Serine/threonine-protein phosphatase 2A physically interacts with human telomerase reverse transcriptase hTERT and regulates its subcellular distribution
Abstract
Telomerase plays fundamental roles in bypassing cellular aging and promoting cancer progression by maintaining telomere homeostasis and telomere-independent activities. However, the molecular mechanisms by which telomerase provokes aging and cancer are far from being fully understood. In a search for proteins interacting with human telomerase reverse transcriptase hTERT by the yeast two-hybrid screen using hTERT T-motif as bait, we identified PP2A scaffolding subunit PR65 alpha isoform as an hTERT interacting partner. We showed that both PP2A catalytic subunit PP2AC and scaffolding subunit PR65 interacted with hTERT in vivo and in vitro and inhibited telomerase activity. In addition, we found that PP2A prevented the interaction of hTERT with 14-3-3θ signaling protein, an hTERT binding partner that is required for nuclear localization of hTERT. Activation of PP2A by overexpression of PP2AC or PR65 led to cytoplasmic accumulation of hTERT, which was reversed by treatment with PP2A inhibitor okadaic acid. Together, these observations suggest that PP2A regulates hTERT subcellular localization, in addition to its inhibitory effects on telomerase activity. J. Cell. Biochem. 114: 409–417, 2013. © 2012 Wiley Periodicals, Inc.
A telomere is a special structure consisting of tandem telomeric DNA repeats and regulatory proteins. These structures protect chromosomes ends and play an important role in genomic stability [Blackburn, 1991; McEachern et al., 2000]. Telomerase is an RNA-dependent DNA polymerase essentially composed of the catalytic subunit (hTERT) that adds the telomeric repeats onto the end of chromosome and a RNA component (Telomerase RNA, hTR) serves as a template for telomeric DNA synthesis [Blackburn, 2000; Cong et al., 2002].
hTERT is the determinative factor for telomerase activity. Most human somatic cells have undetectable telomerase activity due to transcriptional repression of the catalytic subunit hTERT gene during early embryonic development, but telomerase is reactivated during tumorigenesis and presents in over 90% of tumor cells [Cong et al., 2002]. In addition to the transcription regulation of hTERT expression which is the rate-limiting step of telomerase activation, the post-translational modification of hTERT, the assembly of the active telomerase holoenzyme, and transport to the nucleus are also important for the regulation of telomerase activity [Collins, 2006]. It has been reported that serine/threonine protein kinase Cα (PKCα) can phosphorylate hTERT and activate telomerase activity in breast cancer cells [Li et al., 1998]. Consistently telomerase activity is enhanced by the protein kinase C (PKC) activator phorbol myristate acetate and inhibited by the PKC inhibitor bisindolylmaeimide I in peripheral blood mononuclear cells [Bodnar et al., 1996]. The protein kinase Akt is also found to be involved in activation of telomerase activity by phosphorylating two potential phosphorylation sites of hTERT [Kang et al., 1999]. The 14-3-3 signaling protein, which is important in controlling cellular signaling cascades to regulate intracellular localization of their binding partners [Yang et al., 1999], is associated with telomerase and controls the intracellular localization of hTERT. 14-3-3 is required for efficient accumulation of hTERT in the nucleus [Seimiya et al., 2000].
PP2A is a major serine/threonine phosphatase involved in many essential aspects of cellular function including cell-cycle regulation, development, multiple signal transduction regulation, and tumorigenesis [Virshup, 2000; Lechward et al., 2001; Janssens et al., 2005]. The holoenzyme of PP2A consists of three components including a 65 kDa scaffolding subunit (PR65), a 36 kDa catalytic subunit (PP2AC), and a variable regulatory subunit [Xu et al., 2006]. Earlier studies demonstrate that incubation with recombinant protein PP2A inhibits nuclear telomerase activity in vitro and this inhibition is prevented by the PP2A inhibitor okadaic acid in human breast cancer cells [Li et al., 1997; Kang et al., 1999], but the mechanism is still unclear.
In a search for proteins interacting with human telomerase by the yeast two-hybrid screen with the hTERT T-motif (495–604 aa) as the bait, we identified PP2A scaffolding subunit PR65 alpha isoform interacting with hTERT T-motif. We found that both PP2AC and PR65 interact with hTERT in vivo and in vitro. PP2A prevents the interaction of hTERT with 14-3-3θ protein, regulates hTERT subcellular localization, and specifically inhibits telomerase activity.
MATERIALS AND METHODS
Cell Culture, Transfection, and Antibody
HeLa and U2OS cells was cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Hyclone), 1% L-glutamine, 100 U/ml penicillin and 100 ug/ml streptomycin at 37°C and 5% CO2. Transfection was done with Lipofectamine 2000 (Invitrogen, Paisley, UK) according to the manufacture's protocol. The following antibodies were used: antibodies against β-actin and β-tubulin were from Cell Signaling (Beverly, MA), the Rabbit monoclonal antibodies of hTERT were from Abcam (ab32020, Cambridge, UK), antibodies against 14-3-3θ and histone H2B were from Santa Cruz Biotechnology (Santa Cruz, CA), and antibodies against PP2AC and PR65 were from Bethyl Laboratories (Montgomery, USA).
Plasmid Construction
hTERT expression plasmid flag-hTERT was constructed by cloning the full-length hTERT cDNA into p3xFlag-CMV vector. hTERT T motif (495–604 aa) fragment was obtained by PCR using specific primers (forward: 5′-GAAGTTCG AATTCCTGGGGAAG-3′, reverse: 5′-GATGCTGCCTGTCGACTGCTTC-3′) from full-length hTERT cDNA. Then this fragment was cloned into GST fusion protein expression vector pEGST-6p-1 and yeast expression vector pGBKT7 DNA-BD. PR65 yeast expression vector pGBKT7-PR65 was obtained from the yeast Two-hybrid screen using the HeLa cDNA library, then PR65 cDNA fragment was subcloned into pcDNA3.1 and p3xFlag-CMV vector. Two fragments of PR65 cDNAs encoding amino acids 1–168 and 168–589 were subcloned into pGBKT7 vector, respectively. PP2AC expression construct pcDNA3.0-PP2AC was provided by Xing-Zhi Xu (College of Life Sciences, Capital Normal University, China), PP2AC cDNA was then cloned into p3xFlag-CMV vector.
Yeast Two-Hybrid Screen
hTERT T-motif was used as the bait for identification of the hTERT interacting proteins. pGBKT7-hTERT T-motif construct was transformed by the lithium acetate method into the AH109 yeast strain. The yeast strain Y187 was transformed with a HeLa cDNA library fused to the activation domain vector pGADT7 (Clontech Palo Alto, CA). Then the two yeast strains were mated and screened following the manufacturer's protocol (Clontech).
To confirm the interaction of hTERT T-motif and PR65, AH109 yeast strain harboring pGBKT7-hTERT T-motif was mated with Y187 yeast strain which pre-transformed pGADT7 expression vectors contained different region of PR65 coding sequences. To eliminate false positives, relevant clones were tested by co-transformation of AH109 yeast with either pGBKT7-hTERT T-motif or empty pGBKT7 vectors.
Cell-Free Transcription and Translation (TNT) and GST Pull-Down Assay
PR65 and PP2AC were expressed in vitro using plasmids containing PR65 or PP2AC cDNA under the T7 promoter (pcDNA3.1-PR65 and pcDNA3.0-PP2AC) in a rabbit reticulocyte lysate system (Promega Madison, WI). The system was incubated at 30°C for 90 min according to the manufacturer's instructions. Escherichia coli BL21 were transformed with pGEX-6P1-hTERT T-motif plasmids or empty vector and growth at 30°C for 3 h in LB medium with 100 µg/ml ampicillin and 0.1 mM IPTG. Bacteria were sonicated 6 times for 15 s on ice in buffer B (50 mM Tris–Hcl pH 8.0, 100 mM Nacl, 5 mM DTT, 1% N-lauryl Sacrosine, and protease inhibitor cocktail). The extracts were centrifuged twice at 12,000g for 15 min. Aliquots of supernatants were incubated with 50 µl glutathion-sepharose beads for 4 h at 4°C and washed twice with 500 µl of ice-cold buffer B to isolate GST proteins. About 40 µg PR65 or PP2AC proteins from cell-free transcription and translation system were incubated with GST alone or GST-hTERT T-motif bound to glutathion-sepharose beads overnight at 4°C. Beads were washed five times with 200 µl of ice-cold buffer B. An equal amount of bound proteins was separated in each lane by SDS–PAGE and analyzed by Western blot using anti-RPE65 (1:5,000) or anti-PP2AC (1:5,000) rAb.
Okadaic Acid Treatment and PP2A Phosphatase Assay
Okadaic acid (Sigma–Aldrich Steinheim, Germany) was dissolved in DMSO to a final concentration of 1 µM and added to synchronized cells to a final concentration of 1 nM for 5 h. After incubation, the cells were washed three times in PBS and prepared for PP2A activity analysis or immunofluorescence.
PP2A assay was performed using the malachite green-based PP2A immunoprecipitation phosphatase assay kit (Millipore Billerica, MA) following the manufacturer's protocol. In brief, cells were washed with 0.15 M NaCl and lysate with 1% NP-40, 10 mM Hepes, pH 7.5, 0.15 M NaCl, and 10% glycerol. Fifty to 100 µg of proteins were diluted in 50 mM Tris–HCl, pH 7.0, 100 mM CaCl2, and immunoprecipitated was performed using 4 µg of anti-PP2A antibody and 30 µl of protein A-agarose beads. Immunoprecipitates were washed and used in the phosphatase reaction following the manufacturer's protocol.
Telomerase Activity Assays
Telomerase activity was detected by using TRAPEZE® Telomerase Detection Kit (Millipore). Cell extracts (200 ng of protein) were added to telomerase extension reactions and incubated for 30 min at 30°C. PCR was performed using the TS primer and ACX primer for 30 cycles (denaturation at 94°C for 30 s, annealing at 59°C for 30 s, and extension at 72°C for 1 min). As an internal telomerase assay standard, NT and TSNT primers were added to the PCR mixture. Telomerase products were resolved by electrophoresis on a 12% non-denaturing polyacrylamide gel. Bands were then visualized by staining with SYBR DNA gel stain (Invitrogen). The telomerase activity was quantified with the telomerase activity in the cells transfected by pcDNA3.1 plasmid set as 1. Data were expressed as the mean ± standard error from three independent experiments.
Immunoblotting and Immunoprecipitation
For immunoblotting, cell lysates were prepared in RIPA buffer (50 mM Tris · Cl, pH 8.0, 100 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, and protease inhibitor cocktail (Roche Basel,Switzerland). Total protein concentration was measured with BCA kit, an equal amount of samples (20–60 µg) was separated on 12% SDS–PAGE and then transferred to PVDF membrane (Millipore). The membranes were incubated at 4°C overnight with primary antibodies. AP-conjugated anti-mouse or rabbit secondary antibody were used for detection by BCIP/NBT color development substrate (Promega). For subcellular fractions, cells were lysed using subcellular proteome extraction kit (Calbiochem) and analyzed by immunoblotting. For immunoprecipitation, cells were lysed in NP-40 buffer (50 mM Tris · Cl, pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, and protease inhibitor cocktail). Clarified extract was incubated with 20–30 µl M2 anti-FLAG resin (Sigma) for 1–2 h at 4°C. Analysis of associated proteins was performed by immunoblotting.
Immunofluorescence
Cells were plated on six-well plate with glass coverslips, fixed with 4% paraformaldehyde in PBS, and permeabilized in 0.5% TritonX-100. After blocking of non-specific antigens in 5% bovine serum albumin, hybridization with primary antibody at 1:50 dilution was performed at 37°C for 1 h, with FITC-conjugated or TRITC-conjugated secondary antibody at 37°C for 30 min, then cells were stained with DAPI (1 µg/ml) for 5 min. Imaging was performed using an Olympus FluoView confocal laser scanning system, and images were captured using Olympus FluoView (FV300) software (Tokyo, Japan).
RESULTS
hTERT Interacts With PP2A Both In Vitro and In Vivo
In a search for proteins capable of interacting with human telomerase reverse transcriptase hTERT, we used hTERT T-motif (495–604 aa) as the bait in a yeast two-hybrid screen. Among 89 positive clones selected, two of them contained the PP2A scaffolding subunit PR65 alpha isoform full-length cDNAs. To confirm the interaction between PR65 and hTERT, we set up a yeast two-hybrid assay again. AH109 yeast strain harboring pGBKT7-hTERT T-motif was mated with the Y187 yeast strain which pre-transformed pGADT7 expression vectors contained different regions of PR65 coding sequences. The yeast cells transformed by pGADT7 expression vectors contained the full length or the amino-terminal (1–168 aa) of PR65 were able to grow on the selective medium, however the yeast cells transformed by pGADT7 expression vectors contained carboxyl-terminal (168–589 aa) of PR65 or empty vector were unable to grow on the selective medium (Fig. 1A). This result indicates that the amino-terminal of PR65 (1–168aa) interacts with hTERT T-motif in yeast.
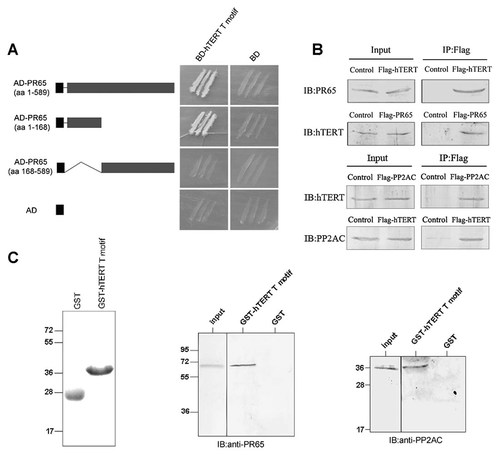
Interaction of hTERT with PR65 and PP2AC. A: Analysis of the physical interaction between hTERT T-motif and PR65 using the yeast two-hybrid assay. Full-length and two fragments of PR65 were analyzed for binding to hTERT T-motif. AD and BD empty vectors were used as negative controls. The growth on the selective dropout media (−Leu, −His, −Trp, and −Ade) plate indicates the activation of the reporter genes. B: In vivo binding analysis by co-immunoprecipitation (IP) of hTERT with PR65 and PP2AC. HeLa cells were transfected with Flag-hTERT, Flag-PR65, and Flag-PP2AC expression constructs, respectively, and subjected to immunoprecipitation with anti-Flag resin, followed by immunoblotting with anti-PR65, anti-PP2AC, or anti-hTERT antibodies. Control cells were transfected with p3XFLAG-CMV empty vectors. C: GST or GST-hTERT T-motif fusion proteins were expressed in E. coli BL21 cells and immobilized on glutathione-Sepharose. PR65 and PP2AC was obtained by cell-free transcription and translation (TNT) system in vitro and incubated with GST or GST-hTERT T-motif proteins. Bound proteins were detected by immunoblotting with anti-PR65 or PP2AC antibodies.
We next investigated whether hTERT and PR65 interacted in mammalian cells by co-immunoprecipitation experiments. HeLa cells were transfected with plasmids expressing Flag-tagged hTERT or empty vectors as control. Cell lysates were immunoprecipitated using the anti-Flag antibody and subsequent analyzed by immunoblotting with anti-PR65 or anti-PP2AC antibody. As shown in Figure 1B, the Flag-tagged hTERT interacted with endogenous PR65 and PP2AC in HeLa cells. On the other hand, we can readily detect the immunoprecipites of endogenous hTERT using Flag antibody in HeLa cells transfected by the Flag-tagged PR65 or PP2AC expression constructs (Fig. 1B). These reciprocal immunoprecipitation experiments suggest that the core enzyme of PP2A including PR65 and PP2AC interacts with hTERT in HeLa cells. Furthermore, the interaction between hTERT and PP2A was further confirmed by the GST pull-down assay. PR65 and PP2AC were produced by in vitro transcription and translation system, and then the in vitro translated PR65 or PP2AC was incubated with bacterially purified GST-hTERT T-motif or GST immobilized on Glutathione Sepharose, respectively. The specific interaction of GST-hTERT T-motif with either PR65 or PP2AC was visualized by immunoblotting using the antibodies against PR65 or PP2AC. As shown in Figure 1C, both PR65 and PP2AC were found to be specifically associated with GST-hTERT T-motif fusion protein but not with GST (Fig. 1C). Taken together, this demonstrates that hTERT T-motif specifically interacts with PP2A both in vivo and in vitro.
PP2A Specifically Inhibits Telomerase Activity in HeLa Cells
Earlier report indicated that the recombinant PP2A inhibits the telomerase activity in vitro [Li et al., 1997]. To investigate whether PP2A regulates telomerase activity in cells, we overexpressed either PP2A or PR65 by transiently transfected different amounts of PP2AC or PR65 expression constructs into HeLa cells, respectively. In both cases, we observed that PP2A activity was increased about 2.5-folds, and inhibited by okadaic acid as expected (Fig. 2A). Consequently we found that the telomerase activity was dose-dependently inhibited by PP2AC and PR65 overexpression (Fig. 2B). To test whether other protein phosphatases regulate telomerase activity, we have overexpressed the catalytic subunit of protein phosphatases PP4 or PP6 in HeLa cells. The results show that the telomerase activity was not affected by PP4 or PP6 overexpression (Fig. 2B), suggesting that PP4 and PP6 are not involved in telomerase regulation in HeLa cells. Thus, our data indicate that PP2A can specifically inhibit telomerase activity in HeLa cells.
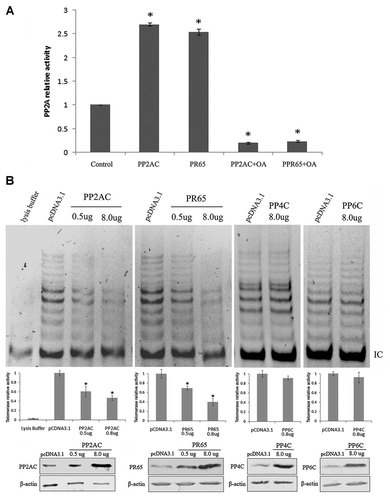
Up-regulation of PP2A phosphatase activity and inhibition of telomerase activity by overexpression of PR65 or PP2AC in HeLa cells. A: Overexpression of PP2AC or PR65 increases PP2A phosphatase activity. HeLa cells were transiently transfected by PP2AC or PR65 expression constructs with indicated amounts, 8 µg of the empty vector used as control. Cells were lysed and PP2A assays were performed 48 h post-transfection using the malachite green-based PP2A immunoprecipitation phosphatase assay kit. The experiments were repeated three times and the results are presented as mean ± SD, *P < 0.05 compared with control. B: Dose-dependent and specific inhibition of telomerase activity by PP2AC and PR65. Telomerase activity detected by using TRAPEZE® Telomerase Detection Kit in HeLa cells transfected with different amounts of cDNA expression constructs of PP2AC, PR65, PP4C, PP6C, empty vector used as control. Endogenous and ectopic expression of these proteins and β-actin was detected by immunoblotting. The telomerase activity was quantified with the telomerase activity in the cells transfected by pcDNA3.1 plasmid set as 1. Data were expressed as the mean ± standard error from three independent experiments.
PP2A Regulates the Subcellular Localization of hTERT
A large number of evidence indicates that the post-translational modification such as phosphorylation is a crucial mechanism controlling the subcellular localization of proteins, such as BAD and Cdc25 [Kumagai and Dunphy, 1999; Pastorino et al., 1999; Yang et al., 1999]. It has been reported that telomerase shuttles between subcellular compartments in response to specific stimuli [Haendeler et al., 2003, 2004], but the mechanism is unclear. Having demonstrated that PP2A directly interacts with hTERT and inhibit telomerase activity, we wondered whether PP2A might affect the subcellular localization of hTERT. To test this hypothesis, we monitored the subcellular distribution of hTERT by immunofluorescence experiments in HeLa cells, in which the levels of PP2A activity was either increased by overexpression of PP2AC or PR65, or inhibited by the inhibitor okadaic acid. The telomerase negative U2OS cells were included to test the specificity of the hTERT antibody. As shown in Figure 3, in control HeLa cells, the endogenous hTERT was mainly located within nucleus, and barely detected in cytoplasm. However when PR65 or PP2AC was overexpressed, the endogenous hTERT appeared to be in both cytoplasm and nucleus of HeLa cells. Moreover, in the presence of the PP2A inhibitor okadaic acid, hTERT retained in nucleus of cells over-expressing PP2AC or PR65 (Fig. 3). There was no immunofluorescence signals detected in the telomerase negative U2OS cells, which confirms the specificity of the hTERT antibody we used. These results demonstrate that the subcellular localization of hTERT is regulated by PP2A activity in HeLa cells.
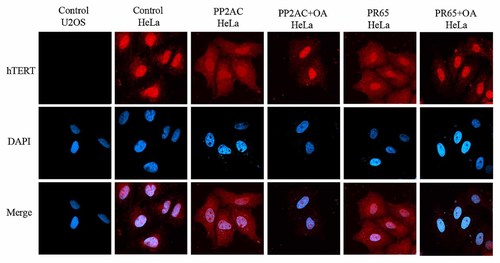
Regulation of subcellular distribution of hTERT by PP2A. HeLa cells were transfected with PP2AC, PR65 expression constructs, empty vector used as control. Cells were fixed and stained with antibody against hTERT (red) and DAPI (blue). DAPI was added to stain nuclei. The okadaic acid (OA) treatment was performed by incubation of cells with 1 nM OA for 5 h before cell fixation. U2OS is an hTERT negative cell line used as a negative control to confirm the specificity of hTERT antibody.
PP2A Increases the Cytoplasmic Distribution of hTERT by Preventing the Interaction of hTERT With 14-3-3θ
To further confirm the subcellular distribution of hTERT induced by PP2A, we performed a cell fractionation experiment in HeLa cells that overexpressed PR65 or PP2A. Consistent with the results of the immunofluorescence experiments, the levels of hTERT were increased in the cytoplasm of cells that overexpressed PR65 or PP2AC. However, the levels of hTERT in the nucleus fraction reduced slightly (Fig. 4A). To clarify whether the levels of hTERT were affected by PP2A, we overexpressed PP2AC by transiently transfecting PP2AC expression construct in HeLa cells. Immunoblotting and RT-PCR results showed that the levels of expression of the hTERT protein and mRNA did not changed in response to the activation of PP2A (Fig. 4B), suggesting that the transcription or translation of hTERT gene was not affected by PP2A in HeLa cells. Given that the majority of hTERT protein localized in the nucleus, the cytoplasmic distribution of hTERT induced by PP2A activation may represent only a small fraction, although the total protein amount is unchanged, the change of hTERT protein level in the nucleus is not significant as visualized by Western blotting.
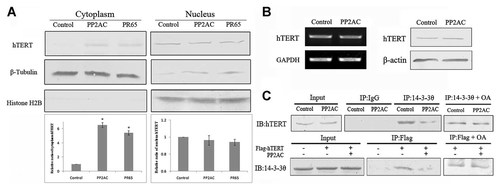
PP2A regulates subcellular distribution of hTERT by modulating the interaction of hTERT with 14-3-3θ. A: The subcellular distributions of hTERT induced by PP2A. Subcellular distributions of hTERT detected by immunoblotting analysis of hTERT in the cytosol and nuclear fractions of HeLa cells transiently transfected with PP2AC or PR65 expression constructs, empty vector was used as control. Expression of β-tubulin and Histone H2B were used as markers for cytosol and nuclear proteins, respectively. The subjacent histogram is the quantification data of immunoblotting results. B: Level of total endogenous hTERT was unchanged by PP2AC overexpression. HeLa cells were transiently transfected by PP2AC expression construct or empty vector as control. Cells were lysed 48 h post-transfection, endogenous hTERT and β-actin was detected by immunoblotting. C: PP2A prevents hTERT from binding with 14-3-3θ in vivo. HeLa cells expressing p3XFLAG-hTERT were transfected by PP2A expression constructs or empty vector as control. The OA treatment was performed by incubation of cells with 1 nM OA for 5 h. Cell lysates were subjected to immunoprecipitation by anti-14-3-3θ or anti-Flag antibodies, immunoprecipites were analyzed by immunoblotting using anti-hTERT or anti-14-3-3θ antibodies.
It has been demonstrated that the 14-3-3 protein interacts with hTERT, promote its nuclear localization in 293T cells. Furthermore, three threonine or serine residues (1030Thr, 1037Ser, and 1041Ser) in hTERT are required for this interaction, and mutations in these three residues result in the cytoplasmic localization of hTERT [Seimiya et al., 2000]. Since PP2A involves in hTERT subcellular localization as we demonstrated above, we reason that the interaction between 14-3-3 and hTERT might be regulated by PP2A. To ascertain this, we carried out a reciprocal immunoprecipitation assay. In consistence with the report [Seimiya et al., 2000], 14-3-3 was detected in the immunoprecipitates with anti-Flag resin in HeLa cells expressed Flag-tagged hTERT, but not in cells transfected by control vector (Fig. 4C). However the amount of 14-3-3θ in immunoprecipitates of hTERT was significantly decreased when PP2AC was overexpressed. Similarly, hTERT was detected in the immunoprecipitates of 14-3-3θ with anti-14-3-3θ resin, and also decreased in immunoprecipitates of 14-3-3θ when PP2AC was overexpressed (Fig. 4C). Moreover, in the presence of the PP2A inhibitor okadaic acid, the interaction of hTERT and 14-3-3θ did not change significantly when PP2AC was overexpressed (Fig. 4C). Together, these data suggest PP2A regulates hTERT subcellular distribution probably by altering hTERT phosphorylation state and thereby modulates the interaction of hTERT with its interacting partner 14-3-3θ required for its nuclear localization [Seimiya et al., 2000].
DISCUSSION
Telomerase reverse transcriptase hTERT is a determinative factor for telomerase activity essential for telomere maintenance. However, increasing evidence indicates that hTERT also involves in telomere-independent biological processes including apoptosis, DNA damage responses, stem cells, and regulation of gene expression [Cong and Shay, 2008]. To elucidate the mechanisms of non-telomeric function of hTERT, we search for binding proteins of hTERT and investigate their interactions. PP2A scaffolding subunit PR65 alpha isoform is one of these binding proteins identified in our yeast two-hybrid screen. In this study, we showed that PP2A interacted with hTERT both in vivo and in vitro, regulated hTERT subcellular localization and specifically inhibited telomerase activity.
The T-motif, a highly conserved telomerase specific domain in the telomerase catalytic subunit, located in the boundary of N terminal and RT domain does not exist in other reverse transcriptases [Nakamura et al., 1997]. T-motif plays an important role in TERT–TR interaction and directly participates in the process of repetitive template copying [Drosopoulos and Prasad, 2010]. It was reported that a region of hTERT (326–620 aa) required for the nucleolar localization of hTERT named as nucleolar localization domain (NoLD) contains the T-motif. Mutation in T-motif (561aa) abrogates the ability of hTERT to localize to nucleolar [Etheridge et al., 2002]. This suggests that T-motif is important for nucleolar localization. By using yeast two-hybrid screen and in vitro and in vivo binding assays, we showed that PP2A interacts with the T-motif of hTERT (495–604 aa), and regulates the subcellular localization of hTERT. This data further supports a functional role of the T-motif in subcellular localization of hTERT prior to telomerase holoenzyme assembly (Fig. 5).
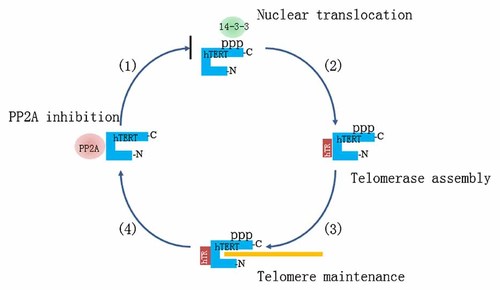
Model of PP2A inhibition of hTERT nuclear translocation, thus assembly with hTR and maintenance of telomeres. (1) PP2A binding the T-motif of hTERT dephosphorylates the C-terminal phosphorylation sites of hTERT, and thus prevents 14-3-3 binding and nuclear localization. (2) In absence of PP2A, hTERT assembles with hTR in a T-motif dependent manner. (3) Telomerase holoenzyme caps and lengths telomere DNA. (4) hTERT recycles from telomere DNA and hTR for dephosphorylation by PP2A by an unknown mechanism.
Post-translational modification of a protein by phosphorylation and dephosphorylation is one of the most common mechanisms for modulating the activity of most eukaryote proteins. It controls a protein behavior such as conformation, stability, subcellular localization, and mode of interaction with interacting protein partners, and thereby extends the range of functions of the protein. Post-translational modification of hTERT is not yet established. TERT can be phosphorylated and telomerase activity is regulated by kinases such as c-Abl, PKC, ERK1/2, and Akt [Li et al., 1998; Kang et al., 1999; Kharbanda et al., 2000; Chang et al., 2006]. On the other hand, protein phosphatases that dephosphorylated hTERT and regulate telomerase activity were also reported. Earlier study has shown that protein Ser/Thr phosphatase PP2A regulates telomerase activity in vitro [Li et al., 1997], but the mechanism is unknown. Recent studies show that nuclear protein tyrosine phosphatase Shp-2 regulates telomerase activity by retaining TERT in the nucleus through dephosphorylation on tyrosine 707 in hTERT [Jakob et al., 2008]. Our data hereby showed that PP2A interacts with the T-motif of hTERT and negatively regulates hTERT nuclear localization and telomerase activity. Collectively, these studies suggest that phosphorylation or dephosphorylation of hTERT is involved in multiple activities of hTERT depending on the specific site of dephosphorylation and net state of phosphorylation.
PP2A core enzyme associates with a variable regulatory subunit to specifically recognize different substrates, thus it is possible that by combining different regulatory subunits, PP2A recognizes different phosphorylation sites on hTERT and regulates its activity under certain conditions. Telomerase improves mitochondrial function to against oxidative stress and apoptosis [Sharma et al., 2003; Indran et al., 2011]. In addition, both acute oxidative stress in 293 cells treated by H2O2 and endogenous chronic oxidative stress in senescent endothelial cells induce the export of hTERT from the nucleus [Kumagai and Dunphy, 1999; Yang et al., 1999], suggesting that the subcellular localization of hTERT plays a role in oxidative stress response. Evidence suggests that PP2A is a critical regulator in the PI3K-Akt and Bcl-2 survival pathways and has a regulatory function in apoptosis [Garcia et al., 2003]. Therefore it is possible that oxidative stress or apoptosis signaling affects the activity of PP2A, which in turn regulates hTERT subcellular distribution and activity.
14-3-3 Protein is a conserved protein family and plays important roles in a wide range of cellular regulatory processes. 14-3-3 Protein is able to bind functionally diverse signaling proteins, and the binding is often dependent on phosphorylation of its partner protein by protein kinases, and is prevented by protein phosphatases [Fu et al., 2000; Muslin and Xing, 2000]. Our results indicate that PP2A affects the interaction of hTERT with 14-3-3θ. PP2A is likely to alter the phosphorylation state of hTERT, thereby inhibiting hTERT interaction with 14-3-3 and thus nuclear translocation. Since the T-motif of hTERT is required for hTR binding, T-motif interaction with PP2A may competitively inhibit hTERT binding to hTR, thus inhibiting telomerase activity (Fig. 5). Given that both PP2AC and PR65 are proposed to be function as tumor suppressor [Wang et al., 1998; Janssens et al., 2005], and telomerase is required for cellular immortality and is permissive for tumorigenesis, the regulation of hTERT subcellular localization or telomerase activity by PP2A may play a role in PP2A tumor suppressor function.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China [31071200, 31171320] and the National Basic Research Program of China [2012CB911203].




