banas et al. [2009 ] |
hAD-MSC | ALB, TDO2, FOXA2 | Glycogen storage, ALB secretion, LDL uptake | CCl4-induced liver injury in nude mice | Decrease of plasma ALT and ammonia |
|
Seeliger et al. [2012 ] |
hAD-MSC | ALB, AFP, CYP1A1, CYP1A2, CYP3A7, CYP3A4, CYP7A1 | Glucose production, urea synthesis, inducible CYP activities | Not tested | Not tested |
|
Sgodda et al. [2007 ] |
rat AD-MSC | Alb, Afp, Ck19, Ck7, Cx43, Cyp1A1, Pepck, Ck18, Cd26 | Glycogen storage, urea synthesis | Portal veins injection to CD26-deficient rats treated with retrorsine | Engrafted into host livers |
|
Schwartz et al. [2002 ] |
mBM-MSC | Gata4, Foxa2, Ck18, Ck19, Foxa2, Alb, Ttr, Cyp2b9, Cyp2b13 | ALB secretion, urea synthesis | Not tested | Not tested |
|
Snykers et al. [2006 ] |
rat BM-MSC | Afp, Alb, Hnf3β, Ck18, Hnf1α, Cyp1a1, Cyp2b1/2 | Glycogen storage, ALB secretion, urea synthesis, CYP1A activity | Not tested | Not tested |
- a BM-MSC, bone marrow-derived mesenchymal stem cell.
- b AD-MSC, adipose tissue-derived mesenchymal cell.
- c UCB-MSC, umbilical cord blood-derived mesenchymal stem cell.
- d UCM-MSC, umbilical cord matrix-derived mesenchymal stem cell.
DIRECT INDUCTION OF HEPATOCYTE-LIKE CELLS FROM FIBROBLASTS
We have successfully induced p19Arf-null mouse tail-tip fibroblasts to proliferative and functional hepatocyte-like (iHep) cells by overexpression of Foxa3, Gata4, and Hnf1α, transcription factors important for both liver development and maintenance of mature hepatic functions [Huang et al., 2011] (Table IV). The three transcription factors (3TFs) were identified as essential for hepatic conversion through a screening of 14 transcription factors important for liver development and function. Deletion of p19Arf, a key player controlling cellular senescence, enabled iHep cells to expand enormously in vitro. Global gene expression profiling showed that iHep cells are closer to primary hepatocytes, but distinctive to fibroblasts. Importantly, iHep cells showed hepatic functions in vitro, including glycogen production, ALB secretion, LDL uptake, and CYP metabolism activity.
| Refs. | Origin | In vitro characterization | In vivo characterization | ||
|---|---|---|---|---|---|
| Expression of hepatic genes | In vitro function | Mouse model | In vivo function | ||
|
Huang et al. [2011 ] |
Mouse fibroblast | Alb, Afp, Tdo2, Ttr, Aat, Trf, Ck18, Ck19, E-cad, Cldn2, Vtn | Glycogen storage, ALB secretion, LDL and ICG uptake, inducible CYP activity | Fah−/−, Rag2−/− mice | 5–80% Repopulation in host livers; 5/12 mice survived 8 weeks after NTBC withdrawal; decrease of plasma tyrosine, bilirubin, ALT |
|
Sekiya and Suzuki [2011 ] |
Mouse fibroblast | Alb, Afp, Mrp2, Mrp4, Comt1, NatZ, MaoA, Tpmt, GS, Gsta4 | Glycogen storage, ALB secretion, LDL and ICG uptake, inducible CYP activity | Fah−/− mice | Repopulation rate not quantified; 6/14 mice survived 10 weeks after NTBC withdrawal; increase of plasma Alb and decrease of bilirubin, ALP and ALT |
The in vivo therapeutic function of iHep cells was demonstrated by rescuing fumarylacetoacetate-hydrolase-deficient (Fah−/−) mice [Grompe et al., 1993]. Fah−/− mice recapitulate a fatal human liver metabolic disease that requires 2-(2-nitro-4-trifluoro-methylbenzyol)-1,3-cyclohexanedione (NTBC) treatment for survival. After withdrawal of NTBC, Fah−/− mice die in around one month because of severe liver failure. Fah−/− mice without NTBC treatment can be rescued by transplantation of primary hepatocytes that repopulate and restore liver functions. Upon receiving iHep cell transplantation, half of the Fah−/− mice survived after withdrawal of NTBC treatment. These recipient mice showed significant increase in body weight and correction of serum levels in total bilirubin, alanine aminotransferase, and aspartate aminotransferase. Histologically, iHep cell repopulation restored normal liver architecture without fusion with recipient cells.
Similar to our findings, another group generated hepatocyte-like cells from wild-type fibroblasts by using two transcription factors (2TFs), Hnf4α and a Foxa family factor [Sekiya and Suzuki, 2011] (Table IV). Hepatocyte-like cells induced by 2TFs showed similar in vitro and in vivo functions as our iHep cells. However, 2TFs induce hepatocyte-like cells at a lower efficiency and with a longer latency of conversion compared to our protocol. Actually, endogenous Hnf4α can be induced by 3TFs in our protocol. Moreover, the combination of Hnf1α and Foxa3 was already enough to induce epithelial iHep colonies in our study although at a low efficiency, while addition of Gata4 significantly enhanced hepatic conversion. Strikingly, hepatocyte-like cells generated from wild type fibroblasts by 2TFs were expandable in vitro, whereas primary hepatocytes and fetal hepatoblasts only show limited proliferative capability in cultures. It would be interesting to address whether the cellular senescence pathway is inactivated during hepatic induction from wild-type fibroblasts. Nevertheless, both groups have demonstrated a novel strategy to convert terminally differentiated cells directly to functional hepatocyte-like cells without producing hepatic progenitor intermediates.
CRITERIA FOR HEPATOCYTE-LIKE CELLS
As discussed before, donor-independent hepatocyte-like cells have possessed several hepatic functions to some extent. It is important to critically analyze the hepatocyte-like cells to confirm that they recapitulate mature hepatocyte in versatile characteristics. As reported in different studies, the hepatocyte-like cells should at least display a hepatic morphology and express hepatic marker genes including liver-enriched transcription factors [Banas et al., 2007; Cai et al., 2007; Si-Tayeb et al., 2010b; Huang et al., 2011] (Table V). A whole genome expression profile would further reveal whether the conversion occurs at the genomic level and is relatively completed. Importantly, hepatocyte-like cells need to be evaluated for their in vitro and in vivo hepatic functions. Hepatocyte-like cells should have the capacity to generate glycogen, secrete ALB, and uptake LDL and ICG. Particularly, hepatocyte-like cells should possess inducible CYP enzyme activities that are essential for detoxification of xenobiotic compounds such as phenacetin, testosterone, and diclofenac (Table V).
| Morphology | Epithelial morphology |
|---|---|
| Gene expression | Plasma proteins: Alb, Ttr, etc. |
| Metabolism enzymes: Aat, Tat, Tdo2, Cyp7a1, etc. | |
| Liver-enrich transcriptional factors: Hnf4α, Foxa2, etc. | |
| Cytoskeletal proteins: CK8, CK18, etc. | |
| In vitro functions | Glycogen storage |
| ALB secretion | |
| Urea synthesis | |
| ICG and LDL uptake | |
| Inducible CYP enzyme activities | |
| In vivo functions | Transplantation of hepatocyte-like cells repopulates livers and improves liver functions of animals with lethal liver diseases |
In vivo function of hepatocyte-like cells could be evaluated in animals with lethal liver diseases. Two-thirds partial hepatectomy and CCl4-induced liver injury are commonly used to create a proliferative stimulus in recipient mice. Retrorsine pretreatment is applied before the surgery to further block proliferation of recipient hepatocytes; however, the repopulation rate of donor hepatocytes is poor in these models [Rajvanshi et al., 1996; Sandhu et al., 2001]. Both FAH-deficiency and µPA-transgenic models as improved genetic liver disease models are often used to achieve high engraftment of donor hepatocytes [Grompe et al., 1993; Rhim et al., 1994]. Recently, NOD-scid, IL2Rγ−/− mice that express a thymidine kinase (TK) transgene specifically in the liver allow high engraftment of human hepatocytes after administration of ganciclovir, which is toxic to TK-expressing hepatocytes [Hasegawa et al., 2011]. These are some examples of excellent models to characterize engraftment, repopulation, and in vivo functions of hepatocyte-like cells.
We would like to emphasize that because hepatocyte-like cells of various origins are likely to be different, the summarized criteria only serve as a reference that may be modified according to specific studies. Primary hepatocytes shall serve as the gold standard for comparison of different types of hepatocyte-like cells. On the other hand, primary hepatocytes have versatile characteristics and functions. It would need great efforts to optimize hepatocyte-like cells to fully recapitulate primary hepatocytes. A more practical approach in future is to improve hepatocyte-like cells for specialized functions. For example, iHep cells have already expressed several CYP genes and acquired CYP1a, CYP2c, and CYP3a activities. Further optimization of iHep cells to express drug transporter genes and comprehensive CYP enzyme activities should lead to an alternative to primary hepatocytes for the early stages of drug discovery.
THE p19Arf/p53 PATHWAY IN CELL FATE CONVERSION
During the generation of iHep cells, we observed an extensive cell death, senescence, and proliferation arrest in wild-type fibroblasts upon overexpression of the three hepatic transcription factors. Cell fate conversion is a dramatic process that is extremely rare in nature. It is conceivable that cells with defined fate or identity should be preserved to maintain the physiological function of a given tissue. To overcome the barrier during lineage conversion, we inactivated cell-cycle-dependent kinase inhibitor p19Arf in fibroblasts and successfully generated mouse iHep cells that are capable of expanding without losing genetic stability. Mechanistically, p19Arf has been shown to bind directly to MDM2 and inhibit the MDM2-dependent degradation of p53 [Kamijo et al., 1998; Pomerantz et al., 1998; Zhang et al., 1998]. It is likely that p19Arf activates p53, thus leading to irreversible growth arrest during hepatocyte culture. Transient disruption of the p19Arf/p53 pathway appears to be a novel way to overcome hepatic conversion-induced cell death and to endow hepatocyte-like cells with proliferative capacity.
Besides the effect to promote proliferation of iHep cells, disruption of the p19Arf/p53 pathway also shows strong impact on increasing the efficiency of iPS cell generation. The p19Arf is activated during the passage of primary mouse fibroblasts with increasing cellular senescence. Deletion of p19Arf significantly increases the induction efficiency of iPS cells from mouse fibroblasts [Utikal et al., 2009]. On the other hand, the reprogramming process during iPS cell induction activates p53-mediated cell death [Marion et al., 2009]. Remarkably, inactivation of the p53 pathway dramatically improves the efficiency and kinetics of iPS cell generation [Zhao et al., 2008; Hong et al., 2009; Kawamura et al., 2009; Li et al., 2009; Marion et al., 2009; Utikal et al., 2009].
As a tumor suppressor, p53 plays essential roles in mediating cell cycle arrest and triggering senescence and apoptosis upon oncogenic stimulation [Levine and Oren, 2009]. It is interesting that the p19Arf/p53 pathway is found to suppress both tumorigenesis and cell fate conversion. Apparently, cell fate conversion and tumorigenesis are two different processes. During the induction of iHep and iPS cells, forced expression of specific transcription factors induces cell fate conversion and disturbs the steady state of the cells of origin, whereas genetic mutations are believed as the major cause of tumorigenic transformation. More importantly, iHep cells did not form tumors after subcutaneous xenograft in NOD-scid mice [Huang et al., 2011]. However, if tumorigenic transformation is considered as an extreme example of cell fate conversion during pathologies, we speculate that the p19Arf/p53 pathway may be employed by normal cells as a common mechanism to maintain the cell fate (Fig. 2). Apparently, future studies need to validate this hypothesis and to identify how the p19Arf/p53 pathway is activated during lineage conversion.
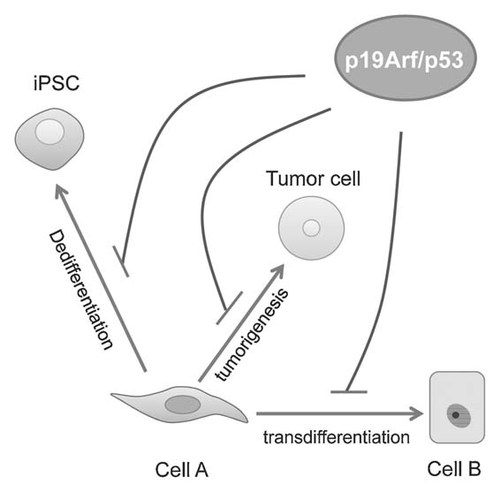
A proposed role of the p19Arf/p53 pathway in cell fate maintenance. As a tumor suppressive pathway, the p19Arf/p53 pathway plays essential role in protecting cells from tumorigenic transformation. p19Arf/p53 deficiency has also been shown to dramatically improve the dedifferentiation efficiency from somatic cells to iPS cells. Our data demonstrate that the p19Arf, a key activator of the p53 pathway, limits transdifferentiation of fibroblasts to iHep cells. Here, we hypothesize a general role of the p19Arf/p53 pathway in maintenance of cell fate.
PERSPECTIVES
Multiple strategies have been developed to generate hepatocyte-like cells in a liver organ-independent manner. Human hepatocyte-like cells have been successfully obtained from ES cells, iPS cells, and MSCs via hepatic differentiation in vitro (Table VI). Direct lineage conversion by defined factors holds new potential for regenerative medicine (Table VI). Specifically, iHep cells can proliferate extensively in vitro, which facilitates transplantation of a large and homogeneous cell mass. Forced direct conversion to hepatic lineage offers several other advantages over ES or iPS cell-derived hepatocyte-like cells. iHep cells are generated directly from fibroblasts without reverting to the pluripotent status, therefore, reducing the complexity of experimental manipulation. Further, transplantation of iHep cells avoids the contamination with stem cells capable of teratoma formation (Table VI). The protocol to generate iHep cells is still preliminary regarding therapeutic application. For example, iHep cells show relatively low hepatic functions in vitro compared with primary hepatocytes, which could be improved by optimizing culture conditions. However, our preliminary data implicate that iHep cells are more mature compared with mouse ES cell-derived hepatocyte-like cells [Li et al., 2010]. Nonetheless, a rigorous comparison of iHep cells with other surrogate hepatocyte-like cells would be necessary, so that when a specialized hepatic function is desired one can decide which type of hepatocyte-like cells to use.
| ESC-derived hepatocytes | iPSC-derived hepatocytes | MSC-derived hepatocytes | Induced hepatocytes from fibroblasts | |
|---|---|---|---|---|
| Cell of origin | Embryonic stem cells | Fibroblasts or other terminally differentiated cells | Mesenchymal stem cells | Fibroblasts |
| Tumorigenicity | Possible to generate teratoma if not fully differentiated | Possible to generate teratoma if not fully differentiated | No | Noa |
| Autologous transplantation | No | Yes | Yes | Yes |
| Cell amount | Need to expand ES cells to achieve large amount of hepatocyte-like cells | Need to expand iPS cells to achieve large amount of hepatocyte-like cells | Need to obtain large amount of MSC from patients | iHep cells are expandable in vitro |
| Procedure | 2–3 weeks, need first differentiate to definitive endoderm | 3–4 weeks, need to generate iPS cells | 3–4 weeks | 1–2 weeks, direct lineage conversion |
- a p19Arf-null iHep cells showed no tumorigenicity upon transplantation to immune deficient mice.
Primary hepatocytes dramatically lose major hepatic functions and gene expression after a few days of culture. To maintain the hepatic functions, great efforts have been made to establish culture conditions recapitulating the in vivo micro-environment, such as co-culture with extracellular matrixes and other types of cells and addition of growth factors and small molecule compounds [Sahi et al., 2010]. Nevertheless, it remains largely unsolved how to maintain their hepatic functions during the long-term in vitro culture. Future studies are expected to improve their in vitro functions through optimization of extracellular matrix in cultures. Alternatively, it will be interesting to generate hepatic progenitor cells from ES and iPS cells or from direct lineage conversion, which could be expanded in vitro and further differentiated into fully mature hepatocytes [Li et al., 2010].
From an application point, it is obvious that generation of human iHep cells takes the top-priority. Mouse iHep cells are surely a stepstone for generation of human iHep cells. However, the factors for inducing human iHep cells are not necessary to be identical to the factors used for mouse iHep cells. Latest studies on human iN cells have shown that transcription factors for mouse iN cells cannot be directly translated to human [Pang et al., 2011; Qiang et al., 2011]. To induce proliferative human iHep cells is another key issue. During mouse iHep induction process, inactivation of p19Arf (p14Arf in human) dramatically enhances mouse iHep cell proliferation in vitro. Although the mechanistic regulations of cellular senescence is not identical between human and mouse cells, inactivation of the p14Arf/p53 pathway seems to be a feasible method to endow human iHep cells proliferative capacity as well.
Shortly after the emergence of iPS cell technology, several groups generated terminally differentiated cells including hepatocytes from patient-specific iPS cells as in vitro disease models [Yusa et al., 2011]. It will also be of great interest to find out whether iHep cells could be an alternative strategy to generate patient-specific hepatocytes. These patient-specific iHep cells could be transplanted to severe immunodeficient mice to generate a humanized liver disease model, which will be a powerful tool for liver disease modeling and drug discovery. Furthermore, upon genetic modification the mutated protein responsible for the disease are corrected in the iPS cell-derived cells, which hold the promise to be transplanted back to the patients for therapy [Hockemeyer and Jaenisch, 2011]. With the technology of targeted gene correction, it is possible to correct the mutations of disease-causing genes in patient-specific iHep cells, therefore, providing another unlimited source of cells for autologous cell-based therapies.
Acknowledgements
We would like to thank Guoyu Pan and Zhiying He for suggestions and Lan He for editing the manuscript. Lijian Hui laboratory is supported by Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01020308), National Key Basic Research and Development Program of China (2012CB945001) and the National Natural Science Foundation of China (91019014).




